2020 Volume 43 Issue 9 Pages 1413-1420
2020 Volume 43 Issue 9 Pages 1413-1420
The gut–liver axis may be involved in non-alcoholic steatohepatitis (NASH) progression. Pathogen-associated molecular patterns leak through the intestinal barrier to the liver via the portal vein to contribute to NASH development. Active vitamin D3 (1,25(OH)2D3) is a potential therapeutic agent to enhance the intestinal barrier. Active vitamin D3 also suppresses inflammation and fibrosis in the liver. However, the adverse effects of active vitamin D3 such as hypercalcemia limit its clinical use. We created a nano-structured lipid carrier (NLC) containing active vitamin D3 to deliver active vitamin D3 to the intestine and liver to elicit NASH treatment. We found a suppressive effect of the NLC on the lipopolysaccharide-induced increase in permeability of an epithelial layer in vitro. Using mice in which NASH was induced by a methionine and choline-deficient diet, we discovered that oral application of the NLC ameliorated the permeability increase in the intestinal barrier and attenuated steatosis, inflammation and fibrosis in liver at a safe dose of active vitamin D3 at which the free form of active vitamin D3 did not show a therapeutic effect. These data suggest that the NLC is a novel therapeutic agent for NASH.
Non-alcoholic fatty liver disease (NAFLD) represents a spectrum of disorders ranging from simple steatosis to non-alcoholic steatohepatitis (NASH), which can progress to fibrosis, cirrhosis and, finally, to hepatocellular carcinoma.1,2) The global prevalence of NAFLD is estimated to be 25.2%, which includes a larger percentage (59.1%) of patients who progress to NASH.3) There is an urgent need to develop an efficacious drug that can arrest or reverse NAFLD progression.
Recently, accumulating evidences has suggested that the gut–liver axis contributes to liver inflammation for NAFLD development.2,4–6) Increased intestinal permeability leads to translocation of pathogen-associated molecular patterns (PAMPs) originating from intestinal bacteria, which increases PAMPs concentration in the portal circulation to the liver. Then, PAMPs stimulate immune cells in the liver to induce expression of proinflammatory cytokines which aid progression of liver inflammation.7,8) Moreover, these cytokines stimulate the intestinal epithelium to enhance the permeability to further increase the amount of PAMPs in the portal circulation.9) This positive feedback in the gut–liver axis has been postulated to contribute to NAFLD progression.4)
Extensive research has shown that the liver steatosis and inflammatory damage are strongly associated with a low serum vitamin D3 in the serum of patients with NASH.10,11) Thus, supplementation with vitamin D3 is expected to show a therapeutic effect upon NASH. Vitamin D3 is converted to 25-hydroxycholecalciferol (25(OH)D3) and then the active form, 1,25-dihydroxycholecalciferol (1,25(OH)2D3), by different enzymes present in many tissues, especially in the liver and kidney. Active vitamin D3, 1,25(OH)2D3, serves as the ligand for the vitamin D3 receptor to regulate expression of various genes in many cell types.12)
In terms of NAFLD amelioration, active vitamin D3 suppresses activation of immune cells13) and collagen synthesis in hepatic stellate cells14) to contribute directly to the pathogenic symptoms of NAFLD. Furthermore, active vitamin D3 can enhance the intestinal barrier which aids NAFLD treatment via gut–liver axis. For instance, active vitamin D3 enhances the tight junctions between cells in intestinal epithelia15) and maintains the balance of the gut microbiome by activating secretion of antimicrobial peptides from resident macrophages and Paneth cells.16)
Despite these promising features of active than pre-active vitamin D3 for NAFLD treatment through direct and indirect effects, previous clinical trials that evaluated the effects of oral vitamin D3 supplementation in NAFLD patients used pre-active 25(OH)D317,18) because application of active 1,25(OH)2D3 has the risk of adverse effects such as hypercalcemia.19) Only one clinical trial has used active vitamin D3 for NAFLD treatment, and showed superior results to those of other trials using pre-active vitamin D3.20) Thus, to use active vitamin D3 for NAFLD treatment without risk, a formulation to deliver active vitamin D3 to the intestine and liver effectively to show a therapeutic effect at a low dose has been anticipated.
Previously, we developed a nano-sized formulation of active vitamin D3 suitable for intestinal absorption using a nano-structured lipid carrier (NLC).21) The latter comprises solid and liquid lipids and can incorporate hydrophobic drugs for protection from acidic conditions in the stomach, but can release drugs gradually in response to the lipases in intestinal juice.22) We applied the vitamin D3-containing NLC for the treatment of inflammation in the gastrointestinal tract and found that our NLC maintained a high concentration of active vitamin D3 in the intestine after oral administration then ameliorated inflammation by normalizing the structure of intestinal epithelia.21) These features of the active vitamin D3-containing NLC were expected to aid NAFLD treatment by efficiently delivering active vitamin D3 to intestine and then liver via systemic circulation. Hence, we examined here the therapeutic effect of the NLC on NAFLD in vitro and in vivo.
NLC containing 1,25(OH)2D3 was prepared by an oil in water emulsion method as pervious described.21) Briefly, 30 mg glycerol monostearate, 4 mg L-α phosphatidylcholine, 1 mg L-3-phosphatidyl-L-serine sodium, 2 mg α-tocopherol and 20 µg 1,25(OH)2D3 were dissolved in 1 mL chloroform-toluene mixed solution. Then the organic solution was dropped into aqueous solution containing the 24 mL 1.25% Pluronic F127 and 10 mg ascorbic acid. The two phases were mixed by homogenization and ultrasonication successively. The size and ζ-potential of NLC were checked by Zeta sizer Nano to be 110 nm and −17.0 mV, respectively.
Cell CultureCaco-2 cells (Riken, Japan) were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% fetal bovine serum (FBS), 4.5 mg/mL glucose, 100 U/mL penicillin, 100 U/mL streptomycin, supplemented with 0.1 mM MEM non-essential amino acid. RAW264.7 macrophages were transfected with secreted alkaline phosphatase (SEAP) as a reporter gene under the transcriptional control of a nuclear factor-kappaB (NF-κB) response element21) and cultured with the DMEM medium supplemented with 500 µg/mL G418. The both cells were incubated at 37°C, 5% CO2 humidified environment.
Transepithelial Electrical Resistance (TEER) MeasurementCaco-2 cells were cultured in 12-well Transwell™ insert plate filters with 0.4 µm pore size (Corning Inc., NY, U.S.A.) to establish the Caco-2 monolayer. After about 21 d, the Caco-2 cells were fully differentiated and formed integral Caco-2 monolayer (TEER value >500 Ω·cm2). The TEER value of Caco-2 monolayer was measured using a Millicell-Electrical Resistance System (ERS) instrument (Millipore, Germany). Lipopolysaccharide (LPS) (3 ng/mL) and NLC (0.042 µg/mL 1,25(OH)2D3) were added into the part of Transwell™ insert. After incubation for three days, the culture medium was changed with DMEM containing 10% FBS and TEER values were recorded.
Cell Co-culture SystemThe RAW264.7 macrophages transfected with SEAP were seeded into 12-well multiple plate and incubated for overnight. The Transwell™ insert which have integral Caco-2 monolayer were transferred into the 12-well multiple plate preloaded with RAW264.7 cells. The medium was changed to RPMI 1640. The RAW264.7 macrophages were stimulated by LPS (100 ng/mL), followed by addition of the NLC (0.042 µg/mL 1,25(OH)2D3) as shown in Fig. 1C. After 36 h incubation, the TEER value of this system was checked. The medium of the lower phase was collected for measurement of the SEAP level following reported protocol.21)

(A) Caco-2 monolayer in a Transwell™. (B) Protective effect of the NLC on the LPS-stimulated relative epithelial resistance of Caco-2 cells. (C) Co-culture of Caco-2 cells and RAW264.7 macrophages. (D) Suppressive effect of the NLC on LPS-induced NF-κB activation of RAW264.7 cells. (E) Protective effect of the NLC on the relative epithelial resistance of Caco-2 cells in the presence of LPS-stimulated RAW264.7 cells. Data are the mean ± S.E.M. (n = 4 per group). * p < 0.05, *** p < 0.001.
Six-week-old C57BL/6J male mice were purchased from Kyudo Co., Ltd. (Saga, Japan). Mice were maintained in a temperature and light-controlled facility. All studies were approved by the Animal care of Kyushu University. Mice were fed methionine-choline deficient (MCD) diet (Charles River Laboratories Japan Inc., Japan) to establish the NASH model. In normal diet group, mice were fed normal chow diet (CLEA Japan Inc., Japan). Mice were divided into four groups: normal diet (0.0131% EtOH), MCD diet (0.0131% EtOH), MCD diet + free-D3 (0.262 µg/mL 1,25(OH)2D3), and MCD diet + NLC (0.262 µg/mL 1,25(OH)2D3). During the experiment, mice were orally gavaged with each sample at a dose of 5 mL/kg body weight three times a week. After 2, 4, 6 or 8 weeks, mice were sacrificed and blood, liver and intestine tissues were collected and stored at −80°C until the following measurements.
In Vivo Intestinal Permeability AssayThe intestinal permeability was determined by the serum signal intensity of fluorescein isothiocyanate (FITC)-dextran (4 kDa, Sigma-Aldrich, U.S.A.) which was from the mice after orally administration of FITC-dextran as previously reported.23) In brief, mice feed with MCD diet after 2, 4, 6 or 8 weeks were fasted without diet and water for 8 h before sacrifice. At 4 h before sacrifice, the mice were orally applied with 60 mg/mL FITC-dextran at a dose of 10 mL/kg body weight. Mice were anesthetized by isoflurane and then collect the serum for measurement of fluorescence intensity.
Liver Histology and Serum BiochemistryLiver tissues were fixed in 10% formaldehyde solution. Then, the sections were embedded in paraffin block, cut into slices of 4 µm in thickness and stained with hematoxylin and eosin (H&E) or Masson’s trichrome (Sigma-Aldrich) by following the manufacturer’s instructions. Steatosis, inflammation, and fibrosis index were evaluated based on a literature.24) Scoring criteria are summarized in Table S1. The blood samples were centrifuged at 2000 × g for 10 min to obtain the serum. The serum level of alanine aminotransferase (ALT) and triglyceride were measured with the Fuji DRI-CHEM 4000 V system (FUJIFILM, Japan).
Real Time RT-PCRLiver and intestine tissues were extracted by RNAiso Plus (TaKaRa Inc., Japan) according to manufacturer’s protocol and 5 µg RNAs were reversed using the SuperScript III First-Strand Synthesis System (Thermo Fisher Scientific, U.S.A.). The primer sequences of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, toll-like receptor (TLR)4, Claudin1, ZO-1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) are summarized in Table S2. The LightCycler FastStart DNA Master SYBR Green kit (Roche Inc., Switzerland) was used for PCR amplification reactions. The relative amount of target gene expression compared to GAPDH and determined by comparative CT method.
Statistical AnalysesData are expressed as mean ± standard error of the mean (S.E.M.) and analyzed using the statistical software GraphPad prism 8. Groups were compared by one-way ANOVA test followed by the statistically significant. p < 0.05 was considered significant difference.
First, we examined the effect of the NLC on an LPS-induced increase in permeability of an intestinal epithelia model, monolayer of Caco-2 cell25–27) (Fig. 1A). Vitamin D3 has been reported to enhance the tight junctions between epithelial cells by activating expression of related proteins.28) An NLC containing the active form of vitamin D3, 1,25(OH)2D3, was prepared according to our previous report.21) The size of the NLC was adjusted to be 110 nm and its surface charge was negative (−17 mV) because of the stabilizer of phosphatidylserine. The size and charge were suitable for endocytic uptake by epithelia and macrophages via scavenger receptors.22) LPS and NLC were added to the Caco-2 monolayer and incubated for 3 d, then the membrane permeability was evaluated by TEER. The NLC suppressed the LPS-induced increase in permeability of the Caco-2 monolayer (Fig. 1B). Hence, after endocytic uptake of the NLC in Caco-2 cells, active vitamin D3 was released from the NLC to enhance the tight junctions between cells.
Next, we investigated the suppression of monolayer permeability mediated by inflammatory cytokines secreted from LPS-stimulated macrophages. RAW264.7 macrophages were cultured beneath the Caco-2 monolayer (Fig. 1C), this scenario mimicked the intestinal environment.29,30) Here, we used transfected RAW264.7 macrophage which expresses SEAP reporter gene under the transcriptional control of an NF-κB response element. The NLC were added in each layer and LPS were added in the lower layer, then cells were cultured for 36 h. The NLC suppressed the LPS-induced activation of macrophage, and the permeability of the monolayer was maintained (Figs. 1D, E). These results suggested that active vitamin D3 was released from the NLC in macrophages and suppresses their LPS-induced activation, thereby reducing the monolayer permeability.
NLC Ameliorated MCD-Induced Intestinal Leakage and Liver InjuryTo investigate whether NLC could ameliorate intestinal leakage and liver injury, we employed a NASH model in mice induced by consumption of the MCD diet. Mice developed steatohepatitis rapidly in week 1, followed by inflammation in week 2 and fibrosis by week 6.31,32) This NASH model also reproduces the increase in intestinal permeability.23,32) C57BL/6 mice fed the MCD diet were administered the NLC or free active vitamin D3 by oral gavage three times a week. The dose of active vitamin D3 was approx. 11 ng/mouse/d, which is much lower than a dose to cause hypercalcemia (50 ng/mouse/d).33,34) The MCD diet induced a loss in body weight and liver weight (Figs. 2A, B) even though the amount of food intake was identical to that of the group eating a normal diet (Fig. S1). This observation was attributed to an increase in energy expenditure in this model.35) Treatment with NLC and free active vitamin D3 did not improve the loss of body and liver weight, just like most NASH drugs under development.36,37)

Changes in (A) body weight, (B) liver weight, and the serum level of (C) FITC-dextran, (D) ALT, and (E) triglyceride of mice consuming a normal or MCD diet treated with the NLC or free vitamin D3. Mice were treated with each diet throughout the experiment. The NLC or free vitamin D3 (equal vitamin D3 dose: 0.262 µg/mL) was applied via the oral route three times a week. For C, serum was prepared after 4 h of oral application of FITC-dextran. Data are the mean ± S.E.M. (n = 5 per group). * p < 0.05, ** p < 0.01, *** p < 0.001; and “ns” denotes “not significant.” (Color figure can be accessed in the online version.)
To evaluate whether NLC could reduce the intestinal permeability, the leakage of orally administered FITC-labelled dextran (4 kDa) from the intestine to the blood circulation was used.23) The hydrodynamic diameter of 4 kDa dextran is 2.8 nm.38) The serum fluorescence intensity derived from FITC-dextran of each treatment group is summarized in Fig. 2C. In mice fed a normal diet, FITC-dextran leakage was negligible until 8 weeks (data not shown). Mice fed MCD diet showed a significant increase in the serum concentration of FITC-dextran from 2 weeks. The treatment groups using free vitamin D3 and the NLC showed a significant reduction in intestinal permeability. The difference between free vitamin D3 and the NLC was not significant even though we reported that the NLC can maintain a high concentration of vitamin D3 in the intestine for ≥12 h compared with that for a group given free vitamin D3.21)
To evaluate the liver injury wrought by the MCD diet, serum levels of ALT and triglyceride were measured. The increase in serum levels of ALT induced by the MCD diet was suppressed by the NLC from 2 weeks, whereas free vitamin D3 did not show this effect (Fig. 2D). Hence, the NLC ameliorated MCD diet-induced hepatic injury. A similar superior effect of the NLC over that of free vitamin D3 was observed in the MCD diet-induced reduction of serum triglyceride concentration from 6 weeks (Fig. 2E). This observation suggested that the NLC maintained hepatocyte function to secrete triglycerides into blood.
NLC Reduced NASH-Like Pathologic SymptomsLiver damage was evaluated by a histology scoring system24) of liver sections stained by H&E (Fig. 3A). Liver steatosis and inflammation were increased during the MCD diet feeding (Figs. 3B, C). Oral application of free active vitamin D3 did not affect the progress of these pathologic features, whereas NLC treatment displayed a significant reduction in these features throughout the observation period.

The treatment procedures were same with Fig. 2. (A) H&E staining of liver tissues taken from mice fed MCD diet at 2, 4, 6, and 8 weeks. Arrows show representative inflammatory foci. (B) Steatosis score and (C) inflammation score evaluated from liver histology. Data are the mean ± S.E.M. (n = 5 per group). * p < 0.05, ** p < 0.01; and “ns” denotes “not significant.” (Color figure can be accessed in the online version.)
Progression of hepatic fibrosis was evaluated using Masson’s Trichrome staining, which dyed collagen fibers blue (Fig. 4A). The fibrosis index is summarized in Fig. 4B. The fibrosis index of mice fed a normal diet was <2% throughout the observation (data not shown). The liver fibrosis induced by the MCD diet time-dependently increased from 4 weeks. NLC treatment suppressed the fibrosis from 6 to 8 weeks, but free vitamin D3 treatment showed no effect on liver fibrosis.
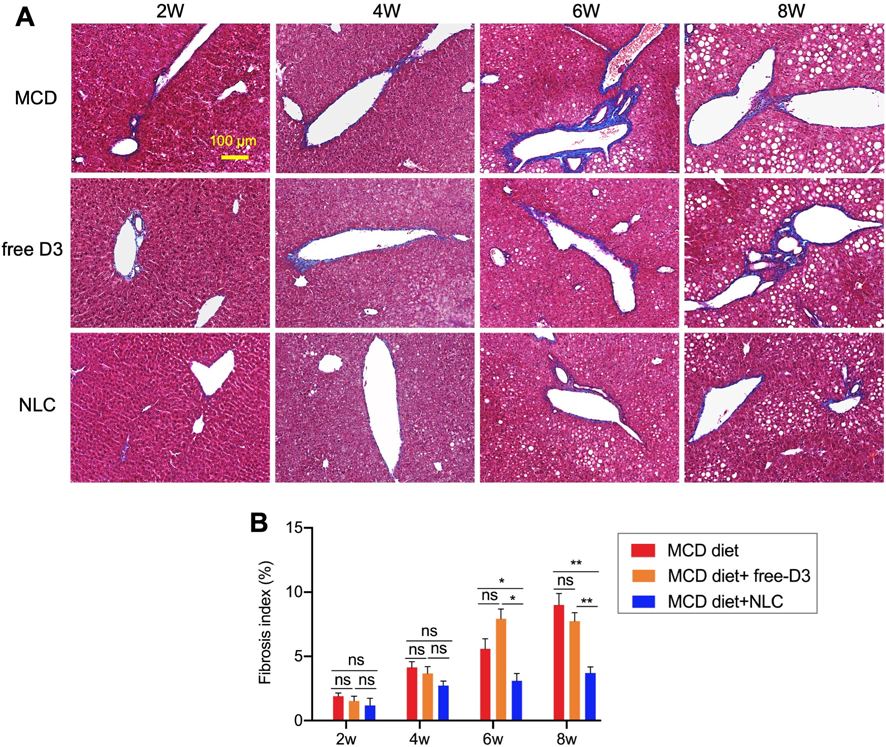
The treatment procedures were same with Fig. 2. (A) Masson’s trichome staining of liver tissues taken from mice fed MCD diet at 2, 4, 6 and 8 weeks. (B) The fibrosis index was evaluated from liver histology. Data are the mean ± S.E.M. (n = 5 per group). * p < 0.05, ** p < 0.01; “ns” denotes “not significant.” (Color figure can be accessed in the online version.)
The inflammation level in the liver was evaluated by the gene expression of inflammatory cytokines and TLR4 that is the receptor for LPS. Expression of IL-6 and IL-1β was significantly reduced by NLC treatment (Fig. 5). Expression of TNF-α and TLR4 also slightly reduced by the NLC, but not significantly so. As reported previously, proinflammatory cytokines such as IL-6 and IL-1β secreted in response to hepatic injury enhance intestinal permeability.39,40) Thus, suppression of such cytokine expression in the liver would aid suppression of intestinal permeability to progress NASH via the gut–liver axis.

The treatment procedures were same with Fig. 2. Six weeks after treatment in mice, the liver was resected to evaluate gene expression. Data are normalized to GAPDH expression and are the mean ± S.E.M. (n = 4–5 per group). * p < 0.05; “ns” denotes “not significant.”
Protective effect of the NLC on intestinal permeability was evaluated by the gene expression of intestinal tight junction proteins, Claudin1 and ZO-1 which play a key role for maintenance of the tight junction.41) MCD diet significantly downregulated the expression of Claudin1 and ZO-1. The NLC treatment reversed the expression of these genes (Fig. 6). Together with the dextran leakage study (Fig. 2C), the NLC was suggested to protect the increase in intestinal permeability induced by the MCD diet.

The treatment procedures were same with Fig. 2. Six weeks after treatment in mice, the intestine was resected to evaluate gene expression. Data are normalized to GAPDH expression and are the mean ± S.E.M. (n = 4–5 per group). * p < 0.05.
Recent drug development for NAFLD treatment targets pathologic symptoms in the liver such as steatosis, inflammation and fibrosis.2) However, accumulating evidence has shown that the gut–liver axis plays a key part in NAFLD development. The leaked PAMPs that reach the liver through the portal vein worsen liver inflammation. It has been reported that intervention of dysbiosis in the intestine ameliorates NAFLD symptoms by improving the intestinal permeability.9,32) However, drug for NAFLD treatment that targets improvement in the intestinal barrier has not been reported.
Vitamin D3 can suppress the inflammation and fibrosis in the liver14) as well as enhance the intestinal permeability.15) Previously, we designed an NLC containing active vitamin D3 to treat a model of intestinal inflammation and found that the NLC ameliorated intestinal inflammation in mice by suppressing activation of intestinal resident macrophages as well as by inducing mucosal healing at a safe dose of active vitamin D3.21) This mucosal healing effect of the NLC was expected to contribute to the therapy of NASH via gut–liver axis.
First, we examined the effect of the NLC on a co-culture system which mimicked the intestinal environment. The NLC suppressed the LPS-induced increase in epithelial permeability by working directly on endothelia and macrophages (Fig. 1). Kupffer cells in the liver secrete inflammatory cytokines, which reach the intestinal epithelia to enhance permeability.42) Thus, the anti-inflammatory effect of the NLC on macrophages could be a promising way to suppress the “positive loop” of enhancement of the intestinal permeability in NAFLD.
Next, we used the NLC to treat a NASH model in mice induced by the MCD diet. Mice in this model reproduce NASH-like pathologic features43) and intestinal permeability.23) The NLC and free active vitamin D3 suppressed intestinal permeability as evaluated by leakage of FITC-dextran of hydrodynamic size 2.8 nm (Fig. 2C). In addition, the NLC reversed the gene expression of the tight junction proteins in the intestine which were reduced by the MCD diet (Fig. 6). Although the NLC and free vitamin D3 showed similar level of suppression effect on the permeability of FITC-dextran, it was found later that only the NLC showed therapeutic effect on NASH model mice. This result indicates that the NLC may ameliorate the intestinal permeation which cannot be detected by 4 kDa FITC-dextran. Actually, some PAMPs have molecular weights less than 4 kDa; lipid A (a lipid part of LPS; 1.8 kDa) and lipoteichoic acid (0.8 kDa). The permeation of these small PAMPs through intestinal epithelia may contribute to the difference in the NASH development between mice treated with the free vitamin D3 and NLC.
In the MCD diet-induced NASH model, pathologic symptoms proceed in the order of steatosis, inflammation, and fibrosis.31) The NLC alleviated or delayed progress of such symptoms. The therapeutic effect of the NLC was observed in the suppression of steatosis (Fig. 3B), inflammation (Fig. 3C) and insult (Figs. 2D, E) from 2 weeks. As a result of the suppression of the liver insult, fibrosis was suppressed by the NLC from 6 weeks (Fig. 4). The therapeutic effects observed in the present study are superior to those in previous reports in which pre-active vitamin D3 were employed.44–49) Thus, the therapeutic effects of the NLC would be derived from retaining a high concentration of active vitamin D3 in the intestine and liver. The NLC is known to be degraded by lipase secreted from pancreas to release the content.50) Thus, most of our NLC would also be degraded in lumen to release vitamin D3, which will be absorbed through epithelia then delivered to the liver through the systemic circulation. Some fraction of our NLC may not be degraded completely and may be absorbed via M cells as a particle. This partially degraded particle would also reach to the liver through the systemic circulation.
Most of NASH drugs under development target the pathological symptoms in the liver. For example, agonists of farnesoid X receptor, which are the representative drugs for the NASH treatment, work to maintain functions of hepatocytes and suppress stellate cells activation.51) The advantage of active vitamin D3 over these drugs is that active vitamin D3 target the pathological symptoms both in the liver and the intestine.
We utilized NLC containing active vitamin D3 to target the intestine and liver for treatment of NASH model mice. At a safe dose at which free vitamin D3 did not show a therapeutic effect, NLC suppressed intestinal permeability and ameliorated NASH symptoms. This simple formulation of vitamin D3 prepared from approved materials and could be a practical therapeutic agent that act on the liver and intestine for NAFLD therapy via the gut–liver axis.
The authors would like to thank Prof. Koji Hase at Keio University for helpful discussion.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.