2020 Volume 43 Issue 7 Pages 1096-1103
2020 Volume 43 Issue 7 Pages 1096-1103
P19 pluripotent embryonic carcinoma (EC) stem cells are derived from pluripotent germ cell tumours and can differentiate into three germ layers. Treatment of these cells in suspension culture with retinoic acid induces their differentiation into neurons and glial cells. Hence, these cells are an excellent in vitro model to study the transition from the upper blastoderm to the neuroectoderm. However, because of the complex nature of the techniques involved, the results are highly dependent on the skills of the experimenter. Herein, we developed a simple method to induce neuronal differentiation of adherent P19 EC cells in TaKaRa NDiff® 227 serum-free medium (originally N2B27 medium). This medium markedly induced neuronal differentiation of P19 EC cells. The addition of retinoic acid to the NDiff® 227 medium further enhanced differentiation. Furthermore, cells differentiated by the conventional method, as well as the new method, showed identical expression of the mature neuronal marker, neuronal nuclei. To determine whether our approach could be applied for neuronal studies, we measured histone deacetylase 8 (HDAC8) activity using an HDAC8 inhibitor and HDAC8-knockout P19 EC cells. Inhibition of HDAC8 activity suppressed neuronal maturation. Additionally, HDAC8-knockout cell lines showed immature differentiation compared to the wild-type cell line. These results indicate that HDAC8 directly regulates the neuronal differentiation of P19 EC cells. Thus, our method involving P19 EC cells can be used as an experimental system to study the nervous system. Moreover, this method is suitable for screening drugs that affect the nervous system and cell differentiation.
P19 pluripotent embryonic carcinoma (EC) stem cells were originally derived from a teratocarcinoma in C3H/HE mice and are the malignant counterparts of embryonic stem (ES) cells in both humans and mice.1) P19 EC cells can differentiate into three germ layers, which in turn can differentiate into various cell types including neurons, astroglia, and cardiomyocytes.2–6) Therefore, EC cells are similar in many ways to ES cells cultured from the inner cell mass of the early embryo.7) This differentiation can be regulated by the addition of drugs and modulation of the culture conditions. For example, P19 EC cells aggregate to form an embryoid body (EB) in suspension culture. Moreover, P19 EC cells can be induced to differentiate into neurons and glial cells by adding a specific concentration of retinoic acid (RA) to the culture medium.2)
The P19 EC cell model system offers several important advantages over other cell models. For example, P19 EC cells can be used to conduct preliminary studies on induced pluripotent stem (iPS) cells or ES cells that are expensive and difficult to maintain. These advantages render P19 EC cells a promising in vitro model for studying the molecular mechanisms underlying pluripotent stem cell differentiation.
P19 EC cells are a convenient tool for analysis of neuronal differentiation. However, EB formation and the separation process require the use of sophisticated techniques, i.e., controlled trypsinisation and pipetting to induce EB dispersion. Thus, untrained experimenters often cause cell death owing to excessive pipetting, or unequal neural differentiation from insufficient EB dispersion. Therefore, induced differentiation of P19 EC cells in suspension culture yields variable results depending on the skills of the experimenter.
Establishment of a consistent experimental system is important in studies involving P19 EC cells. In particular, there is a need to develop a simple method to induce neuronal differentiation of these cells. EBs are formed during a critical period where there is dynamic fluctuation in the expression of transcription and growth factors such as fibroblast growth factor 8 (FGF8).8,9) Hence, stimulation that induces EB formation is necessary for differentiating adhered P19 EC cells.
Several groups have reported neuronal differentiation under adherent conditions using a medium containing FGF8 and similar stimulants.10,11) However, these techniques used multiple growth media and additives depending on the differentiated stage and require substantial preparation. Herein, we describe a simple protocol to induce neuronal differentiation of adherent P19 EC cells using only TaKaRa NDiff® 227 medium (originally N2B27 medium; TaKaRa Bio, Shiga, Japan),12,13) which is widely used for neuronal differentiation of iPS cells. In addition, we examined whether our method could be used to analyse various metabolic effects on the nervous system.
Histone deacetylase 8 (HDAC8) is a class I HDAC family member that is involved in several diseases including neuroblastoma,14) the Cornelia de Lange syndrome,15) and the Rett syndrome-related disorder.16) We showed previously that HDAC8 accelerated neuronal differentiation of P19 EC cells using the classical suspension method.17) We also demonstrated that inhibition of HDAC8 activity suppressed EB formation and neuronal differentiation of P19 EC cells.17) However, it was unclear whether HDAC8 was directly involved in neuronal differentiation, as in the conventional method EBs are formed before neuronal differentiation. Thus, by establishing a method of neuronal differentiation without EB formation, it was possible to determine whether HDAC8 controlled neuronal differentiation directly or through EB formation. To this end, we developed a simple method for neural differentiation of P19 EC cells under adherent culture conditions. Moreover, we analysed HDAC8 activity in these cells after neuronal differentiation.
The HDAC8 inhibitor, NCC-149 (i.e., HDAC8i; developed by Drs. Suzuki and Miyata18,19)), was obtained from Tokyo Chemical Industry (Tokyo, Japan). Two HDAC8 knockout (KO) P19 EC cell clones (KO35 and KO41) were established previously.17) P19 EC cells were kindly provided by Dr. Miura (Nagoya City University).
Neuronal Differentiation of P19 EC Cells Using Cell Suspension Cultures (Minimum Essential Medium Eagle-Alpha Modification (MEMα) Method)P19 EC cells were cultured in an MEMα (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) supplemented with 10% foetal bovine serum (Invitrogen, Carlsbad, CA, U.S.A.) and 1% penicillin/streptomycin (Nacalai Tesque, Kyoto, Japan) at 37°C in a humidified atmosphere of 5% CO2. These cells were passaged at least twice before differentiation was induced.
Neuronal differentiation was induced according to methods described in previous studies.17,20) Briefly, P19 EC cells (1 × 106) were added to 10-cm dishes (Iwaki, Shizuoka, Japan) containing medium supplemented with 0.5 µM all-trans RA (FUJIFILM Wako Pure Chemical Corporation) and cultured for 4 d in suspension to form EBs [0–4 d in vitro (DIV)]. Cells were harvested by centrifugation at 200 × g for 5 min, then trypsinised. Next, 2 × 105 cells were seeded on poly-L-lysine-coated 6-well plates (Nippon Genetics, Tokyo, Japan) in the absence of RA (4–6 DIV). A schematic representation of the method is shown in Fig. 1a.

Experimental schemes of neuronal differentiation using the conventional method (MEMα method) (a) and the NDiff® 227 method (NDiff method) (b). Phase-contrast images of P19 EC cells at different stages of differentiation are shown (scale bar: 100 µm). Arrowheads indicate elongated neurites. DIV = days in vitro; EB = embryoid body; RA = retinoic acid.
NDiff® 227 (TaKaRa Bio) medium containing 1% penicillin/streptomycin was used to induce cell differentiation. NDiff® 227 (N2B27) medium is a mixture of N2 medium (Dulbecco’s modified Eagles/F12 medium supplemented with modified N2) and B27 medium (neurobasal medium supplemented with B27).12) In addition, it is a serum-free complete synthetic medium. Therefore, serum was not added for neuronal differentiation in NDiff® 227. Briefly, P19 EC cells were cultured as described above before differentiation was induced. To induce neuronal differentiation, 1 × 105 cells were plated in poly-L-lysine-coated 12-well plates (Nippon Genetics) containing NDiff® 227 medium supplemented with 0.5 µM RA. The culture medium was replaced daily with fresh medium. Differentiated cells were harvested and analysed by Western blotting. A schematic representation of the method is shown in Fig. 1b.
Western Blot AnalysisWestern blot analysis was conducted according to the method described in our previous studies.17,20) The following primary antibodies were used: neuronal nuclei (NeuN; Cell Signaling Technology, Danvers, MA, U.S.A.), HDAC8 (Sigma-Aldrich, St. Louis, MO, U.S.A.) and β-tubulin (FUJIFILM Wako Pure Chemical Corporation).
ImmunocytochemistryImmunocytochemistry was performed as described previously.20) Briefly, cells were fixed in 4% paraformaldehyde, followed by a phosphate-buffered saline (PBS) wash and treatment with 0.1% Triton X-100 in PBS for 5 min at room temperature. Cells were then blocked using 1% bovine serum albumin in PBS for 20 min at room temperature, then incubated with Tuj1 (βIII-tubulin) (1 : 100; Cell Signaling Technology) and NeuN (1 : 100) antibodies for 2 h at room temperature. Cells were then incubated with Alexa Fluor 488-conjugated anti-mouse immunoglobulin G (IgG) or Alexa Fluor 555-conjugated anti-rabbit IgG for 2 h at room temperature. Nuclei were then stained with 4-6-diamidino-2-phenylindole (DAPI).
Other MethodsProtein concentrations were determined using a bicinchoninic acid assay kit (TaKaRa Bio) with bovine serum albumin as the standard. Cell images were observed using a BZ-X710 microscope (Keyence, Osaka, Japan). The neurite length of P19 EC cells was measured using the Analysis Application Measurement Module (BZ-H3M; Keyence) in a BZ-X710 microscope. A total of 140 neurites were measured. Statistical significance of the experiments was determined using Welch’s t-test. p < 0.05 was considered significant.
Figure 1 shows the simple P19 EC cell monolayer culture-based differentiation protocol that we developed using NDiff® 227 medium supplemented with RA (NDiff method). In the conventional MEMα method, RA stimulation of P19 EC cells in suspension culture induced differentiation into neuronal cells (Fig. 1a). Similarly, the NDiff method efficiently induced neuronal differentiation of P19 EC cells grown in a monolayer culture (Fig. 1b). Neurite outgrowth observed in cells differentiated using the NDiff method confirmed that neuronal differentiation occurred (Fig. 1b: arrowhead).
Determination of Differentiation Activity and Establishment of Optimal Differentiation Conditions for the NDiff MethodWe compared the differentiation potential of the NDiff method with that of the conventional MEMα method. The MEMα method requires the addition of RA. To determine the effect of RA treatment on neuronal differentiation by the NDiff method, we measured expression of the mature neuronal marker, NeuN, in cells cultured in NDiff® 227 media in the presence (RA(+)) or absence (RA(−)) of RA. The expression of NeuN in the RA(+) condition was higher than that in the RA(−) condition (Figs. 2a–c). Next, the differentiation efficiency of the NDiff method in the presence of RA was compared with that of the MEMα method. The difference in the expression level of NeuN between both methods was not significant (Figs. 2d, e).
To increase the efficiency of P19 EC cell differentiation, we examined different culture conditions and determined the optimum cell seeding density in a 12-well plate. We seeded 1 × 104, 5 × 104, and 1 × 105 cells in the presence and absence of RA. NeuN expression was highest at a seeding density of 1 × 105 cells both in the presence and absence of RA (Figs. 3a, b). We also examined the optimal time required for neuronal differentiation. NeuN expression on 6 DIV was significantly higher than that on 0 DIV. At 8 DIV, a further increase in NeuN expression was observed; however, the increase was not significant compared to that at 6 DIV (Figs. 3c–e). These results suggest that a culture period of 6 DIV may be suitable for the NDiff method. Next, the effect of varying the frequency of NDiff® 227 medium replenishment on NeuN expression was assessed. No significant difference in NeuN expression was observed between daily and alternate day replenishment of the culture medium (Figs. 3f–h). Hence, optimal cell culture conditions for the NDiff method were: RA(+), cell seeding density of 1 × 105, 6 DIV of differentiation, and daily medium replenishment.

Phase-contrast images of P19 EC cells differentiated using the MEMα or NDiff method with (RA(+)) or without (RA(−)) RA. scale bar: 100 µm (a). NeuN expression levels in RA(+) and RA(−) conditions in cells differentiated using the NDiff method (b), and quantitation of the Western blot results (c). Comparison of NeuN expression levels in cells differentiated by the MEMα and NDiff methods (d). Quantitation of NeuN expression relative to that of β-tubulin, n.s. = not significant. (e). The amount of cell extract protein used for the Western blot experiments was 10 µg. RA = retinoic acid.

Estimation of the cell seeding density (a) and quantitation of the results (b). Time-lapse (2-DIV step) phase-contrast images of P19 EC cells differentiated using the NDiff method. scale bar: 100 µm. (c). Western blot analysis of NeuN expression (d) and quantitative results of NeuN Western blot analysis by Image J software, ** p < 0.01, n.s. = not significant. (e). Phase-contrast images of differentiated P19 EC cells following medium replenishment. scale bar: 100 µm. (f). Western blot analysis of NeuN expression (g) and quantitative results, n.s. = not significant. (h). The amount of cell extract protein used for the Western blot experiments was 10 µg. DIV = days in vitro; RA = retinoic acid.
We examined the expression of neuronal lineage markers by immunostaining. Although the density of differentiated cells was different between cells differentiated by the two methods, Tuj1 (βIII-tubulin), a neurite marker, was expressed on neurites in cells differentiated by both methods (Fig. 4a). Similarly, NeuN was expressed in the nucleus of cells differentiated by both methods (Fig. 4a). To study morphological differences between cells differentiated by the two methods, we analysed neurite length. There were no differences in the average neurite length in cells differentiated by the two methods (Fig. 4b). These results suggested that the NDiff differentiation method induced neuronal differentiation and neurite growth similar to the conventional MEMα method.

Immunofluorescence of the anti-Tuj1 (Alexa Fluor 488) and anti-NeuN (Alexa Fluor 555) antibodies, and DAPI fluorescence, were observed with a BZ-X710 microscope. scale bar: 100 µm. (a). Neurite length measured from Tuj1 fluorescence images. A scatter plot was created from the measurements and the results are shown in box plots. A total of 140 neurites were measured, n.s. = not significant. (b). (Color figure can be accessed in the online version.)
In our previous study with suspension cells, we showed that HDAC8 was necessary for neuronal differentiation as inhibiting its activity or HDAC KO resulted in reduced EB formation and caused neurodevelopmental failures.17) In this study, we analysed the expression of HDAC8 before and after neuronal differentiation in monolayer cultures to determine how HDAC8 activity contributed to this process. HDAC8 expression was observed both in undifferentiated and differentiated P19 EC cells (Fig. 5a), suggesting that it might contribute to differentiation. Next, we used an HDAC8 inhibitor to confirm the role of HDAC8 in differentiation by the NDiff method. NeuN expression was significantly reduced in a concentration-dependent manner upon treatment with the HDAC8 inhibitor (Figs. 5b–d). Finally, we measured NeuN expression in wild-type and HDAC8 KO P19 EC cells differentiated using the NDiff method. The results demonstrated that NeuN expression in the HDAC8 KO clones was lower than that in wild-type cells. Therefore, HDAC8 was required for neurodevelopment and was not limited to EB formation (Figs. 6a–c). These results suggest that our new NDiff method may be potentially used to determine drug efficacy and study neuronal differentiation.
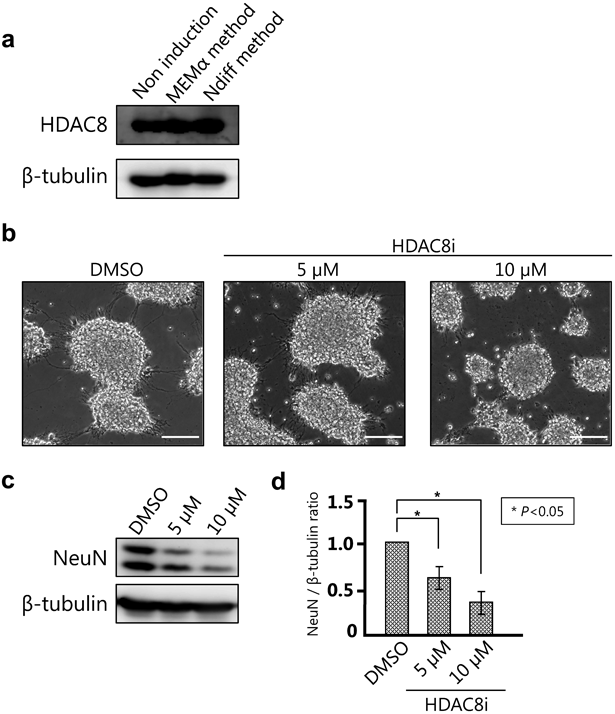
Expression of HDAC8 in P19 EC cells before and after differentiation (a). P19 EC cells were treated with either 5 or 10 µM NCC-149 during neuronal differentiation using the NDiff method. Images of the differentiated cells (6 DIV) are shown in panel b (scale bar: 100 µm). NeuN expression levels are shown in panels c and d. * p < 0.05. The amount of cell extract protein used for the Western blot experiments was 10 µg. DIV = days in vitro; HDAC = histone deacetylase.

Two independent HDAC8 KO P19 EC cell clones (KO35 and KO41) were differentiated using the NDiff method. NeuN expression in these clones was compared with that in the wild-type cells. Images of differentiated cells (6 DIV) are shown in panel a (scale bar: 100 µm). NeuN expression levels are shown in panels b and c. * p < 0.05, *** p < 0.001. The amount of cell extract protein used for the Western blot experiments was 10 µg. DIV = days in vitro; HDAC = histone deacetylase.
In this study, we developed a simple method to induce neuronal differentiation of P19 EC cells using NDiff® 227 medium. We showed that this method was better than the conventional MEMα method owing to its potential to differentiate cells in a monolayer culture (Figs. 1b, 2, 3). Moreover, the NDiff method can be used to measure HDAC8 activity in differentiated cells. Importantly, cells differentiated by this method showed the same elongation of neurites as those by the conventional MEMα method (Fig. 4). Therefore, our new method is useful for analysing neuronal differentiation and morphogenesis irrespective of the skill of the experimenter.
P19 EC cells derived from transplanted early mouse-embryonic epiblast cells express pluripotent cell-specific markers.21–23) Thus, neuronal induction/differentiation of P19 EC cells provides an excellent in vitro model for studying the transition process from the upper blastoderm to neuroectoderm.2,3) Maintenance of P19 EC cells is more time- and cost-effective than that of iPS and ES cells. Therefore, our method can be potentially used for initial screening of compounds and genes that control neuronal differentiation.
Using HDAC8 inhibitor and HDAC8 KO experiments, we showed that HDAC8 directly regulated the neuronal differentiation of P19 EC cells (Figs. 5, 6). In our previous study using suspension cells, we demonstrated that inhibition of HDAC8 activity suppressed EB formation and neuronal differentiation.17) Results from the current study demonstrated that the NDiff and conventional MEMα methods can be used together to study the role of different proteins in neuronal differentiation.
Our results showed that RA treatment induced neuronal differentiation in cultured cells (Fig. 2b, c). It is well-known that RA treatment suppresses cell proliferation of various tumours and cancers of the breast, lung, liver, and blood.24–27) In our hands, P19 EC cells died by overgrowth after 6 DIV in the absence of RA (data not shown). We hypothesise that cells overgrow because of the inability of RA to reduce cell proliferation. In addition, RA arrests the cell cycle in the G1 phase,28) which in turn can induce cell differentiation.29) These findings suggest that the addition of RA to induce neuronal differentiation may be an effective strategy. Moreover, neuronal differentiation of P19 EC cells by RA involves continuous expression of various genes. For example, a previous study demonstrated that the expression of several cell cycle-related genes was dramatically increased after RA stimulation until day 1.30) Therefore, we propose that RA enhances the neuronal differentiation potential of the NDiff method by triggering early cell cycle arrest through the regulation of gene expression.
The validity of using NDiff® 227 medium (originally known as N2B27 medium12,13)) for neuronal differentiation was considered. N2B27 medium is a mixture of equal volumes of N2 and B27 medium.12) N2 medium can induce neuronal differentiation and morphogenesis,12,31,32) while B27 medium protects nerve cells and helps in cell maturation.33,34) A previous study reported differentiation of P19 EC cells by culturing them in N2 medium containing FGF8 and RA, followed by maturation of the differentiated neuronal cells by culturing them in B27 medium.11) Our new method requires only a single medium – NDiff® 227 medium containing RA. Furthermore, our method does not require the addition of serum for neuronal differentiation.
Another study reported the use of serum-free N2B27 medium to form EBs from P19 EC cells and showed a rapid and efficient induction of differentiation of approx. 95% of the neural stem cells.35) In the default neuronal differentiation induction model, the bone morphogenetic protein (BMP) signalling pathway plays an important role in determining the fate of ectodermal cells and their differentiation into either epidermis or neural tissue.36,37) The low levels of serum BMP used in the conventional method can block neuronal differentiation of ES and P19 EC cells.38,39) However, NDiff® 227 medium completely inhibits BMP signalling and provides a favourable environment for neuronal differentiation of P19 EC cells.13) Therefore, the NDiff method is considered effective to achieve neuronal differentiation of P19 EC cells despite the adherent cell culture condition.
In conclusion, this report describes a novel method to induce neuronal differentiation of P19 EC cells. This method can induce neuronal differentiation, as well as the conventional MEMα method, as shown by NeuN expression (Fig. 2) and neurite outgrowth by Tuj1-staining (Fig. 4). Moreover, this simple method can be used to study the differentiation of stem cells that are difficult to maintain, such as the ES cells. In addition, because it is possible to differentiate nerves only by changing the growth medium, experimental error is minimised. Therefore, this method can be used to induce neuronal differentiation and achieve neuronal maturation of P19 EC cells in vitro.
We thank Dr. T. Suzuki. and Dr. N. Miyata for initial experiments. We are grateful to Dr. Y. Miura for providing the P19 EC cells. This work was supported in part by JSPS KAKENHI, Grant Number 19K08264 (T.I.), MEXT-Supported Program for the Strategic Research Foundation at Private Universities (2015–2019 to T.I.) and Takeda Science Foundation.
The authors declare no conflict of interest.