2020 Volume 43 Issue 9 Pages 1375-1381
2020 Volume 43 Issue 9 Pages 1375-1381
Adipogenic differentiation is a complex process by which fibroblast-like undifferentiated cells are converted into cells that accumulate lipid droplets. We here investigated the effect of gene deletion of calcium-independent phospholipase A2γ (iPLA2γ), a membrane-bound PLA2 enzyme, on adipogenic differentiation in mice. Since iPLA2γ knockout (KO) mice showed reduced fat volume and weight, we prepared mouse embryonic fibroblasts (MEF) from wild-type (WT) and iPLA2γ KO mice and examined the effect of iPLA2γ deletion on in vitro adipogenic differentiation. iPLA2γ increased during adipogenic differentiation in WT mouse-derived MEFs, and the differentiation was partially abolished in iPLA2γ KO-derived MEFs. In KO-derived MEFs, the inductions of peroxisome proliferator activator receptor γ (PPARγ) and CAAT/enhancer-binding protein α (C/EBPα) were also reduced during adipogenic differentiation, and the reductions in PPARγ and C/EBPα expressions and the defect in adipogenesis were restored by treatment with troglitazone, a PPARγ ligand. These results indicate that iPLA2γ might play a critical role in adipogenic differentiation by regulating PPARγ expression.
Adipocytes play important roles in the control of lipid homeostasis and energy balance by storing and mobilizing triacylglycerol. Increasing the number and size of adipocytes can result in an expansion of the white adipose tissue (WAT) mass. The adipocyte maturation process is called adipogenesis, and involves the differentiation of fibroblast-like preadipocytes into mature adipocytes. Adipogenic differentiation is a complex process associated with coordinated changes in gene expression, cell morphology, and hormone sensitivity.1) Several key transcription factors involved in this process have been identified: peroxisome proliferator activator receptor γ (PPARγ), CAAT/enhancer-binding proteins (C/EBPs), and sterol regulatory element-binding protein (SREBP) all play central roles in adipogenic differentiation.2–4)
Bioactive lipid mediators such as prostaglandins (PGs) and lysophosphatidic acid (LPA) are also involved in the regulation of adipogenic differentiation. PGE2, PGF2α, and LPA inhibit adipogenesis,5–7) whereas PGD2 and its metabolite, 15-deoxy-Δ12,14-PGJ2, bind to PPARγ as a ligand and promote adipogenesis.8,9) Prostacyclin (PGI2) affects the induction of adipogenic differentiation.10,11)
Phospholipase A2 (PLA2) catalyzes the cleavage of the sn-2 position of glycerophospholipids to yield free fatty acids and lysophospholipids, and it has an important role in membrane remodeling as well as lipid mediator signaling. To date, approximately 55 types of PLA2 enzymes have been identified and divided into several families: secretory PLA2 (sPLA2), calcium-dependent cytosolic PLA2 (cPLA2), calcium-independent PLA2 (iPLA2), platelet-activating factor acetyl hydrolases (PAF-AHs), lysosomal PLA2 and adipose PLA2s.12) The iPLA2 family is also referred to as the patatin-like phospholipase domain-containing protein (PNPLA) family, since its members share homology with patatin, a lipid acyl hydrolase in plants.12,13)
Among the iPLA2 isozymes, independent phospholipase A2γ (iPLA2γ/PNPLA8) is a membrane-associated protein that contains four methionine residues that can potentially encode 88, 77, 74 and 63 kDa polypeptides. iPLA2γ is localized to the mitochondria, peroxisome and endoplasmic reticulum, and its localization led to the suggestion that iPLA2γ is associated with the regulation of energy metabolism in mitochondria and maintains their functions through the membrane lipid metabolism of those organelles.14) iPLA2γ knockout (KO) mice have been established and show many phenotypes, such as growth retardation, muscle weakness and mitochondrial dysfunction.14–19) Regarding fat accumulation, iPLA2γ KO mice exhibited reduced lipid accumulation in adipose tissue even when fed a normal-fat diet.18,19) Moreover, Su et al. reported that knockdown of iPLA2γ in 3T3-L1 fibroblast cells inhibited hormone-induced adipogenic differentiation.20) However, the involvement of iPLA2γ in adiopogenesis has not been fully elucidated.
In the present study, we investigated the effects of iPLA2γ gene deletion on the adiposity of mice in vivo, and we then isolated mouse embryonic fibroblasts (MEFs) from iPLA2γ-KO mice and examined their phenotypes, including in vitro adipogenic differentiation. The results revealed that the gene deletion of iPLA2γ suppressed the hormone-induced adipogenic differentiation of MEFs and their lipid accumulation. The inductions of C/EBPα, C/EBPβ, C/EBPδ and PPARγ, which are adipogenic differentiation markers, were attenuated in iPLA2γ-KO MEFs. These results indicated that iPLA2γ might play a critical role in adipogenic differentiation by regulating the expression of PPARγ.
The detail of the construction of the pnpla8−/− (iPLA2γ-KO) mice were described previously.17) All mice were housed in climate-controlled (21°C) specific pathogen-free facilities with a 12-h light-dark cycle, with free access to standard laboratory food (Picolab mouse diet 20; Laboratory Diet, Brentwood, MO, U.S.A.) and water. All procedures involving animals were performed under approved institutional guidance.
Computed Tomography (CT) AnalysisThe fat volume was analyzed in the 2- to 4-month-old wild-type (WT) and iPLA2γ KO mice using a CT system (eXplore Locus, GE Healthcare, London, ON, Canada). Mice were anesthetized with 2% isoflurane (Dainippon Sumitomo Pharmaceutical, Osaka, Japan) and scanned for 10 min under the following conditions: resolving power, 93 µm; view number, 400; voltage, 80 kVp; and electric current, 450 µA. CT images were analyzed using MicroView 2.0 software (GE Healthcare, U.S.A.).
HistopathologyFor histopathology, tissue sections were fixed in 10% formalin, embedded in paraffin, and stained with hematoxylin-eosin by a standard method. For Oil Red O staining, adipose tissues were embedded in OCT compound and cryosectioned.
Cell Culture and in Vitro Adipogenic DifferentiationPrimary MEFs were prepared from 13.5-d-old embryos obtained from heterozygous iPLA2γ mice intercrosses as described previously.21) Briefly, after dissection of the head and visceral organs for genotyping, embryos were minced and trypsinized for 30 min at 37°C. Embryonic fibroblasts were then plated and maintained in high-glucose Dulbecco’s modified Eagle medium (HG-DMEM) (GIBCO, Life Technologies, Grand Island, NY, U.S.A.) with 10% fetal calf serum (FCS) (Life Technologies), 100 U/mL penicillin and 100 µg/mL streptomycin at 37°C in an atmosphere of 5% CO2. All experiments were performed with WT and iPLA2γ KO MEFs at 2 − 3 passages. For adipogenic differentiation, 2-d-postconfluent cells (day 0) were treated with HG-DMEM supplemented with 10% FCS, 1 µM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine and 1 ng/mL insulin for 2 d (all from Sigma, St. Louis, MO, U.S.A.). The medium was renewed every 2 d with HG-DMEM containing 10% FCS and 1 ng/mL insulin.
For the visualization of lipid accumulation, cells were stained with Oil Red O.21,22) Briefly, cells on 12-well plates were washed with phosphate-buffered saline (PBS), fixed with 3.7% formaldehyde solution (Wako Pure Chemical Corporation, Osaka, Japan) for 10 min and then rinsed three times with distilled water. The fixed cells were treated with 60% isopropyl alcohol (Wako) for 1 min and then stained with 0.3% Oil Red O in 60% isopropyl alcohol solution for 10 min. After staining, the cells were washed three times with water and left to dry. We first evaluated Oil Red O staining with an optical microscope (×100). The number of Oil Red O positive cells was counted in 5 randomly-selected microscopic fields of view. Furthermore, to quantify the retention of Oil Red O, stained adipocytes were extracted with 1 mL of 4% Igepal CA-630 (Sigma) in isopropyl alcohol for 15 min, and absorbance of supernatant was measured by spectrophotometry at 520 nm.
Measurement of Prostanoids by Electrospray Ionization (ESI)-MS/MSCulture media from MEFs were isolated and adjusted to pH 3.0 with 1 M HCl. An internal standard (50 pg of LTB4-d4) was added to the samples, and then the samples were passed through a Sep-Pak C18 cartridge (Waters, Milford, MA, U.S.A.). The retained PGs were eluted with 3 mL of ethyl acetate/methanol (9 : 1 (v/v)). The sample solvents were evaporated and then resuspended in 100 µL of mobile phase A and injected into a LC-ESI-MS system. All MS analyses were performed using a Prominence HPLC system (Shimadzu, Kyoto, Japan) equipped with a linear ion trap quadrupole mass spectrometer (QTRAP5500, AB Sciex, Framingham, MA, U.S.A.). The quantitation of prostanoids was performed using LC-MS/MS via multiple-reaction monitoring in negative-ion mode, as described previously.23)
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and ImmunoblottingTissue homogenates or cell lysates (10 µg protein equivalents) were subjected to SDS-PAGE using 7.5, 12 or 15% gels under reducing conditions. The separated proteins were electroblotted onto nitrocellulose membranes (Schleicher & Schuell, Keene, NH, U.S.A.) with a semidry blotter (Bio-Rad Laboratories, Hercules, CA, U.S.A.) according to the manufacturer’s instructions. After blocking with 5% (w/v) skim milk in 10 mM Tris–HCl, pH 7.4, containing 150 mM NaCl and 0.05% Tween 20, the membranes were probed with the respective antibodies (1 : 5000 dilution) for 2 h, followed by incubation with horseradish peroxidase-conjugated anti-mouse immunoglobulin G (IgG) (1 : 5000 for PPARγ) and anti-rabbit IgG (1 : 5000 for C/EBPα, C/EBPβ, C/EBPδ, and iPLA2γ). After being washed, the membranes were visualized with Western Lightning Chemiluminescence Reagent Plus (PerkinElmer, Inc. Life Sciences, Boston, MA, U.S.A.). Antibodies against PPARγ, C/EBPα, C/EBPβ and C/EBPδ were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). Rabbit polyclonal antibody against human iPLA2γ were prepared as described previously.17)
Quantitative RT-PCR (Q-PCR)RNA extraction, cDNA synthesis and Q-PCR were carried outaccording to standard protocols in our laboratory, as previously described.17) The primer pairs were 5′-GCC CAC CAA CTT CGG AAT C-3′ and 5′-TGC GAG TGG TCT TCC ATC AC-3′ for mouse pparg; 5′-GAG CCT TCA CTG TCT GTT GGA A-3′ and 5′-CTG CTA CAG CCA GAT TCA GAA CTG-3′ for mouse cd36; 5′-GCG GCC CAA TCC TAT CCT-3′ and 5′-AGG TTG AAG TGG GTC AAG CAA-3′ for mouse ap2; and 5′-TTC GTA TTG CGC CGC TAG A-3′ and 5′-CTT TCG CTC TGG TCC GTC TT-3′ for mouse 18s ribosomal RNA.
StatisticsThe data were statistically evaluated by unpaired Student’s t-test or Tukey’s test, and p-values <0. 05 were accepted as significant.
Since our previous research demonstrated that iPLA2γ-KO mice exhibited lower body weights than WT mice,17) we examined the abdominal fat volume by performing a CT scan analysis. The results revealed that the total fat volume and the volumes of visceral fat and subcutaneous fat in the KO mice were significantly lower than those in their WT littermates (Fig. 1A). The weights of white and brown fat tissues were also reduced in the KO mice (Fig. 1B). Hematoxylin-eosin staining and Oil Red O staining of fat tissues demonstrated that the sizes of the white and brown adipocytes in the iPLA2γ-KO mice were reduced by more than 60% relative to those in the WT mice (Fig. 1C). These results suggested that iPLA2γ might be involved in adipose differentiation.

A: CT images of abdominal fat in WT (a) and iPLA2γ KO (b) mice at 4 months of age. Yellow areas represent visceral fat. (c) Visceral and subcutaneous fat volumes of WT (white bars) and KO mice (black bars) revealed by a CT scan analysis. Quantitative data are means ± standard deviation (S.D.). * p < 0.05 vs. WT (n = 6). B: Weights of white adipose tissue (WAT) and brown adipose tissue (BAT) in WT (white bar) and iPLA2γ KO (black bar) mice (left). The ratio of WAT or BAT weight to total body weight (right). Quantitative data are means ± S.D. * p < 0.05 vs. WT (n = 6). C: Histological analysis of WAT and BAT from WT and iPLA2γ KO mice. a, b, e, f: Hematoxylin eosin staining. c, d, g, h: Oil Red O staining. Scale bar: 100 µm.
To investigate the involvement of iPLA2γ in adipogenesis, we next employed an adipogenic differentiation system from MEFs in vitro and examined the effects of iPLA2γ deficiency on the differentiation. In our system, MEFs were prepared from 13.5-d embryos of WT or iPLA2γ-KO mice, and primed with a differentiation-inducing cocktail (containing insulin, dexamethasone and IBMX) for 2 d, followed by treatment with insulin for an additional 7 d. The adipose differentiation status was confirmed by staining with Oil Red O (Fig. 2A).
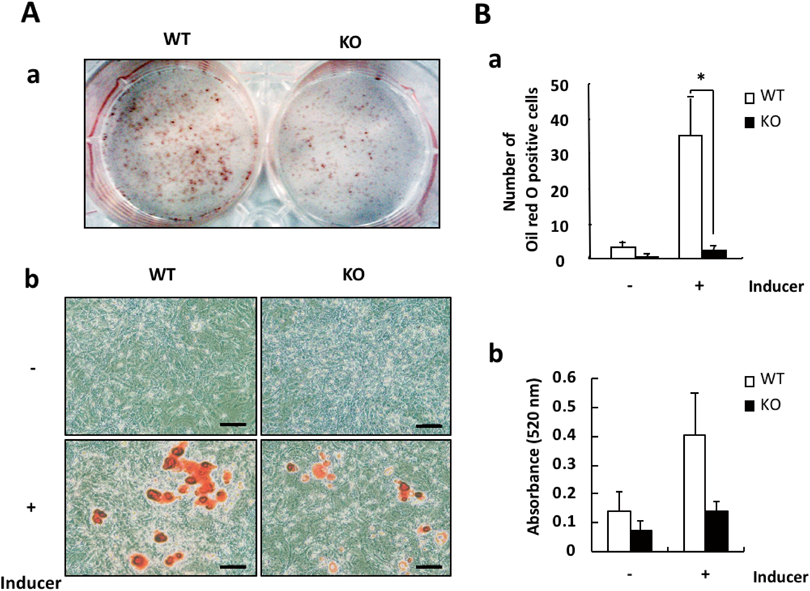
A: Staining of cells induced by an adipogenic inducer with Oil Red O to visualize the extent of adipose conversion. (a) Representative whole well images of Oil Red O staining. (b) Representative magnified images. The scale bar is 100 µm. B: (a) The number of Oil Red O positive cells per field of view (n = 3). (b) Quantification of Oil Red O staining (n = 4). Data are means ± S.D. * p < 0.05.
As shown in Fig. 3A, the level of iPLA2γ protein was somewhat down-regulated by the induction of adipogenic differentiation, and it then began to increase 4 d after the induction. iPLA2γ was increased during adipogenic differentiation and was markedly expressed in the differentiated adipocytes. We further observed that the in vitro adipogenic differentiation of MEFs was suppressed by iPLA2γ deficiency (Fig. 2A). The number of Oil Red O-stained cells differentiated from KO mouse-derived MEFs was significantly lower than that from WT MEFs (Fig. 2B). Su et al. also reported that iPLA2γ expression was induced during a hormone-induced differentiation of 3T3-L1 cells into adipocytes, and that the knockdown of iPLA2γ inhibited this adipogenic differentiation.20) Taken together, these results indicate that iPLA2γ might be critical for the differentiation of fibroblasts into adipocytes after hormone stimulation.
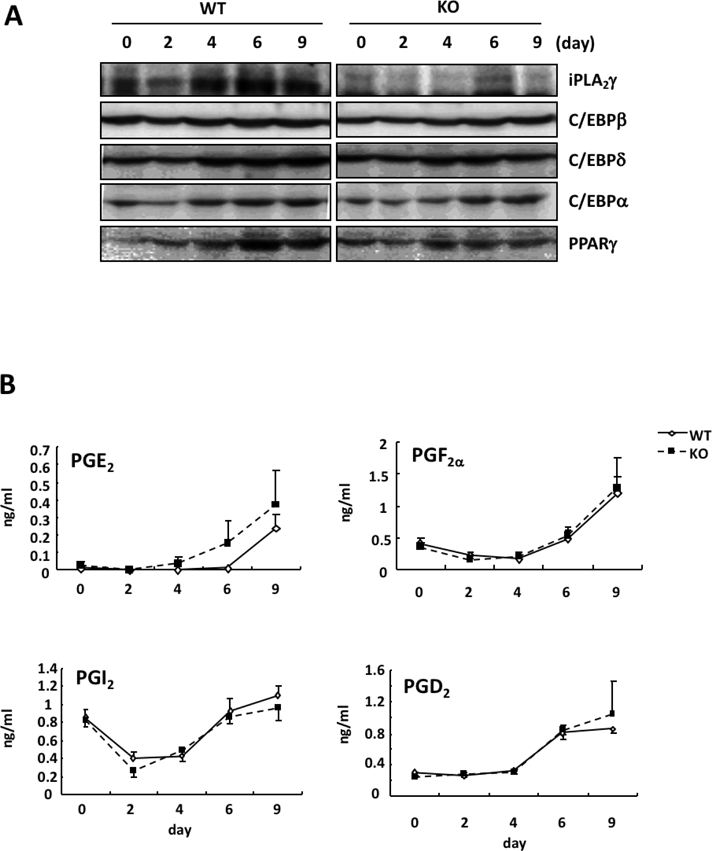
A: Western blot analysis of iPLA2γ, C/EBPα, C/EBPβ, C/EBPδ and PPARγ of cell lysates prepared from the differentiated WT or iPLA2γ-deficient MEFs. MEFs were induced to differentiate for 0, 2, 4, 6 or 9 d. Representative results of three experiments are shown. B: PGE2, PGF2α, PGI2 and PGD2 production during adipogenic differentiation. Their concentrations were measured in the culture medium prepared from the differentiated WT or iPLA2γ-deficient MEFs by an LC-MS/MS analysis. Data are means ± S.D. (n = 4).
Several prostanoids, such as PGE2, PGF2α, PGD2 and PGI2, are known to be involved in the regulation of adipogenic differentiation.5,6,8–11) We further investigated whether iPLA2γ gene deletion affected the production of prostanoids from MEFs. As shown in Fig. 3B, the amounts of PGE2, PGF2α and PGD2 in culture media from MEFs were increased during adipogenic differentiation. In addition, the amount of 6-ketoPGF1α (a stable metabolite of PGI2) was down-regulated by the induction and then began to increase 4 d after the induction. The alteration in PGI2 production was correlated with that in iPLA2γ expression during adipogenic differentiation. However, these prostanoid productions were not affected by iPLA2γ gene deletion.
iPLA2γ Deficiency Inhibited the PPARγ Expression and Activation during the Differentiation of MEFs into AdipocytesWe further examined the effects of iPLA2γ deficiency on the expression of adipocyte-related genes during adipogenesis. The expressions of the adipocyte-related genes, aP2 and CD36, were also suppressed in iPLA2γ-KO mouse-derived cells (Fig. 4A). Since the expressions of both aP2 and CD36 is regulated by the transcriptional factor PPARγ,24,25) iPLA2γ might be involved in the regulation of PPARγ transcriptional activity during adipogenic differentiation. In fact, we observed that the expression levels of PPARγ were also reduced in the iPLA2γ KO mice (Figs. 3A, 4A).

A: The Pparγ, ap2 and cd36 mRNA levels of WT (white bar) and iPLA2γ KO (black bar) MEFs which were differentiated for 9 d were determined by the real-time RT-PCR (n = 4). 18S rRNA was used as the reference gene (* p < 0.05). B: WT (white bar) and iPLA2γ KO (black bar) MEFs were induced by an inducer with 10 µM of troglitazone or rosiglitazone. Lipid accumulation was quantified by Oil Red O staining on day 9 (n = 4). Data are means ± S.D. * p < 0.05 by Tukey’s test. C: Western blot analysis of C/EBPα, C/EBPβ, C/EBPδ and PPARγ of cell lysates prepared from iPLA2γ-deficient MEFs which were differentiated for 0, 2, 4, 6 or 9 d in the presence or absence of 10 µM of troglitazone. Representative results of three experiments are shown.
It has been reported that during the adipogenic differentiation of fibroblasts, the expression of C/EBPβ and C/EBPδ is induced at the early stage of adipogenesis, and then C/EBPβ and C/EBPδ elicit the expression of PPARγ, which in turn leads to the induction of C/EBPα.24) Therefore, we next investigated the effect of iPLA2γ deficiency on the expressions of these C/EBPs. In WT mouse-derived MEFs, the C/EBPβ and C/EBPδ levels were up-regulated along with the induction of iPLA2γ, and then C/EBPα and PPARγ were increased. The induction of C/EBPβ and C/EBPδ at the early stage of adipogenic differentiation was not affected in the iPLA2γ KO mouse-derived MEFs, but the induction of C/EBPα and PPARγ at the late stage was reduced in the KO cells (Fig. 3A). These results suggest that iPLA2γ is involved in a late stage of the differentiation.
To further reveal whether the suppressive effect of iPLA2γ deficiency on adipogenic differentiation was caused by a suppression of PPARγ activity, we next examined the effects of two PPARγ agonists, troglitazone and rosiglitazone, on the adipogenesis of iPLA2γ KO MEFs. In this experiment, MEFs prepared from iPLA2γ KO embryos were primed with a differentiation-inducing cocktail and the PPARγ agonists, troglitazone or rosiglitazone for 2 d, and then the retention of Oil Red O was quantified. As shown in Fig. 4B, treatment with troglitazone or rosiglitazone rescued the adipogenic differentiation of iPLA2γ-deficient MEFs. The expressions of the PPARγ target genes aP2 and CD36 in the KO MEFs were also up-regulated by troglitazone treatment.
We next investigated the effect of troglitazone treatment on the expression levels of transcriptional factors involved in adipogenesis in iPLA2γ KO mouse-derived MEFs. The induction of C/EBPβ and C/EBPδ at the early stage of adipogenesis was not affected by troglitazone, but the expression of C/EBPα was increased. The induction of C/EBPα might thus be mediated by PPARγ activation. On the other hand, the PPARγ expression was not changed by the troglitazone treatment. These results indicated that iPLA2γ might be involved in PPARγ activation as well as in PPARγ-independent PPARγ expression. Our findings also suggested that in iPLA2γ KO MEFs, the defect of PPARγ activation might be critical for its impairment in adipogenic differentiation.
In the present study, we showed that gene deletion of iPLA2γ suppressed the adipogenic differentiation of MEFs. As described above, Su et al. also reported that the knockdown of iPLA2γ expression inhibited a hormone-induced differentiation of 3T3-L1 cells into adipocytes.20) It was further shown that the suppression of the adipogenic differentiation of both MEFs and 3T3-L1 cells was rescued by the addition of PPARγ agonists. These results indicated that iPLA2γ produces certain lipid mediators that act as active ligands of PPARγ to induce adipogenic differentiation. Several bioactive lipids have been described as PPARγ ligands. It was shown that PGD2 and its metabolite, 15-deoxy-Δ12,14-PGJ2, bind to PPARγ as a ligand and promote adipogenesis.8,9) Fujimori et al. reported that lipocalin-type PGD synthase (L-PGDS), which is involved in PGD2 synthesis, was increased at a late stage of the adipocyte differentiation of 3T3-L1 cells and L-PGDS knockdown suppressed the differentiation of these cells into adipocytes.26)
However, as shown in Fig. 3B, PGD2 production during the adipogenic differentiation of MEFs was not affected by iPLA2γ gene deletion. Although the possibility that an iPLA2γ-mediated production of PGD2 and its metabolite in an intracellular specific site of MEFs plays an important role in adipogenic differentiation cannot be excluded, other bioactive lipids the productions of which are mediated via iPLA2γ might be critical for adipogenesis. iPLA2γ has been shown to mediate various types of lipid mediators other than prostanoids.14) It was reported that iPLA2γ is involved in the production of atypical eicosanoid-lysolipids.27) Such novel types of lipids might regulate adipogenesis. Further studies are needed to clarify the involvement of iPLA2γ in adipogenic differentiation.
This work was supported in part by a Grants-in-Aid for Scientific Research (B) (No. 16H05108) from the Japan Society for the Promotion of Science, and by the Private University Research Branding Project from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
The authors declare no conflict of interest.