Abstract
Acute biliary pancreatitis (ABP) with a high mortality rate is an incurable digestive system disease induced by abnormal bile acid regurgitation due to the biliary obstruction. Dehydrocholic acid (DA) alleviates the severity of cholestatic hepatitis related to biliary inflammation, suggesting DA is potential to develop for the incurable ABP management. Here we identified DA potency and explored the underlying mechanism in ABP. Our data showed that DA administration not only reduced typically clinicopathological parameters including serum levels of amylase and lipase but also suppressed pancreatic tissue edema, necrosis and trypsin activation in ABP mice. We also found that DA significantly reduced the necrosis of pancreatic acinar cells induced by sodium taurocholate (NaT). Further experimental data showed the significant inhibitions of DA on mitochondrial membrane potential depolarization, ATP exhaustion, calcium overload and reactive oxygen species (ROS) erupted in acinar cells induced by NaT, indicating DA could avert acinar cell death through protecting the mitochondrial function, scavenging excessive oxidative stress and balancing calcium. The comprehensive study found DA elevated the expression of transcription factor EB (TFEB) in vitro thus to increase the functional lysosome content. Indeed, DA decreased the Microtubule-associated protein light chain 3 (LC3) II/I ratio as well as ubiquitin-binding protein p62 and Parkin expressions in vivo and in vitro, revealing autophagy restoration maybe through the improvement of TFEB-mediated lysosome biogenesis. These data indicate that DA improves ABP through the mitochondrial protection, antioxidant ability enhancement and autophagy recovery. In conclusion, our study proposes a potential therapy strategy for the incurable ABP.
INTRODUCTION
Acute pancreatitis (AP) is a sudden local inflammation occurring in pancreas with increasing morbidity which could be life-threatened. Gallstone-induced AP, termed as acute biliary pancreatitis (ABP), accounts for approximately 40% of AP incidence.1) Several AP cases would develop into a severe systemic inflammatory response resulting in morbidity from multiple organ failure,2,3) and compared with the general population, people with AP history are prone to growing prevalence of pancreatic cancer.4) However, there is no effective agents for AP therapy due to the unclear pathogenesis. Pancreatic acinar cells synthesize, store and secrete digestive enzymes to sustain exocrine pancreas function, therefore disorder of acinar cells can initiate pancreatitis.5) Bile regurgitation into pancreas induced ABP6) is thought to untimely activate the trypsinogen which could induce the extensive acinar cell necrosis as well as inflammatory cascade and propagation.7) In addition, activated trypsin existed in bile acid might back-diffuse into pancreatic interstitium via a compromised duct epithelium.8)
As the main organelle for ATP synthesis and energy supply,9,10) the dysfunction of mitochondria has been implicated in acinar cell death during AP including ABP.11,12) Our previous studies have clarified that mitochondrial damage occurrence in sodium taurocholate (NaT)-induced ABP showing as the loss of mitochondrial membrane potential and mitochondrial swelling.13,14) Mitochondrial injury in acinar cells resulting from abnormal cell calcium overload represents as the loss of mitochondrial membrane potential and ability to generate ATP,15) which is often accompanied by a series of pathological phenotypes, including massive production of reactive oxygen species (ROS), cell apoptosis and necrosis, as well as the generation and spread of inflammatory mediators.16) It is reported that ROS generated from mitochondrial dysfunction provokes the damage of acinar cells due to the direct harm to intracellular lipid and protein in AP.17) The broken mitochondria are found to be selectively eliminated through mitophagy, a particular mode of autophagy,18) whose impairment could aggravate mitochondrial damage. Recently, reports reveal autophagy disability occurred during ABP, showing as the accumulation of autophagosome consequent upon the decrease capacity of lysosome degradation.19,20)
Lysosome is a single-membrane-bond organelle which contains plenty of hydrolases, especially as Cathepsin B and Cathepsin L.21) Cargoes destined for degradation are internalized by endocytosis or sequestered in autophagosomes, and then fused with lysosome forming autolysosome. Therefore, abnormal lysosome function can lead to the impairment of autophagy degradation.22,23) The transcription factor EB (TFEB) as the master gene controls the lysosome biogenesis and coordinates the massive transcription program to promote autophagy-relative gene upregulation.24) The impairment of TFEB mediating lysosome biogenesis damage in response to aberrant Ca2+ signaling in acinar cells has been demonstrated underlying the pathogenesis of caerulein induced AP.20,25)
Dehydrocholic acid (DA), which is commonly used in clinical for cholestatic hepatitis, could promote the drainage of biliary duct and sustain its cleaning by stimulating the secretion of bile from hepatocytes thereby reducing the incidence of the hepatitis. However, the effect of DA on the incurable ABP related to biliary obstruction is rarely reported in our knowledge. In this study, we examined the role of DA on NaT-induced AP, a classic ABP mouse model which mimics bile regurgitation induced AP elicited by pancreatobiliary obstruction. We also assessed the mitochondrial function through the detections of mitochondrial membrane potential, ATP level and mitochondrial permeability transition pore (mPTP) opening. Further studies focused on autophagy level and lysosome content during ABP were carried out. Overall, our data show that DA is a potential candidate for ABP therapy through autophagy improvement to prevent mitochondrial dysfunction thus to suppress the necrosis of acinar cells.
MATERIALS AND METHODS
MaterialsCollagenase IV, NaT, MitoSOX were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). Hoechst 33342, propidium iodide (PI) were bought from Molecular Probes (Eugene, OR, U.S.A.). Fluo-4 AM, Rhod-2 AM were obtained from Thermo Fisher Scientific (Waltham, MA, U.S.A.). 2′,7′-Dichlorodihydrofluorescein diacetate (H2DCHFDA), JC-1 assay kit, ATP assay kit, bicinchoninic acid (BCA) protein assay kit and RIPA lysis buffer were purchased from Beyotime Biotech (Shanghai, China). Malondialdehyde (MDA) assay kit and superoxidase dismutase (SOD) assay kit were gained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Boc-Gln-Ala-Arg-7-amido-4-methylcoumarin hydrochloride was purchased from Peptide (Ibaraki, Japan) and protease inhibitor cocktail tablets were from Roche Diagnostics (Mannheim, Germany). Antibodies were bought from Cell Signaling Technology Inc. (Beverly, MA, U.S.A.). DA and other reagents were obtained from common commercial sources.
AnimalsAll animal experiments and methods were carried out with the prior ethics approvals of Ethics Committee of West China Hospital of Sichuan University and under the guidance of ARRIVE guideline. Specified pathogen free (SPF) male C57BL/6 mice (8–12 weeks of age, 25–30 g) were hosted in a monitored room with a 12 h light/dark cycle under the temperature of 22°C and fed with standard food and water. All animals were allowed to acclimatize for at least 1 week preceding the experiments with free gain of water and food.
Acinar Cell PreparationPrimary pancreatic acinar cells were obtained from C57BL/6 mice using a collagenase digestion procedure as described.26) In brief, fresh pancreas obtained from C57BL/6 male mouse was digested by collagenase IV (200 U/mL) incubation for 19 min at 37°C. After incubation, pancreas was disintegrated mechanically and filtered through a 100 µm cell strainer and primary acinar cells were obtained by centrifuged at 110 × g for 2 min. Acinar cells were cultured in N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid (HEPES) buffer which contained (in mM) 140 NaCl, 1.13 MgCl2, 4.7 KCl, 1 CaCl2, 10 D-glucose, 10 HEPES (adjusted to pH 7.35 using NaOH) and used within 4 h. Each pancreas provides approximately 17.5 × 106 cells.
NaT-Induced Acinar Cell Death Measurement in VitroFresh isolated acinar cells were stimulated by NaT (5 mM) with or without DA at 37°C and then stained with fluorescent dyes Hoechst 33342 (50 µg/mL) and PI (1 µM) respectively for total cell and dying cells counting.13) Images were captured by fluorescence microscopy ZEISS AX10 imager A2/AX10 cam HRC (Jena GmbH, Germany). The total number of cells absorbing PI was counted from each condition to provide the percentage (necrosis %), with a minimum of 1000 cells counted.
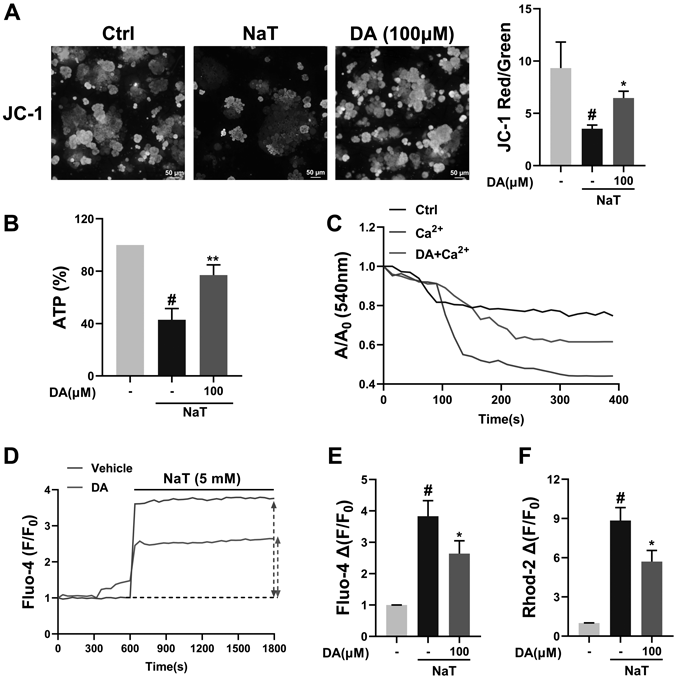
Mitochondrial Membrane Potential (ΔΨm) MeasurementIsolated acinar cells treated with NaT at the presence or absence of DA (100 µM) were prepared for ΔΨm measurement by JC-1 mitochondrial membrane potential kit. Briefly, isolated acinar cells were stimulated by NaT with or without DA, and then stained by JC-1 dye. The fluorescent intensity was detected by fluorescence microscopy ZEISS AX10 imager A2/AX10 cam HRC (Jena GmbH). The depolarization of ΔΨm was expressed as the ratio of red/green fluorescence intensity. Data acquisition and processing were from five separate isolates per condition.
ATP Level MeasurementThe ATP level of acinar cells were detected by ATP assay kit under the direction of manufacturer’s specification. Primary acinar cells were treated by NaT (5 mM) or DA (100 µM). After that, cells were extracted from culture system and resuspended in 200 µL lysis buffer, and centrifuged at 12000 × g for 5 min at 4°C. Luminescence in the supernatant was tested by SynergyMx multifunctional Microplate Reader (Gene Company Ltd., China). The data were normalized to protein content and equality to vehicle as 100%.
Total and Mitochondrial ROS MeasurementsTotal and mitochondrial ROS in acinar cell treated with NaT and/or DA were determined respectively by H2DCFDA and MitoSOX fluorescent dyes. In brief, acinar cells were pre-treated by DA (100 µM) and then stained with H2DCFDA or MitoSOX for 20 min and the fluorescence was recorded as F0 (F0 as the baseline). Then acinar cells were incubated with NaT (5 mM) and the fluorescence was recorded as F by a Synergy Mx multifunctional Microplate Reader for 25 min. The data were expressed as F0/F.
Calcium Content MeasurementCalcium contents in cytoplasm and mitochondria were determined by Fluo-4 AM and Rhod-2 AM under the guidance of manufacturers’ instructions as described before.27,28) Isolated primary acinar cells suspended in HEPES buffer without Ca2+ were incubated with DA (100 µM) and stained respectively by Fluo-4 AM and Rhod-2 AM for 30 min. After staining, cells were washed for twice and the fluorescence intensity was dynamically recorded with the treatment of NaT (5 mM) by a Synergy Mx multifunctional Microplate Reader (BioTek Instruments, Inc., U.S.A.). The data were expressed as F0/F (F0 as the baseline before NaT addition).
Mitochondrial Swelling MeasurementIsolated acinar cell mitochondria were utilized to determine the protective effect of DA on mitochondrial damage as previous study described.13) Isolated pancreas mitochondria were pretreated with 10 µM DA for 15 min and stimulated by 0.3 mM free Ca2+. The absorbance at 540 nm was detected for 400 s after its addition.
MDA and SOD Activity MeasurementsThe MDA level and SOD activity of acinar cells with or without NaT and DA were determined by MDA assay kit and SOD assay kit as the manufacturers’ instructions.
Lysosome Content MeasurementLysoTracker Red (Beyotime) was used to stain lysosome based on the manufacturer’s protocol. In brief, acinar cells were stimulated by NaT with or without DA before LysoTracker Red (2 µM) 20 min-incubation. After staining termination, cells were washed for twice by HEPES buffer and the images were captured by a fluorescence microscope (Zeiss, Axiovert 200, Germany).
Experimental ABP ModelBefore induction of ABP, all the mice were fasted for 18 h and divided into four different groups: the control group (Ctrl), the ABP group (NaT), DA groups (25, 50 mg/kg). Mice were retrograde injected of 3% NaT (4 mL/kg) or an equivalent volume of saline respectively as ABP group or control group. DA (25, 50 mg/kg) was given by intraperitoneal (i.p.) injection 1, 3 and 6 h after the surgery for three times. Normal saline with the equal volume of drug groups was given to control and NaT group by i.p. All the mice were sacrificed at 24 h after surgery.
Histopathology AnalysisPancreas tissue was separated and fixed in 4% paraformaldehyde overnight. Then tissue was dehydrated by ethanol and embedded in paraffin. After sectioned, the haematoxylin and eosin (H&E) stain was carried out. The pathological sections were observed by an optical microscope (ZEISS, Jena GmbH). Histopathological scores of pancreas and lung including edema, inflammatory infiltration and necrosis were judged blindly by two pathologists from 0 to 3 (see supplementary material Table S1).
Serum Amylase and Lipase MeasurementsWhole blood obtained from mice was centrifuged at 986 × g for 15 min. And the serum amylase and lipase were determined by a fully automatic biochemical analyzer (Roche, Mannheim, Germany).
Myeloperoxidase (MPO) and Trypsin Activity MeasurementsThe MPO and trypsin activities of pancreas were tested as the previous study described.14) The quantification of total protein contents was determined by BCA protein assay kit.
Western Blot AnalysisProteins of acinar cells and pancreas were homogenized in a RIPA lysis buffer including freshly added phenylmethylsulfonyl fluoride (PMSF) and protease inhibitors. After target proteins were separated by 10–15% polyacrylamide gel, they were transferred onto Polyvinylidene difluoride (PVDF) membrane followed by blocking and incubation with primary antibodies as well as secondary antibodies sequentially.29) The flashers were observed by enhanced chemiluminescence (ECL) Western blotting reagents.
Statistical AnalysisAll the statistical analyses in the study were performed in Prism software 8.0 as one-way ANOVA followed by Student’s t-tests (GraphPad Software Inc.). All the experiments were repeated at least for three independent times and data were expressed as means ± standard deviation (S.D.). p < 0.05 was considered as a significant difference.
RESULTS
Effect of DA on NaT-Induced Acinar Cell Death in VitroPrimary acinar cells isolated from mice pancreas were stained with Hoechst and propidium iodide (PI) to evaluate acinar cell death (Fig. 1A). We first detected the effect of DA on normal acinar cells, and found DA had no significant cytotoxicity of acinar cells, as quantified in Fig. 1B. The death of acinar cells induced by NaT was remarkably decreased by DA incubation especially at the dose of 100 µM (Fig. 1C).
Palmitoleic acid ethyl ester (POAEE) is well-known to induce pancreatic acinar cell injury due to the excessive consumption of ethanol.30) Therefore, we also treated the acinar cells with POAEE to induce damage, and the effects of DA with varying concentrations were evaluated. The results indicated that DA could suppress the acinar cell necrosis induced not only by NaT but also POAEE (Supplementary Fig. 1), suggesting that DA did not replace with NaT to prevent the damage of acinar cells.
Effect of DA i.p. on NaT-Induced ABP in VivoC57BL/6 male mice were utilized to induce experimental ABP by NaT retrograde injection, and DA (25, 50 mg/kg) was administrated by i.p. for three times after the surgery (Fig. 2A). The pathological changes of pancreas from mice were determined by H&E staining. As Fig. 2B shows, conspicuous damage occurred in pancreas from mice with NaT injection (3%) such as edema, inflammation infiltration, and necrosis compared with control mice. DA administration (25, 50 mg/kg) significantly ameliorated NaT-induced pancreatic injury in a dose-dependent manner (Figs. 2C–E).
Effects of DA on Serum Amylase and Lipase as Well as Pancreatic Trypsin and MPO in ABP MiceSerum amylase and lipase as the clinicopathological parameters for ABP diagnosis are also utilized to determine the severity of experimental ABP.31) As Figs. 3A and B show, the ascendant serum levels of amylase and lipase in NaT-treated mice were both inhibited by DA treatment. Untimely activation of pancreatic trypsin is considered as the cornerstone of pancreatic injury leading to events related to acinar cell death.7) NaT injection increased the trypsin activity in pancreas, while DA treatment decreased its activation (Fig. 3C). To evaluate pancreatic oxidative injury, we determined pancreatic MPO after DA treatment at the concentration of 25 or 50 mg/kg, and found DA suppressed the significant increase of pancreatic MPO level in ABP mice (Fig. 3D).
Effect of DA on Acinar Cell Mitochondrial Damage in Response to NaT StimulationMitochondrial damage is a core event resulting in acinar cell death.32) Mitochondrial membrane potential (ΔΨm) was detected using JC-1 membrane potential assay kit. The depolarization of ΔΨm represented as the decreased ratio of red/green fluorescence intensity. As Fig. 4A shows, the decreased fluorescence ratio induced by NaT was recovered with DA treatment. DA interference also improved the exhaustion of ATP resulted from NaT-treatment (Fig. 4B). The swelling of isolated mitochondria in response to Ca2+ stimulation was decreased by DA treatment (Fig. 4C). We also detected the cytoplasmic and mitochondrial calcium levels, and found that DA incubation significantly prevented calcium overload both in mitochondria and cytoplasm compared with NaT-only-treated acinar cells (Figs. 4D–F).
Effect of DA on NaT-Induced Acinar Cell Oxidative StressOxidative stress due to mitochondrial damage is a pathological event resulting in the acinar cell damage.33) We detected the ROS generated from mitochondria, and found the suppression of DA on excessive mitochondrial ROS (mtROS) production in response to NaT (Figs. 5A, C). Total ROS detected by the general oxidative stress indicator H2DCFDA was scavenged with the interference of DA (Figs. 5B, D). We also found that DA reduced the elevated MDA, a lipid peroxidation index, in acinar cells treated with NaT (Fig. 5E). Moreover, DA significantly recovered the activity of SOD, a scavenger of ROS, which was suppressed in NaT-treated acinar cells (Fig. 5F).
Effect of DA on Autophagy in NaT-Induced ABP in Vitro and in VivoGiven the consideration of autophagy blockage during AP,21,34,35) we detected protein expressions associated with autophagy by Western blot. As shown in Figs. 6A and B, compared with acinar cells treated with NaT alone, DA incubation down-regulated the LC3 II/I ratio and p62 expression, revealing the autophagy damage was remarkably improved. The increased expression of Parkin in NaT-treated acinar cells,36) a recognized receptor of mitophagy, was suppressed by DA coincubation. The similar results of protein expressions were observed in pancreas tissues obtained from ABP mice with or without DA administration (Figs. 6C, D).
To explore the mechanism by which DA restored autophagy blockage, we measured the functional lysosome amount by LysoTracker Red. As our consideration, DA could increase the functional lysosome content in acinar cells treated with NaT (Fig. 6E). TFEB as a master controller of autophagy is discovered to mediate the lysosome biogenesis37) which is impaired in experimental AP and pancreatic patients.20,25) We found that DA up-regulated the expression of TFEB in vitro and in vivo compared with NaT-treatment (Figs. 6A–D), indicating that DA could alleviate lysosome biosynthesis damage in ABP acinar cells which contribute to autophagy recovery in acinar cells through increasing TFEB expression.
DISCUSSION
In this report, we verified for the first time the effect of DA on ABP, an uncurable disease with no specific medical therapy, by using NaT-induced ABP mouse model which represents bile regurgitation-induced AP caused by the obstruction of biliary tract.38) We also demonstrated that DA performed protection on NaT-ABP through the improvement of mitochondrial dysfunction, oxidative stress and autophagy blockage. Furthermore, DA was found to increase TFEB-mediated lysosome biogenesis, which might be the underlying therapeutic mechanism of DA on ABP.
Mitochondrion plays a pivotal role in the pathogenesis of AP including ABP.12) Mitochondrial membrane potential (MMP) depolarization and ATP exhaustion have been proved in copious researches about AP.13,36) Our results showed that DA treatment significantly suppressed NaT-induced MMP depolarization and ATP exhaustion in acinar cells. Plenty of researchers have verified the regulatory effect of calcium in a wide range of disease progression, including in AP.39,40) Researches about calcium release-activated calcium channel protein 1 (ORAI)31) and endoplasmic reticulum (ER)-calcium release receptor inositol trisphosphate receptor (IP3R)41) revealed the key role of calcium in acinar cell physiology and ABP pathology. In this research, DA was first demonstrated to regulate the calcium oscillation as the prohibition of calcium overload both in acinar cell cytoplasm and mitochondria in response to NaT. As such, calcium signaling regulation in acinar cells could be the underlying mechanism of acinar cell mitochondrial protection of DA during ABP.
Mitochondrion with disrupted respiratory chain causes the generation of excessive ROS, leading to acinar cell oxidative damage and death.42) Our data confirmed that DA restrained ROS eruption stimulated by NaT both in mitochondria and cytoplasm, represented respectively as the descendent fluorescent density of MitoSOX and H2DCFDA. Acinar cells with oxidative stress are susceptible to lipid peroxidation leading to the production of MDA.43) DA was certified to suppress the MDA level and restore the SOD activity compared with ABP model in vitro. These findings further provide the evidence that the antioxidation is relative to the protective effect of DA against NaT-induced ABP.
Recently, it is proposed that autophagy impairment is the major pathological event in AP patients and animal models.25) Autophagy blockage mediated by gene modification contributes to the cause and development of pancreatitis.19,44) Damaged mitochondrion elimination relies on a special form of autophagy termed as mitophagy,45) whose impairment could exacerbate the cell phenotypes relative to mitochondrial damage including oxidative stress, cell apoptosis and necrosis. We observed that DA reduced the ratio of LC3II/I and the accumulation of p62 compared to NaT groups. And consistent with the research,36) as the critical mediator of mitophagy, Parkin expression was increased in ABP acinar cells and pancreas tissue. DA treatment down-regulated its expression compared with NaT-only treated groups. Lysosome is widely known in autophagy clearance46) and the previous studies have demonstrated that lysosome impaired during AP and associated with human pancreatitis.47,48) Wang and colleagues demonstrated that TFEB-mediated lysosomal biogenesis was impaired during pancreatitis which promoted the development of the disease.37) We stained acinar cells by LysoTracker Red to evaluate the lysosome and autolysosome structures and detected the expression of TFEB in acinar cells and pancreas, and as our hypothesis, DA could increase TFEB-mediated lysosome biogenesis. Our results indicate DA could alleviate TFEB-mediated lysosome biogenesis thus to restore the damaged autophagic flux during ABP model in vitro and in vivo. However, the more precise regulatory mechanism underlying the protective effect of DA on TFEB-mediated lysosome biogenesis and relative autophagy program needs to be done in near future.
To sum up, our researches support a role of DA to improve ABP such as reducing pancreatic histopathological alterations as well as serum amylase and lipase by function as an autophagy guard by ameliorating TFEB-mediated lysosome biogenesis. DA improved mitophagy damage and mitochondrial dysfunction thus to suppress oxidative stress and acinar cell necrosis. Taken together, we provide proof of concept that DA is potential for the therapy of ABP, a disease without specific medical therapy.
Acknowledgments
We would like to show our appreciation to Bin Zhou for providing ChemiDoc™–XRS imaging system, Jingyao Zhang and Fulai Xue for using fluorescence microplate reader, Yi Zhang for the user guidance of automatic fluorescence microscope. We also thank Guang Yang, Xijing Yang and Jianjun Chen for providing animal care. This work was financed by Grant-in-Aid for Scientific Research from the National Major Scientific and Technological Special Project for “Significant New Drugs Development” (Grant Nos. 2019ZX09201005-005-001, 2019ZX09201005-005-004), the National Natural Science Foundation of China (Grant Nos. 81673710, 81973580), the National Science Foundation for Young Scientists of China (Grant No. 81803866), the Project funded by China Postdoctoral Science Foundation (Grant Nos. 2018M640932, 2019T120854, 2019M663533), the Applied Basic Research Programs of Department of Science and Technology of Sichuan Province (Grant No. 2019YJ0095), the Post-Doctor Research Project, West China Hospital, Sichuan University (Grant Nos. 2018HXBH061, 2019HXBH024), Innovative Chinese Medicine and Health Products Research Academician Workstation of Academician Zhang Boli and Academician Zhu Beiwei, West China Hospital, Sichuan University (Grant Nos. HXYS19001, HXYS19002), and Postdoctoral Interdisciplinary Research Project of Sichuan University (Grant No. 2019JCJX3245).
Conflict of Interest
The authors declare no conflict of interest.
Supplementary Materials
The online version of this article contains supplementary materials.
REFERENCES
- 1) Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet, 371, 143–152 (2008).
- 2) Koutroumpakis E, Wu BU, Bakker OJ, Dudekula A, Singh VK, Besselink MG, Yadav D, Mounzer R, van Santvoort HC, Whitcomb DC, Gooszen HG, Banks PA, Papachristou GI. Admission hematocrit and rise in blood urea nitrogen at 24 h outperform other laboratory markers in predicting persistent organ failure and pancreatic necrosis in acute pancreatitis: a post hoc analysis of three large prospective databases. Am. J. Gastroenterol., 110, 1707–1716 (2015).
- 3) Lagoo JY, D’Souza MC, Kartha A, Kutappa AM. Role of Ulinastatin, a trypsin inhibitor, in severe acute pancreatitis in critical care setting: a retrospective analysis. J. Crit. Care, 45, 27–32 (2018).
- 4) Zhang X, An R, Tian H, Zhao J. Increased risk of pancreatic cancer after acute pancreatitis: a meta-analysis of prospective cohort studies. Clin. Res. Hepatol. Gastroenterol., 43, 39–41 (2018).
- 5) Habtezion A, Gukovskaya AS, Pandol SJ. Acute pancreatitis: a multifaceted set of organelle and cellular interactions. Gastroenterology, 156, 1941–1950 (2019).
- 6) Criddle DN, Murphy J, Fistetto G, Barrow S, Tepikin AV, Neoptolemos JP, Sutton R, Petersen OH. Fatty acid ethyl esters cause pancreatic calcium toxicity via inositol trisphosphate receptors and loss of ATP synthesis. Gastroenterology, 130, 781–793 (2006).
- 7) Saluja A, Dudeja V, Dawra R, Sah RP. Early intra-acinar events in pathogenesis of pancreatitis. Gastroenterology, 156, 1979–1993 (2019).
- 8) Metz J, Forssmann WG. Exocrine pancreas under experimental conditions. Cell Tissue Res., 173, 221–235 (1976).
- 9) Spinelli JB, Haigis MC. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol., 20, 745–754 (2018).
- 10) Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell, 148, 1145–1159 (2012).
- 11) Maleth J, Rakonczay Z Jr, Venglovecz V, Dolman NJ, Hegyi P. Central role of mitochondrial injury in the pathogenesis of acute pancreatitis. Acta Physiol., 207, 226–235 (2013).
- 12) Odinokova IV, Sung KF, Mareninova OA, Hermann K, Gukovsky I, Gukovskaya AS. Mitochondrial mechanisms of death responses in pancreatitis. J. Gastroenterol. Hepatol. Res., 23 (Suppl. 1), S25–S30 (2008).
- 13) Shen Y, Wen L, Zhang R, Wei Z, Shi N, Xiong Q, Xia Q, Xing Z, Zeng Z, Niu H, Huang W. Dihydrodiosgenin protects against experimental acute pancreatitis and associated lung injury through mitochondrial protection and PI3Kgamma/Akt inhibition. Br. J. Pharmacol., 175, 1621–1636 (2018).
- 14) Zhang R, Wen L, Shen Y, Shi N, Xing Z, Xia Q, Niu H, Huang W. One compound of saponins from Disocorea zingiberensis protected against experimental acute pancreatitis by preventing mitochondria-mediated necrosis. Sci. Rep., 6, 35965 (2016).
- 15) Yadav D, Slivka A, Sherman S, Hawes RH, Anderson MA, Burton FR, Brand RE, Lewis MD, Gardner TB, Gelrud A, Disario J, Amann ST, Baillie J, Lawrence C, O’Connell M, Lowenfels AB, Banks PA, Whitcomb DC. Smoking is underrecognized as a risk factor for chronic pancreatitis. Pancreatology, 10, 713–719 (2010).
- 16) Kowaltowski AJ, Vercesi AE. Mitochondrial damage induced by conditions of oxidative stress. Free Radic. Biol. Med., 26, 463–471 (1999).
- 17) Tsai K, Wang SS, Chen TS, Kong CW, Chang FY, Lee SD, Lu FJ. Oxidative stress: an important phenomenon with pathogenetic significance in the progression of acute pancreatitis. Gut, 42, 850–855 (1998).
- 18) Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ., 20, 31–42 (2013).
- 19) Antonucci L, Fagman JB, Kim JY, Todoric J, Gukovsky I, Mackey M, Ellisman MH, Karin M. Basal autophagy maintains pancreatic acinar cell homeostasis and protein synthesis and prevents ER stress. Proc. Natl. Acad. Sci. U.S.A., 112, E6166–E6174 (2015).
- 20) Wang S, Ni HM, Chao X, Wang H, Bridges B, Kumer S, Schmitt T, Mareninova O, Gukovskaya A, De Lisle RC, Ballabio A, Pacher P, Ding WX. Impaired TFEB-mediated lysosomal biogenesis promotes the development of pancreatitis in mice and is associated with human pancreatitis. Autophagy, 15, 1954–1969 (2019).
- 21) Mareninova OA, Hermann K, French SW, O’Konski MS, Pandol SJ, Webster P, Erickson AH, Katunuma N, Gorelick FS, Gukovsky I, Gukovskaya AS. Impaired autophagic flux mediates acinar cell vacuole formation and trypsinogen activation in rodent models of acute pancreatitis. J. Clin. Invest., 119, 3340–3355 (2009).
- 22) Settembre C, Fraldi A, Rubinsztein DC, Ballabio A. Lysosomal storage diseases as disorders of autophagy. Autophagy, 4, 113–114 (2008).
- 23) Settembre C, Fraldi A, Jahreiss L, Spampanato C, Venturi C, Medina D, de Pablo R, Tacchetti C, Rubinsztein DC, Ballabio A. A block of autophagy in lysosomal storage disorders. Hum. Mol. Genet., 17, 119–129 (2008).
- 24) Ide S, Beroza GCH, Kanamori M, Kikuchi A, Huynh T. TFEB Links autophagy to lysosomal biogenesis. Science, 1429, 24–6303 (2011).
- 25) Zhu ZD, Yu T, Liu HJ, Jin J, He J. SOCE induced calcium overload regulates autophagy in acute pancreatitis via calcineurin activation. Cell Death Dis., 9, 50 (2018).
- 26) Criddle DN, Gerasimenko JV, Baumgartner HK, Jaffar M, Voronina S, Sutton R, Petersen OH, Gerasimenko OV. Calcium signalling and pancreatic cell death: apoptosis or necrosis? Cell Death Differ., 14, 1285–1294 (2007).
- 27) Lewarchik CM, Orabi AI, Jin S, Wang D, Muili KA, Shah AU, Eisses JF, Malik A, Bottino R, Jayaraman T, Husain SZ. The ryanodine receptor is expressed in human pancreatic acinar cells and contributes to acinar cell injury. Am. J. Physiol. Gastrointest. Liver Physiol., 307, G574–G581 (2014).
- 28) Camello-Almaraz C, Salido GM, Pariente JA, Camello PJ. Role of mitochondria in Ca(2+) oscillations and shape of Ca(2+) signals in pancreatic acinar cells. Biochem. Pharmacol., 63, 283–292 (2002).
- 29) Dong K, Chen X, Xie L, Yu L, Shen M, Wang Y, Wu S, Wang J, Lu J, Wei G, Xu D, Yang L. Spautin-a41 attenuates cerulein-induced acute pancreatitis through inhibition of dysregulated autophagy. Biol. Pharm. Bull., 42, 1789–1798 (2019).
- 30) Voronina SG, Barrow SL, Simpson AW, Gerasimenko OV, da Silva Xavier G, Rutter GA, Petersen OH, Tepikin AV. Dynamic changes in cytosolic and mitochondrial ATP levels in pancreatic acinar cells. Gastroenterology, 138, 1976–1987 (2010).
- 31) Wen L, Voronina S, Javed MA, et al. Inhibitors of ORAI1 prevent cytosolic calcium-associated Injury of human pancreatic acinar cells and acute pancreatitis in 3 Mouse Models. Gastroenterology, 149, 481–492.e487 (2015).
- 32) Shalbueva N, Mareninova OA, Gerloff A, Yuan J, Waldron RT, Pandol SJ, Gukovskaya AS. Effects of oxidative alcohol metabolism on the mitochondrial permeability transition pore and necrosis in a mouse model of alcoholic pancreatitis. Gastroenterology, 144, 437–446.e436 (2013).
- 33) Booth DM, Mukherjee R, Sutton R, Criddle DN. Calcium and reactive oxygen species in acute pancreatitis: friend or foe? Antioxid. Redox Signal., 15, 2683–2698 (2011).
- 34) Gukovsky I, Gukovskaya AS. Impaired autophagy underlies key pathological responses of acute pancreatitis. Autophagy, 6, 428–429 (2010).
- 35) Sah RP, Garg P, Saluja AK. Pathogenic mechanisms of acute pancreatitis. Curr. Opin. Gastroenterol., 28, 507–515 (2012).
- 36) Biczo G, Vegh ET, Shalbueva N, Mareninova OA, Elperin J, Lotshaw E, Gretler S, Lugea A, Malla SR, Dawson D, Ruchala P, Whitelegge J, French SW, Wen L, Husain SZ, Gorelick FS, Hegyi P, Rakonczay Z Jr, Gukovsky I, Gukovskaya AS. Mitochondrial dysfunction, through impaired autophagy, leads to endoplasmic reticulum stress, deregulated lipid metabolism, and pancreatitis in animal models. Gastroenterology, 154, 689–703 (2018).
- 37) Chao X, Wang S, Zhao K, Li Y, Williams JA, Li T, Chavan H, Krishnamurthy P, He XC, Li L, Ballabio A, Ni HM, Ding WX. Impaired TFEB-mediated lysosome biogenesis and autophagy promote chronic ethanol-induced liver injury and steatosis in mice. Gastroenterology, 155, 865–879.e812 (2018).
- 38) Lerch MM, Gorelick FS. Gorelick, Fred SJG. Models of acute and chronic pancreatitis. Gastroenterology, 144, 1180–1193 (2013).
- 39) Peng TI, Jou MJ. Oxidative stress caused by mitochondrial calcium overload. Ann. N. Y. Acad. Sci., 1201, 183–188 (2010).
- 40) Frick TW. The role of calcium in acute pancreatitis. Surgery, 152 (Suppl 1), S157–S163 (2012).
- 41) Boehning D, van Rossum DB, Patterson RL, Snyder SH. A peptide inhibitor of cytochrome c/inositol 1,4,5-trisphosphate receptor binding blocks intrinsic and extrinsic cell death pathways. Proc. Natl. Acad. Sci. U.S.A., 102, 1466–1471 (2005).
- 42) Booth DM, Murphy JA, Mukherjee R, Awais M, Neoptolemos JP, Gerasimenko OV, Tepikin AV, Petersen OH, Sutton R, Criddle DN. Reactive oxygen species induced by bile acid induce apoptosis and protect against necrosis in pancreatic acinar cells. Gastroenterology, 140, 2116–2125 (2011).
- 43) Altavilla D, Famulari C, Passaniti M, Campo GM, Macri A, Seminara P, Marini H, Calo M, Santamaria LB, Bono D, Venuti FS, Mioni C, Leone S, Guarini S, Squadrito F. Lipid peroxidation inhibition reduces NF-kappaB activation and attenuates cerulein-induced pancreatitis. Free Radic. Res., 37, 425–435 (2003).
- 44) Franco F, Heinrich B, Frank B, Peter R, Büchler MW, Guido K, Jens W. Impaired autolysosome formation correlates with Lamp-2 depletion: role of apoptosis, autophagy, and necrosis in pancreatitis. Gastroenterology, 137, 350–360.e355, 360.e1–360.e5 (2009).
- 45) Tanaka A. Parkin-mediated selective mitochondrial autophagy, mitophagy: Parkin purges damaged organelles from the vital mitochondrial network. FEBS Lett., 584, 1386–1392 (2010).
- 46) Kroemer G, Jäättelä M. Lysosomes and autophagy in cell death control. Nat. Rev. Cancer, 5, 886–897 (2005).
- 47) Wilson J, Apte M, Thomas M, Haber P, Pirola R. Effects of ethanol, acetaldehyde and cholesteryl esters on pancreatic lysosomes. Gut, 33, 1099–1104 (1992).
- 48) Mareninova OA, Sendler M, Malla SR, Yakubov I, French SW, Tokhtaeva E, Vagin O, Oorschot V, Lüllmann-Rauch R, Blanz J, Dawson D, Klumperman J, Lerch MM, Mayerle J, Gukovsky I, Gukovskaya AS. Lysosome-associated membrane proteins (LAMP) maintain pancreatic acinar cell homeostasis: LAMP-2–deficient mice develop pancreatitis. Cell. Mol. Gastroenterol. Hepatol., 1, 678–694 (2015).