2020 Volume 43 Issue 8 Pages 1188-1195
2020 Volume 43 Issue 8 Pages 1188-1195
The immunostimulatory activity of unmethylated cytosine-phosphate-guanine oligodeoxynucleotide (CpG ODN) could be improved via delivery to immune cells expressing Toll-like receptor 9 (TLR9). Previously, we showed that the polypod-like structured nucleic acid (polypodna), a nanostructured DNA comprised of three or more ODNs, was an efficient system for the delivery of CpG ODNs to immune cells. Because some TLR9-positive immune cells express mannose receptors (MR), the uptake of polypodna by immune cells can be further increased by its modification with mannose. In this study, we selected the phosphodiester CpG ODN, ODN1668, which has a sequence identical to CpG1668, and a hexapodna, a polypodna with six pods, to design a hexapodna that harbored ODN1668 or the mannosylated CpG ODN (Man-ODN1668) synthesized via modification of the 5′-terminal of ODN1668 with a synthesized mannose motif. By mixing ODN1668 or Man-ODN1668 with the hexapodna, ODN1668/hexapodna and Man-ODN1668/hexapodna were successfully formed with high yields. However, Man-ODN1668/hexapodna was found to induce a greater tumor necrosis factor-α release from TLR9- and MR-positive mouse peritoneal macrophages and macrophage-like J774.1 cells than Man-ODN1668 or ODN1668/hexapodna. These results indicate that the combination of mannose modification and incorporation into nanostructured DNA is a useful approach for enhancing the immunostimulatory activity of CpG ODN.
Nucleic acid therapeutics are expected to serve as next generation medicines owing to their diverse targets and high specificity. DNA containing the unmethylated cytosine-phosphate-guanine (CpG) motif, which is typically found in bacteria and viruses, is a danger signal that stimulates innate immune cells.1,2) Toll-like receptor 9 (TLR9), an endosomal receptor for CpG DNA, is expressed in antigen-presenting cells (APCs), such as macrophages and dendritic cells.2,3) Ligation of TLR9 triggers innate immune responses that can be useful in different diseases, including cancers.4–6) Synthetic oligodeoxynucleotides (ODNs) containing the CpG motif can mimic the immunostimulatory effects of bacterial and viral DNA. There are three main types of CpG ODNs that exhibit different immune activities,1) and their clinical application as immune adjuvants has been explored. For example, HEPLISAV-B®, which utilizes phosphorothioate-stabilized CpG 1018 as an adjuvant, is a licensed vaccine for the treatment of B-type hepatitis.7,8)
The direct administration of single-stranded CpG ODN should be economic and convenient as CpG ODN coordinates with TLR9 in the single-stranded form.9–11) However, their vulnerability to some enzymes, such as deoxyribonuclease (DNase), and low cellular uptake efficiency highlight the need for suitable carriers for CpG ODN delivery to APCs.12) Recent advances in DNA nanotechnology have provided different delivery systems for nucleic acid therapeutics, such as small interfering RNA, micro RNA, aptamer, and CpG ODN.13) Polypod-like structured nucleic acid (polypodna) is a DNA nanostructure composed of 3 or more ODNs that is used to efficiently deliver CpG ODNs to APCs.14–19) Compared to the conventional, single stranded CpG ODN, CpG ODN loaded onto polypodna leads to higher cellular uptake and immunostimulatory activity.
Previously, we demonstrated that macrophage scavenger receptor 1 (MSR1), one of the scavenger receptors, was involved in the efficient uptake of polypodna according to its structural complexity.20) A further increase in cell specificity and the targeting efficiency of the nanostructured DNAs to immune cells could be achieved via receptor-ligand recognition21) by selecting a combination of receptors expressed on the target immune cells and a high-affinity ligand that selectively binds to the receptor. Among the different receptors, the C-type lectin receptors (CLRs), which often bind to glycan structures in a Ca2+-dependent manner,22) are attractive targets as they are mainly expressed on APCs.23) As a member of the CLRs, mannose receptor (MR), which can specifically recognize saccharides, such as mannose, is mainly expressed on the surface of macrophages and dendritic cells.24) In addition, MR-mediated endocytosis has been examined and applied to achieve cell-specific delivery of bioactive compounds.25–27) Recently, a mannosylated CpG ODN (Man-CpG ODN) that exhibits both enhanced immunostimulatory activity and cellular internalization efficiency was developed.28) Therefore, combining Man-CpG ODN with polypodna could extensively increase the immunostimulatory activity of CpG ODNs.
In 2017, a hexapodna (a polypodna with 6 pods) backbone was designed to serve as a versatile carrier of CpG ODNs.29) By employing this backbone in the present study, we aimed to select a phosphodiester CpG ODN with the same sequence as CpG ODN 1668, called ODN1668, and synthesize mannosylated ODN1668 (Man-ODN1668). Thereafter, we developed a hexapodna loaded with Man-ODN1668 (Man-ODN1668/hexapodna) and evaluated its usefulness to induce cytokine release from macrophages.
All synthetic operations were conducted with a standard Schlenk technique under argon atmosphere. Flash column LC was performed using Kanto Chemical silica gel 60NO (spherical, 40–50 µm; Kanto Chemical Co., Inc., Tokyo, Japan). Analytical TLC was performed on Merck Kieselge1 60 F254 (0.25 mm) plates (Merck KGaA, Darmstadt, Germany). Visualization was accomplished with a 3% H2SO4 solution in methanol followed by heating.
ChemicalsUnless otherwise stated, commercially available chemicals were used as received. Pyridine (dehydrated), dichloromethane (super dehydrated), acetonitrile (super dehydrated), and N-hydroxysuccinimide (NHS) were purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). D-(+)-Mannose, 5-hexen-1-ol, tin (IV) chloride (approx. 1 mol/L in dichloromethane), and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC·HCl) were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Acetic anhydride, sodium periodate, and Celite® 535RVZ were purchased from Nacalai Tesque, Inc. (Kyoto, Japan). All other chemicals were of the highest grade available and were used without further purification.
OligonucleotidesPhosphodiester ODNs were purchased from Integrated DNA Technologies, Inc. (Coralville, IA, U.S.A.) and an Alexa Fluor 488-labeled ODN (Hexa-1) was purchased from Japan Bio Services Co., LTD. (Saitama, Japan). The oligonucleotide sequences were designed according to a previous work29) (Table 1). The ODN1668 with the same sequence as phosphorothioate CpG ODN 1668 and 5′-NH2-ODN1668 was used for Man-ODN1668 synthesis. The complementary sequence to the CpG motif (GAC GTT) in hexa-1 to -6 was designed to have a single base mismatch (AAC TTC) and no CG sequence to avoid TLR9 stimulation by these ODNs.
| Name | Sequence (5′ to 3′) | Base number |
|---|---|---|
| Hexa-1 | TAGCA GCACA TCAGG TTCTG AGCCT TGCTG CAAGC ATCAG GAACT TCATG GA | 52 |
| Hexa-2 | TGCAG CAAGG CTCAG ATCTG CTCAA GCCTG CAAGC ATCAG GAACT TCATG GA | 52 |
| Hexa-3 | TGCAG GCTTG AGCAG ACAGA GCCTT GAGCC TAAGC ATCAG GAACT TCATG GA | 52 |
| Hexa-4 | TAGGC TCAAG GCTCT GGAGG CTCTT AAGCT GCAGC ATCAG GAACT TCATG GA | 52 |
| Hexa-5 | GCAGC TTAAG AGCCT CAGAG CTTGG CATAG CAAGC ATCAG GAACT TCATG GA | 52 |
| Hexa-6 | TGCTA TGCCA AGCTC TACCT GATGT GCTGC TAAGC ATCAG GAACT TCATG GA | 52 |
| ODN1668 | TCCAT GACGT TCCTG ATGCT | 20 |
| 5′-NH2-ODN1668 | /5AmMC6/TCCAT GACGT TCCTG ATGCT | 20 |
All ODNs have a phosphodiester backbone. /5AmMC6/, 5′ amino modifier C6.
Proton and carbon NMR spectra (1H-NMR and 13C-NMR) were recorded on a JEOL JNM-ECZ400 (1H at 399.78 MHz and 13C at 100.53 MHz) spectrometer (JEOL Ltd., Tokyo, Japan), with solvent resonance as the internal standard (1H-NMR, CHCl3 at 7.26 ppm; 13C-NMR, CDCl3 at 77.0 ppm). The following 1H-NMR data were reported: integration, chemical shift multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet), and coupling constants (Hz).
Synthesis of 5-Hexenyl 2,3,4,6-Tetra-O-acetyl α-D-Mannopyranoside (3)D-Mannose pentaacetate (2) was synthesized according to a previous report.30) Briefly, acetic anhydride (63.4 mmol, 6.47 mg, 6.0 mL, 6.34 equivalent (equiv.)) was added dropwise to a solution of D-(+)-mannose (10 mmol, 1.80 g) in pyridine (20 mL) at room temperature (r.t.). The reaction mixture was further stirred at r.t. for 10 h. The resulting mixture was quenched by methanol and concentrated with toluene in vacuo to yield a light-yellow oil (>99%). The crude residue was directly used for the glycosylation of 5-hexen-1-ol. After SnCl4 (1.0 mol/L in CH2Cl2, 0.5 mL, 1.0 equiv.) was added dropwise to a solution of compound 2 (0.53 mmol, 209.9 mg) in CH2Cl2 (7 mL), 5-hexen-1-ol (0.58 mmol, 58.4 mg, 70.0 µL, 1.1 equiv.) was immediately added to the reaction mixture with a vigorous stir. The reaction mixture was further stirred at r.t. for 5 h. The resulting mixture was quenched by sat. NaHCO3 (aq.), poured into a separatory funnel, and extracted three times with CH2Cl2. The organic layer was filtered through Celite® and concentrated. The crude residue was further purified on silica gel column using hexane and ethyl acetate (3 : 1) as eluent to yield the desired product in a colorless oil liquid (62.5 mg, 27%). 1H-NMR (400 MHz, CDCl3): δ = 5.79–5.69 (1H, m), 5.28 (1H, dd, J = 10.0, 3.4 Hz), 5.22 (1H, d, J = 9.9 Hz), 5.18–5.16 (1H, m), 4.98–4.88 (2H, m), 4.74 (1H, d, J = 1.7 Hz), 4.21 (1H, dd, J = 12.2, 5.3 Hz), 4.04 (1H, dd, J = 12.2, 2.4 Hz), 3.99–3.84 (1H, m), 3.63 (1H, dt, J = 12.1, 4.8 Hz), 3.40 (1H, dt, J = 11.8, 4.8 Hz), 2.09 (3H, s), 2.05–1.99 (8H, m), 1.93 (3H, s), 1.60–1.53 (2H, m), 1.44–1.38 (2H, m). 13C-NMR (101 MHz, CDCl3): δ = 170.42, 169.87, 169.69, 169.56, 138.20, 114.67, 97.38, 69.52, 68.96, 68.26, 68.11, 66.07, 62.35, 33.19, 28.49, 25.16, 20.71, 20.54, 20.51, 20.50 ppm. MS (electrospray ionization (ESI)+): Calcd for C20H30O10Na+ [M + Na]+: 453.1731. Found: m/z 453.1733.
Synthesis of 5-[(Tetra-O-acetyl α-D-Mannopyranosyl)oxyl] Pentanoic Acid (4)RuCl3·H2O (aq.) (1.29 mg/mL, 0.48 mL; 3 µmol, 0.62 mg, 2.1 mol %) was added to a solution of compound 3 (0.145 mmol, 62.5 mg) in CH2Cl2/CH3CN (1 : 1, 0.64 mL). Thereafter, NaIO4 (0.6 mmol, 127.9 mg, 4.15 equiv.) was immediately added to the reaction mixture with a vigorous stir. After 2 h, more NaIO4 (0.6 mmol, 127.9 mg, 4.15 equiv.) was added to the reaction mixture, which was further stirred at r.t. for 2 h. The resulting mixture was diluted with H2O, poured into a separatory funnel, and extracted three times with CH2Cl2. The organic layer was dried over MgSO4 and concentrated in vacuo to yield a dark oil liquid. The crude residue was directly used for the subsequent reaction (53.5 mg, 82%, crude yield). 1H-NMR (400 MHz, CDCl3): δ = 5.33 (1H, dd, J = 10.0, 3.4 Hz), 5.28 (1H, d, J = 9.8 Hz), 5.24–5.22 (1H, m), 4.80 (1H, d, J = 1.5 Hz), 4.27 (1H, dd, J = 12.2, 5.4 Hz), 4.10 (1H, dd, J = 12.2, 2.4 Hz), 3.99–3.95 (1H, m), 3.71 (1H, dt, J = 11.1, 5.0 Hz), 3.47 (1H, dt, J = 11.0, 5.0 Hz), 2.42–2.39 (2H, m), 2.15 (3H, s,), 2.10 (3H, s), 2.04 (3H, s), 1.99 (3H, s), 1.75–1.67 (4H, m). 13C-NMR (101 MHz, CDCl3): δ = 178.33, 170.70, 170.12, 169.98, 169.77, 97.57, 69.62, 69.11, 68.47, 67.89, 66.17, 62.52, 33.37, 28.53, 21.31, 20.89, 20.72, 20.69 ppm. MS (ESI+): Calcd for C19H28O12Na+ [M + Na]+: 471.1473. Found: m/z 471.1473.
Synthesis of 2,5-Dioxopyrrolidin-1-yl 5-[(Tetra-O-acetyl α-D-Mannopyranosyl)oxyl] Pentanate (5)1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (0.125 mmol, 24.0 mg, 1.05 equiv.) was added to a solution of compound 4 (0.119 mmol, 53.5 mg) and N-hydroxysuccinimide (0.125 mmol, 14.4 mg, 1.05 equiv.) in CH2Cl2 (1 mL), which was stirred at r.t. for 6 h. The resulting mixture was quenched by H2O, poured into a separatory funnel, and extracted three times using CH2Cl2. The organic layer was washed with brine, dried over Na2SO4, and concentrated in vacuo. The crude residue was directly used for modification of CpG1668 (65.4 mg, >99%, crude yield, dark oil liquid). 1H-NMR (400 MHz, CDCl3): δ = 5.29 (1H, dd, J = 10.0, 3.4 Hz), 5.24 (1H, d, J = 9.8 Hz), 5.21–5.19 (1H, m), 4.78 (1H, d, J = 1.5 Hz), 4.24 (1H, dd, J = 12.2, 5.3 Hz), 4.06 (1H, dd, J = 12.2, 2.2 Hz), 3.92–3.96 (1H, m), 3.71 (1H, dt, J = 11.3, 4.9 Hz), 3.47 (1H, dt, J = 11.2, 4.9 Hz), 2.80 (4H, s), 2.64 (2H, t, J = 7.0 Hz), 2.12 (3H, s), 2.06 (3H, s), 2.01 (3H, s), 1.95 (3H, s), 1.86–1.78 (2H, m), 1.75–1.69 (2H, m) ppm. 13C-NMR (101 MHz, CDCl3): δ = 170.56, 169.95, 169.75, 169.66, 169.08, 168.23, 97.48, 69.49, 68.97, 68.40, 67.47, 66.10, 62.42, 30.47, 28.11, 25.50, 21.33, 20.80, 20.65, 20.61, 20.59 ppm. MS (ESI+): Calcd for C23H31NO14Na+ [M + Na]+: 568.1637. Found: m/z 568.1645.
Synthesis of Mannosylated ODN1668 (Man-ODN1668)After 5′-NH2-ODN1668 (20 nmol, 1 mM in distilled H2O, 20 µL) was added to a mixture of Na2CO3–NaHCO3 buffer (0.33 M, pH = 9, 60 µL) and dimethyl sulfoxide (DMSO) (64 µL), compound 5 (20 µL, 0.5 M in DMSO, 40 µL) was added to the mixture. After moderate mixing of all the reagents using a vortex mixer and centrifugation, the mixture was stored overnight at r.t. for a further reaction. The reactant was purified with a Zeba spin desalting column (7K MWCO, Thermo Fisher Scientific Inc., U.S.A.) according to the manufacturer’s protocol. Thereafter, it was lyophilized to yield a white powder which was resolved in distilled H2O. The formation of Man-ODN1668 was confirmed by matrix assisted laser desorption ionization-time of flight MS (MALDI-TOF MS, JMS-S3000, JEOL Ltd.). MS Calcd: 6501.4. Found: 6501.1. Furthermore, its yield of 85% was confirmed by Nanodrop 2000/2000c (Thermo Fisher Scientific Inc., U.S.A.).
Preparation of PolypodnaAll ODNs involved in polypodna formation were mixed in appropriate molar ratios, heated to 95°C, and then gently cooled to 4°C. The preparation protocol was reported previously.14)
Polyacrylamide Gel Electrophoresis (PAGE)The formation of hexapodnas was confirmed by PAGE (6% polyacrylamide gel), which was carried out at 200 V for 30 min at rt. A total of 50 ng of each DNA sample was added to the gel for PAGE. The 100-bp DNA ladder was purchased from TaKaRa (Tokyo, Japan). ODNs were visualized by staining with ethidium bromide (EtBr; Nippon Gene Co., Ltd., Tokyo, Japan) and observed with the LAS4000 imaging system (FUJIFILM, Tokyo, Japan).
Measurement of Melting TemperatureHexapodnas were diluted in 150 mM NaCl to 250 µg/mL. Melting temperature (Tm) was measured with a spectrophotometer (JASCO J-730, JASCO, Tokyo, Japan) in a quartz cell with an optical path length of 1 mm (JASCO). All samples were scanned from 20°C to 95°C at a rate of 1°C/min and the absorbances were recorded per 0.5°C.
AnimalsC57BL/6N mice (female, 6-week-old) were purchased from Sankyo Labo Service Co., Inc. (Tokyo, Japan). All animal experiments were approved by the Animal Research Committee of the Faculty of Pharmaceutical Sciences, Tokyo University of Science.
Isolation and Culture of Mouse Peritoneal MacrophagesThe elicited macrophages were harvested from the peritoneal cavity of C57BL/6N mice 3 d after an intraperitoneal injection of 2 mL of 2.9% thioglycolate medium (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan). After cells were washed, they were suspended in RPMI 1640 (Nissui Pharmaceutical Co., Ltd.) supplemented with 10% heat-inactivated fetal bovine serum (Gibco, Thermo Fisher Scientific Inc., Waltham, MA, U.S.A.), penicillin G (100 U/mL), streptomycin (100 µg/mL) and L-glutamine (2 mmol/L) (FUJIFILM Wako Pure Chemical Corporation) in a humidified incubator containing 5% CO2 at 37°C. Thereafter, the cells were plated on a 10-cm dish. After incubation for 2 h, nonadherent macrophages were removed via washing with culture medium. As the attached cells were regarded as peritoneal macrophages, they were harvested for further experiments.
Culture of Mouse Macrophage-Like RAW264.7 Cells and J774.1 CellsMouse macrophage-like RAW264.7 cells and J774.1 cells were cultured in RPMI 1640 (Nissui Pharmaceutical Co., Ltd.) supplemented with 10% heat-inactivated fetal bovine serum (Gibco, Thermo Fisher Scientific Inc.), penicillin G (100 U/mL), streptomycin (100 µg/mL) and L-glutamine (2 mmol/L) (FUJIFILM Wako Pure Chemical Corporation) in a humidified incubator containing 5% CO2 at 37°C.
Identification of the Expression of MRThe expression of MR on mouse peritoneal macrophages, RAW264.7 cells and J774.1 cells was identified by direct immunofluorescence staining using anti-CD206 antibody (rat anti mouse CD206: Alexa Fluor 488, Bio-Rad Laboratories Inc., Hercules, CA, U.S.A.) following the manufacturer’s protocol. Briefly, 1 × 106 cells suspended in 90 µL cold (4°C) phosphate buffered saline (PBS)/bovine serum albumin (BSA) buffer were added with 10 µL anti-CD206 antibody, and the mixture was incubated for 1 h at 4°C in dark. Then, the cells were centrifuged, washed with PBS/BSA, and resuspended in 200 µL cold PBS. The fluorescence intensity of the cells was determined using a flow cytometer (Gallios Flow Cytometer; Beckman Coulter, Miami, FL, U.S.A.). Data were analyzed using FlowJo software (version 8.8.4; Beckman Coulter).
Tumor Necrosis Factor (TNF)-α Release from Peritoneal Macrophages and RAW264.7 CellsCells were seeded into a 96-well plate at a density of 1 × 105 cells/well (mouse peritoneal macrophages) or 5 × 104 cells/well (RAW264.7 cells) and incubated for 24 h before treatment. After aspiration of the supernatant, the DNA samples diluted with Opti-modified Eagle’s medium (Opti-MEM, Gibco, Thermo Fisher Scientific) were added to cells at an ODN1668 concentration of 1.2 µM (mouse peritoneal macrophages) or 0.6 µM (RAW264.7 cells). Thereafter, the cells were further incubated for 8 h at 5% CO2 and 37°C. The supernatant was then collected and used to determine the concentrations of TNF-α by enzyme-linked immunosorbent assay (ELISA), following the manufacturer’s protocol (BioLegend, Inc., San Diego, CA, U.S.A.).
IL-6 Release from J774.1 CellsJ774.1 cells (1 × 104 cells/well) were seeded into a 96-well plate and incubated at 37°C for 24 h before treatment. DNA samples diluted with Opti-MEM were added to cells at an ODN1668 concentration of 4.2 nM. Thereafter, the cells were further incubated for 8 h at 5% CO2 and 37°C. The supernatant was then collected and used to determine the concentrations of interleukin (IL)-6 by ELISA following the manufacturer’s protocol (Thermo Fisher Scientific Inc.).
Cellular UptakeAlexa Fluor 488-labaled Hexa-1 was used for the construction of Alexa Fluor 488-labaled DNA samples. Cells were seeded into 24-well plates at a density of 1 × 105 cells/well and incubated for 24 h before treatment. After aspiration of the supernatant, the DNA samples diluted with Opti-MEM were added to cells at a DNA concentration of 1.2 µM (mouse peritoneal macrophages), 0.6 µM (RAW264.7 cells) or 4.2 nM (J774.1 cells). Thereafter, the cells were further incubated for 2 h at 5% CO2 and 37°C. Cells were then washed twice with PBS and harvested. Then, fluorescence intensity of the cells was determined using a flow cytometer (Gallios Flow Cytometer). Data were analyzed using FlowJo software (version 8.8.4).
The synthesis of mannosylated ODN1668 was carried out in two stages: (1) formation of the mannose motif and (2) coupling (Fig. 1). After 5-hexen-1-ol was glycosylated with mannose pentaacetate to yield compound 3, the oxidation of 3 converted the double bond to a carboxylic group. Compound 4 reacted with N-hydroxysuccinimide to yield the mannose motif 5, which was mixed with 5′-NH2-ODN1668 in buffer and hydrolyzed to successfully synthesize Man-ODN1668 (yield, 93%) as identified by MALDI-TOF mass spectrometry.
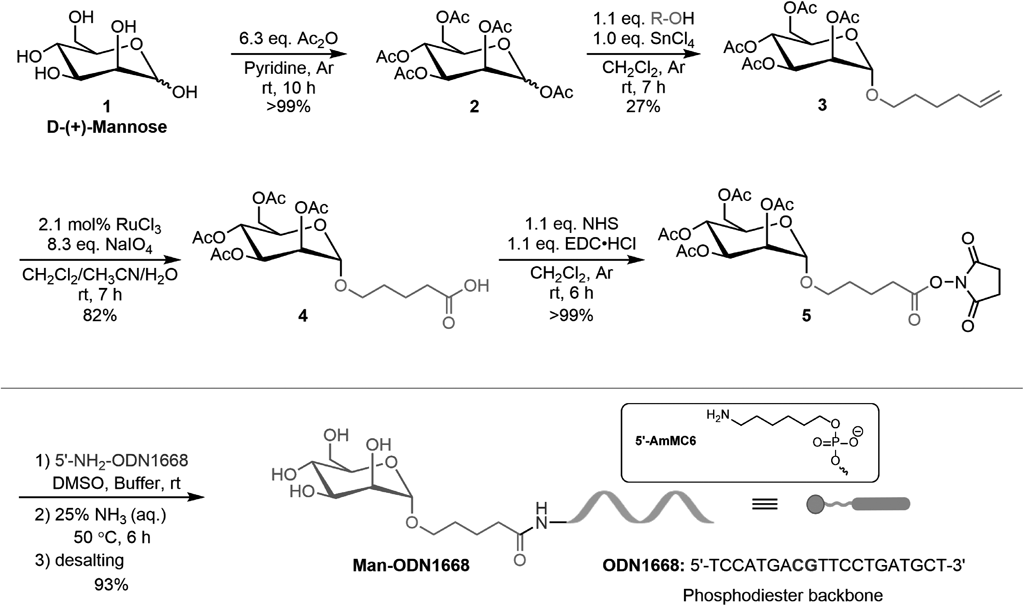
A hexapodna backbone lacking the CG sequences was constructed as previously reported.29) Briefly, ODN1668 or Man-ODN1668 was mixed with six ODNs that form the hexapodna in a stoichiometric ratio of 6 : 1 to obtain six ODN1668 (Man-ODN1668)-loaded hexapodna. Two protocols were employed to confirm the successful construction of the ODN1668-loaded hexapodna (Fig. 2A). In the first protocol, all ODNs were annealed in one step. Conversely, in the second protocol, the hexapodna backbone was first prepared. Thereafter, ODN1668 or Man-ODN1668 was added to the preformed hexapodna. Through PAGE (Fig. 2B), all DNA samples were found to display a single band, indicating that both protocols were efficient for the synthesis of the ODN1668-loaded hexapodna. Due to the addition of ODN1668, the bands of the hexapodna backbone shifted upward. Additionally, due to mannose modification, a further upward shift of the band was observed. Altogether, these findings indicated the successful formation of the Man-ODN1668-loaded hexapodna (Man-ODN1668/hexapodna). In this experiment, we opted to synthesize the ODN1668/hexapodna and Man-ODN1668/hexapodna by annealing all ODNs in one step.
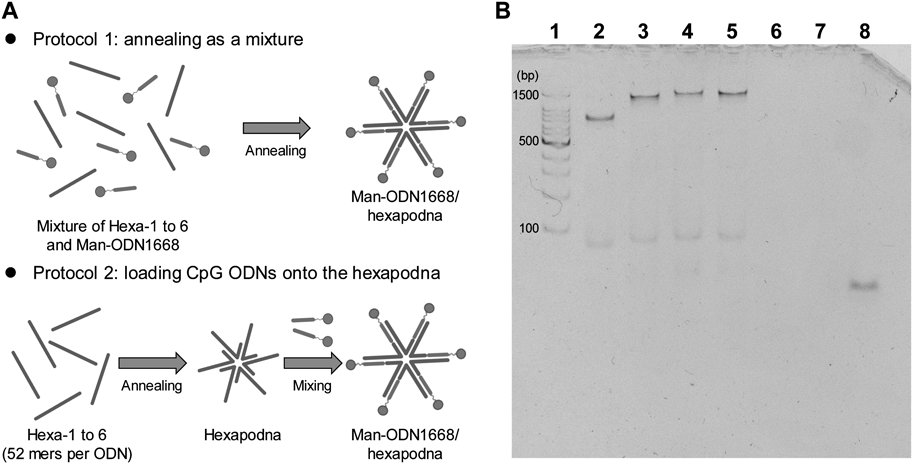
(A) The two protocols employed to synthesize the ODN1668-loaded hexapodna. (B) Hexapodna formation was confirmed by electrophoresis, which was run on a 6% polyacrylamide gel at 200 V for 30 min at room temperature. Lane 1, 100 bp ladder; lane 2, hexapodna; lane 3, ODN1668/hexapodna; lane 4, Man-ODN1668/hexapodna (protocol 1); lane 5, Man-ODN1668/hexapodna (protocol 2); lane 6, ODN1668; lane 7, Man-ODN1668; lane 8, Hexa-6.
To verify whether the thermal stability of ODN1668/hexapodna was altered by the mannose modification of ODN1668, Tm was measured (Table 2). As the Tm values of hexapodna, ODN1668/hexapodna, and Man-ODN1668/hexapodna were comparable, the loading of ODN and mannose modification had a minor influence on the thermal stability of hexapodna.
| Structure | Tm (°C) |
|---|---|
| Hexapodna | 59.3 |
| ODN1668/hexapodna | 57.5 |
| Man-ODN1668/hexapodna | 57.5 |
Prior to the evaluation of the cellular uptake and immunostimulatory activity induced by DNA samples, the expression of MR on mouse peritoneal macrophages, RAW264.7 cells and J774.1 cells was examined by detecting the receptors using anti-CD206 antibody. Flow cytometry results show that almost all mouse peritoneal macrophages and J774.1 cells expressed MR on cell surface (Fig. 3). On the other hand, no significant expression of MR was detected on RAW264.7 cells.
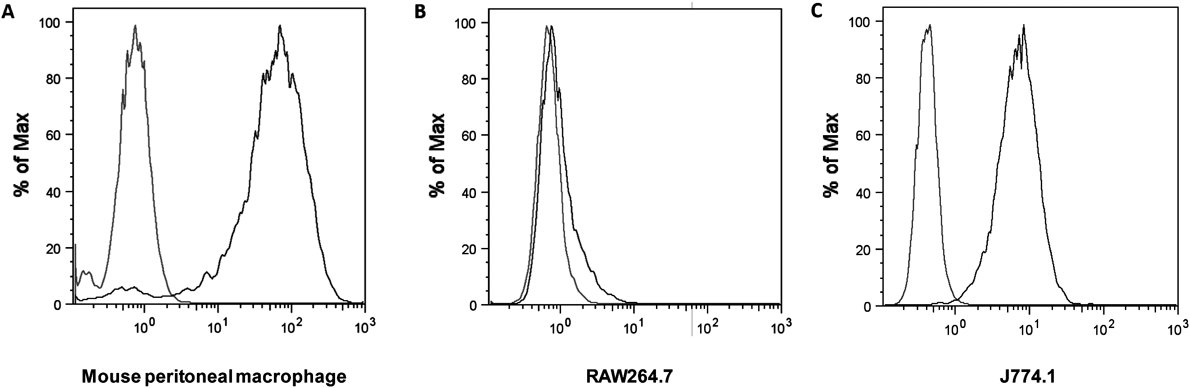
Mouse peritoneal macrophages (A), RAW264.7 cells (B) and J774.1 cells (C) were added with Alexa Fluor 488-anti CD206 antibody, and the cells were subjected to FACS analysis. The gray lines represent control cells (without addition of Alexa Fluor 488-anti CD206 antibody, and the black lines represent cells after labelling. The analysis was performed using 1 × 104 cells per group.
ODN1668, Man-ODN1668, hexapodna, ODN1668/hexapodna, and Man-ODN1668/hexapodna were added to mouse peritoneal macrophages (the amount of each constituent was equivalent to that of ODN1668 (1.2 µM). Figure 4 shows the release of TNF-α from cells at 8 h following the addition of the samples. Mannose modification only slightly affected the release of TNF-α by ODN1668. Moreover, no significant TNF-α release was observed when the hexapodna backbone was added. Compared to single-stranded CpG ODNs, a higher TNF-α release was observed with the ODN1668- or Man-ODN1668-loaded hexapodna. Intriguingly, Man-ODN1668/hexapodna induced a two-fold higher TNF-α release than ODN1668/hexapodna.
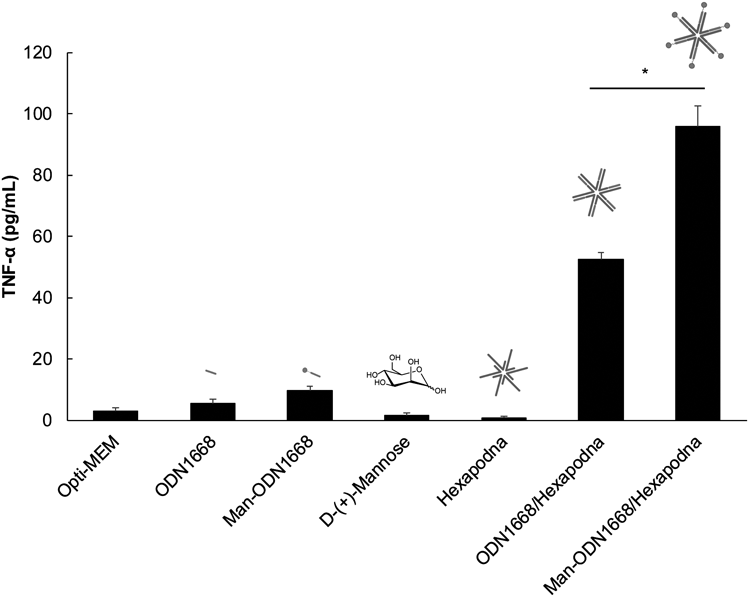
All DNA samples diluted in Opti-MEM were added to mouse peritoneal macrophages at a final concentration of 1.2 µM in ODN1668. After incubation for 8 h, the cell medium (supernatant) was collected and the concentration of TNF-α was measured. Results are expressed as mean ± standard deviation (S.D.) of four independent samples. * p < 0.05, two-tailed unpaired Student’s t-test.
To examine whether Man-ODN1668/hexapodna induces higher cytokine release through MR-mediated pathway, cytokine release from MR-positive J774.1 cells and MR-negative RAW264.7 cells was evaluated. After addition to J774.1 cells, Man-ODN1668/hexapodna induced significantly higher IL-6 release than ODN1668/hexapodna (Fig. 5). In contrast, no significant difference was observed in TNF-α release after addition of ODN1668/hexapodna and Man-ODN1668/hexapodna to RAW264.7 cells (Fig. 6). Taken together with the results of mouse peritoneal macrophages, these results indicate that Man-ODN1668/hexapodna more efficiently induces cytokine release only from MR-positive cells compared with ODN1668/hexapodna.

All DNA samples diluted in Opti-MEM were added to mouse macrophage-like J774.1 cells at a final concentration of 4.2 nM in ODN1668. After incubation for 8 h, the cell media (supernatant) was collected and the concentration of IL-6 were measured. Results are expressed as the mean ± S.D. of four independent samples. * p < 0.05, two-tailed unpaired Student’s t-test.

All DNA samples diluted in Opti-MEM were added to mouse macrophage like RAW264.7 cells at a final concentration of 0.6 µM in ODN1668. After incubation for 8 h, the cell media (supernatant) was collected and the concentration of TNF-α was measured. Results are expressed as the mean ± S.D. of four independent samples. * p < 0.05, two-tailed unpaired Student’s t-test. ns, not significant.
To understand whether the enhanced immunostimulatory activity was correlated with an increased cellular uptake via MR-mediated endocytosis, the uptake of Hex-1, hexapodna, ODN1668/hexapodna and Man-ODN1668/hexapodna, all of which contained Alexa Fluor 488-labeled Hex-1, was examined. Figure 7A shows the mean fluorescence intensity of mouse peritoneal macrophages. There was no significant difference in cellular uptake of hexapodna, ODN1668/hexapodna and Man-ODN1668/hexapodna after addition to mouse peritoneal macrophages. Similar results were obtained using MR-positive J774.1 cells (Fig. 7B) and MR-negative RAW264.7 cells (Fig. 7C). In these two cell lines, the uptake of hexapodna, ODN1668/hexapodna and Man-ODN1668/hexapodna was higher than that of Hex-1, which was in good agreement with previous results.14)
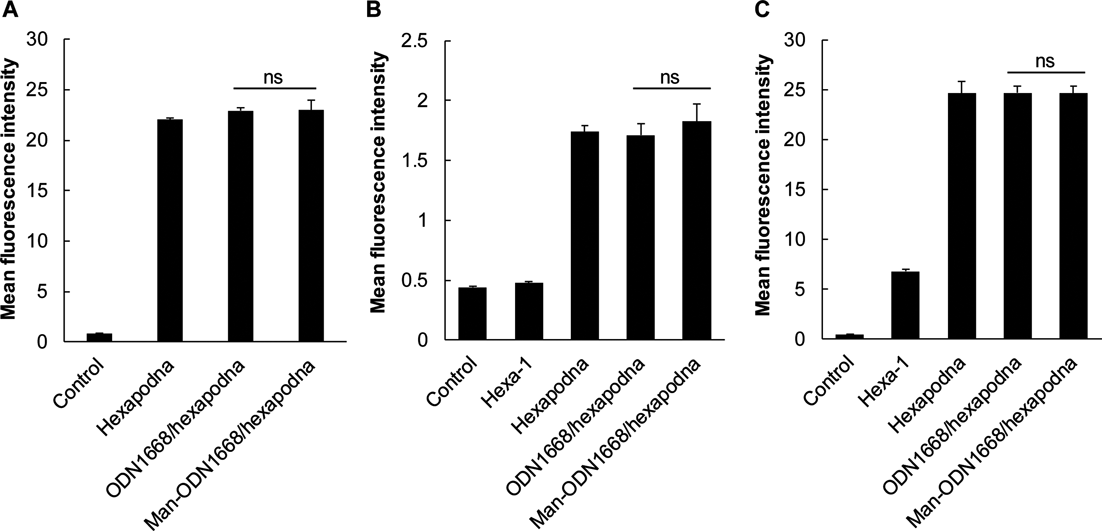
All DNA samples labeled with Alexa Fluor 488 were diluted in Opti-MEM and added to mouse peritoneal macrophages (A), J774.1 cells (B) and RAW264.7 cells (C) at a final concentration of 1.2 µM for mouse peritoneal macrophages, 4.2 nM for J774.1 cells and 0.6 µM for RAW264.7 cells in ODN1668. Mean fluorescence intensity of the cells was determined using a flow cytometer. Results are expressed as the mean ± S.D. of four independent samples. Two-tailed unpaired Student’s t-test. ns, not significant.
One of the important issues in nucleic acid therapeutics is the achievement of efficient delivery to targeted sites. Previously, we demonstrated that polypodna could be employed for the efficient delivery of CpG ODN to APCs.14) In particular, the tripodna and hexapodna lacking CpG sequences were demonstrated to be suitable carriers for CpG ODN delivery.29) As issues, such as stability and targeting efficiency, might exist for the in vivo application of CpG ODN, we developed Man-ODN1668 and succeeded in performing a one-pot synthesis of Man-ODN1668/hexapodna (Fig. 2). Because only one clear high molecular weight band was detected in lanes 2–5, we considered the formation efficiency of nanostructured DNA was high and almost all CpG ODNs were loaded onto the hexapodna. However, faint bands below 100 bp may indicate the formation of incomplete or partial structures with two or more ODNs. Based on the band intensities, the formation efficiency of hexapodna, ODN1668/hexapodna and Man-ODN1668/hexapodna could be assumed to be 90% or higher.
As mouse peritoneal macrophages express both TLR9 and the macrophage mannose receptor, these cells were employed herein.31,32) In addition, J774.1 and RAW264.7 mouse macrophage-like cells were also selected for the evaluation. Although these three types of macrophages are used in many studies as MR-positive cells, the results of the present study showed that mouse peritoneal macrophages and J774.1 cells express MR on cell surface, whereas RAW264.7 cells hardly do (Fig. 3). Based on these results, these three types of macrophages were classified into MR-positive mouse peritoneal macrophages and J774.1 cells and MR-negative RAW264.7 cells. Because J774.1 cells were found very sensitive to CpG ODNs compared with mouse peritoneal macrophages and RAW264.7 cells, a low concentration of CpG ODN (4.2 nM) was used for J774.1 cells.
Mannose modification at the 5′-terminal of ODN not only increases its affinity to the mannose receptors, but also improves its biological stability (Fig. 3). Addition of mannose did not induce significant TNF-α release from mouse peritoneal macrophages. The slightly higher release of TNF-α by single-stranded Man-ODN1668 than single-stranded ODN1668 indicated that the modification hardly disturbed the ODN1668 to interact with TLR9 and this could be explained by the following: first, the affinity of mannosylated ligands to the macrophage mannose receptor is highly dependent on the multivalency of mannose.33) Thus, because Man-CpG ODN has one mannose unit per molecule, its affinity to the receptor could be low. Second, the 5′-terminal modification of ODN with mannose would slightly improve the biological stability of ODN by blocking the access of DNase to the ODN. Compared to single-stranded Man-ODN1668 and ODN1668/hexapodna, Man-ODN1668/hexapodna was effective at inducing TNF-α release. This improvement could be explained by the structural properties of Man-ODN1668/hexapodna which has six Man-ODN1668s incorporated into one hexapodna backbone. This design could increase the density of the mannose units, thereby leading to efficient recognition by the mannose receptor.
The cellular uptake of ODN1668/hexapodna hardly changed after mannose modification irrespective of the expression of MR (Fig. 7). A previous study from our group indicated the cellular uptake of nanostructured DNA by macrophage was related to MSR1-mediated endocytosis.20) Because only CpG ODN released from hexapodna after internalization through endocytosis can be involved in the interaction with TLR9 and following cytokine release,9–11) we considered the different immunostimulatory activity detected with MR-positive and MR-negative cells could be attributed to the following possibilities: (1) the difference in the internalization rate between MSR1-mediated endocytosis and MR-mediated endocytosis after ligand-receptor binding; and (2) prompt release of Man-ODN1668 from hexapodna than ODN1668. Further studies on the relationship between immunostimulatory activity and endocytosis are under investigation.
B-type CpG ODNs, such as CpG ODN 1668, have a full phosphorothioate (PS) backbone with one or more CpG dinucleotides.1) The PS backbone induces a high enzymatic stability of the nucleic acid against DNase and superior membrane permeability. However, PS modification increases the cytotoxicity of the ODNs.34,35) In contrast, natural phosphodiester DNAs, especially short phosphodiester DNAs such as ODNs, are enzymatically unstable and labile to nuclease degradation within the body.12) Thus, the increased immunostimulatory activity achieved by the combination of mannose modification and incorporation into nanostructured DNA would serve as a suitable strategy for increasing immunostimulatory activity and biological stability without increasing the cytotoxicity of CpG ODN.
In the present study, we successfully developed an immune cell-targeted nanostructured DNA to achieve efficient delivery of CpG ODN. Compared to the hexapodna carrying the unmodified CpG ODN, that carrying mannosylated CpG ODN was found to induce a significantly greater release of cytokines from MR-positive mouse peritoneal macrophages and J774.1 cells. Based on our study findings, a useful strategy has been derived to increase the usefulness of CpG ODN as an immune adjuvant.
This work was supported in part by the Japan Science and Technology Agency (JST) Core Research for Evolutional Science and Technology (CREST) [Grant Number JPMJCR1521], Japan, and Grants-in-Aid for Scientific Research (B) [Grant Numbers 23390010, 26293008] from the Japan Society for the Promotion of Science.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.