2021 Volume 44 Issue 1 Pages 18-24
2021 Volume 44 Issue 1 Pages 18-24
Deeper wrinkles and loss of elasticity are one of the skin-aging symptoms. Collagen breakdown by matrix metalloproteinase-1 (MMP-1), which is induced by reactive oxygen species (ROS) and pro-inflammatory cytokines, has been known to be responsible for these skin-aging symptoms. Therefore, much attention has been paid to chemicals to suppress the MMP-1 activity. Epigallocatechin-3-gallate (EGCG), catechin rich in green tea, has been reported to show antioxidant and protect skin from various stimuli such as UV and chemicals. In this study, we evaluated the inhibitory effect of EGCG on MMP-1 gene expression and secretion in tumor necrosis factor-α (TNF-α)-treated human dermal fibroblast cells (Hs68 cells). Pre-treatment with EGCG (10 and 20 µM) suppressed TNF-α-induced MMP-1 expression and secretion. EGCG also reduced the phosphorylation of extracellular signal regulated kinase (ERK) significantly but not that of p38 activation and c-Jun N-terminal kinase (JNK). Besides, EGCG (10 and 20 µM) showed the inhibitory effect on mitogen-activated protein extracellular kinase (MEK) and Src phosphorylation which is reported to be upstream signal proteins of ERK signal pathway. Based on these results, EGCG might have potential activity to slow down the skin-aging through inhibition of collagen breakdown, which remains to be elucidated.
Recently, the prevention of skin-aging has become a popular interest worldwide. Skin-aging is one of the most frequently observed skin symptoms and its causes can be classified as intrinsic, such as aging, and extrinsic environmental factors, such as UV rays and stress.1) Therefore, skin anti-aging methods focusing on the improvement of wrinkles and elasticity and the regeneration of the extracellular matrix (ECM) have received attention. ECM is distributed throughout all tissues and organs, and it is called the extracellular matrix as there are no cells. ECM plays a role in protecting the cells from external forces by forming physical structures as well as by establishing fundamentals of cellular development via biochemical signal transmission. ECM is composed of water, proteins, and polysaccharides. Collagen is the main component of ECM and it is the main substrate protein produced by the skin fibroblasts. The dermis contains a large amount of collagen. The main role of collagen is known for the mechanical rigidity, the resistance of the connective tissues and cohesiveness of the tissues, support for the cellular adhesion, and for inducing cell division. Therefore, the ECM collagen breakdown is related to various skin aging.2) Matrix metalloproteinase 1 (MMP-1), which is a fibroblast collagenase that breaks down collagen, is a component of the MMP family. Increased MMP-1 within ECM induces collagen fiber degradation, and broken-down collagen fragments are further degraded by MMP-3 and MMP-9.3) As the amount of collagen decreases, wrinkles deepen and elasticity is reduced, causing skin sagging and rough skin surface, which are the main causes of skin aging. MMP-1 expression and secretion in skin fibroblasts increase as a result of UV ray exposure, directly, and also interleukin 1-beta (IL-1β) and tumor necrosis factor-α (TNF-α) that are secreted from keratinocytes exposed to UV rays, indirectly.4,5) Increased MMP-1 from the above causes will induce rapid skin aging. As a result, experimental models targeting UV rays to discover cosmetic substances that suppress skin aging or models confirming MMP-1 expression and secretion in fibroblasts treated with inflammatory cytokines are used. Many substances have demonstrated suppressed skin aging through this models.6–8) Epigallocatechin-3-gallate (EGCG, Fig. 1A) is an important component of polyphenol in green tea, and it is well known for its anti-cancer, anti-inflammatory, and antioxidant effects.9,10) In skins, EGCG has been reported for its antiaging effects by suppressing MMP increase induced by fine dust, heat shock, and UVB.11–13) However, there have been no reports of MMP-1 suppression in dermal fibroblasts stimulated by TNF-α. In this study, EGCG effects in suppressing MMP-1 expression and secretion in fibroblasts stimulated by TNF-α have been demonstrated.

(A) Chemical structure of EGCG. (B) DPPH radical scavenging activity of EGCG. Ascorbic acid (AC) was positive control. (C) Cytotoxicity of EGCG in Hs68 cells. (D and, E) Effect of EGCG on intracellular ROS accumulation in Hs68 cells. The scale bar is 400 µm. Cells were incubated with 1, 10, and 20 µM of EGCG for 24 h. Cell viability was measured using MTT assay. # p < 0.05 compared with the vehicle control; * p < 0.05 compared with the control; ** p < 0.01 compared with the control.
Epigallocatechin-3-gallate (EGCG) and human tumor necrosis factor-α were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). The antibodies (phosphorylated extracellular signal regulated kinase (p-ERK), ERK, p-c-Jun N-terminal kinase (JNK), JNK, p-p38, p38, Akt, p-Akt, Src, p-Src, mitogen-activated protein extracellular kinase (MEK), p-MEK, and β-actin) were purchased from Cell Signaling Technology (Danvers, MA, U.S.A.). Human skin fibroblast Hs68 cells were obtained from American Type Culture Collection (ATC C, Manassas, VA, U.S.A.).
Cell CultureCells were grown in Dulbecco’s modified Eagle medium (DMEM) complemented 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin at 37 °C in 5% CO2 incubator.
Cell ViabilityCell viability was assessed using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cells were treated with EGCG at concentrations ranging from 1, 10, and 20 µM for 24 h. And then, MTT solution (5 mg/mL) was added and cells were incubated at 37 °C in 5% CO2 for 3 h. The supernatant was then removed, and 100 µL of dimethyl sulfoxide (DMSO) was added. Finally, absorbance was measured on a microplate reader at 570 nm. The level of cell viability was expressed as % compared with the control group.
2,2-Diphenyl-1-picrylhydrazyl (DPPH)The DPPH radical scavenging ability of EGCG was measured by the slightly modified previous method.14) Samples were dissolved in a solution of 99% methanol (Honey well, U.S.A.) and distilled water at a ratio of 1 : 1 at 0 (control), 1, 10 and 20 µM. Then 2 mL of 0.2 mM DPPH in EtOH was added to 100 µL sample solution. Then absorbance at 517 nm was measured after 20 min of incubation at 25 °C. In the study, ascorbic acid 250 µM was used as a positive control. The inhibition of DPPH radicals by the samples was calculated according to the following equation:
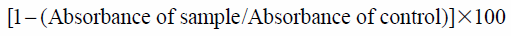 |
The production of ROS by Hs68 cells was measured using the 2,7-dichlorofluorescein diacetate (DCF-DA) dye method. The Hs68 cells were pretreatment with EGCG (1–20 µM) for 45 min prior to the induction with TNF-α (20 ng/mL) for 24 h. After removal of the supernatant, cells were treated with 10 µM DCF-DA in the dark for 45 min and then washed twice with phosphate buffered saline (PBS). The DCF-DA-stained cells were monitored and visualized under the fluorescence microscope using an EVOS fl digital inverted fluorescence microscope (AMG). To quantify the ROS level, cells were seeded on a 6 well plate and treated as described above. Relative fluorescence intensities were measured using a fluorescence spectrophotometer at excitation and emission wavelengths of 485 and 530 nm, respectively. The levels of intracellular ROS were expression as % compared with the control group.
Enzyme-Linked Immunosorbent Assay (ELISA)MMP-1 secretion was measured by MMP-1 Human ELISA kit (GE healthcare Life Sciences; little Chalfont, Buckinghamshire, U.K.) following the manufacturer’s instructions. The Hs68 cells were pretreatment with EGCG (1–20 µM) for 45 min prior to the induction with TNF-α (20 ng/mL) for 24 h. Then the cell supernatant was collected.
Quantitative Real-Time PCR (qRT-PCR)The Hs68 cells were pretreatment with EGCG (1–20 µM) for 45 min prior to the induction with TNF-α (20 ng/mL) for 24 h. Then the cell supernatant was discarded and washed with PBS. Total RNA was extracted from cell lysates using TRIzol reagent (Ambion, U.S.A.), and cDNA was prepared using a Revertra ACE-α- (Toyobo, Japan), according to the manufacturer’s instructions. Quantification of mRNA by qRT-PCR was carried out using Taqman RT-PCR Master Mix. The cDNA was amplified by using the following primers are MMP-1 (Hs00899658_m1), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Hs02786624_g1).
Western Blot AnalysisThe Hs68 cells were pretreatment with EGCG (1–20 µM) for 45 min prior to the induction with TNF-α (20 ng/mL) for 15 min except for the determination of mitogen-activated protein kinases (MAPKs). Then, the cell supernatant was discarded and washed with PBS. The cells were lysed with pre-prep (lysis buffer) including protease and phosphatase inhibitor. Insoluble debris was removed by centrifugation at 12000 rpm for 20 min, and protein content was determined using bicinchoninic acid (BCA) assay. Equal amounts of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were incubated with primary antibodies at 4 °C overnight, then washed with TBST (1 × Tris-buffered saline (TBS) including 0.1% Tween-20), exposed to secondary antibodies for 2 h at room temperature. The membranes were washed 3 times and the protein bands were visualized by the enhanced and quantified using Image J software from the NIH (Bethesda, MD, U.S.A.).
Statistical AnalysisThe SPSS 12.0 version (SPSS Inc., Chicago, IL, U.S.A.) was applied for statistical analysis. Each experiment was done in triplicate and the data are represented as the mean ± standard error of the mean. Statistical analysis of the data was performed using Student’s t-test. Differences with a p < 0.05 were considered statistically significant.
Antioxidant effects of EGCG were measured, as there have been reports of increased cellular ROS production with TNF-α treatment and that ROS affects MMP family protein expression.15,16)
A direct effect of EGCG in radical scavenging was confirmed through DPPH radical. When treated with EGCG 1, 10, and 20 µM, DPPH radical elimination rates were 4.2, 52.5, and 77.9%, respectively, suggesting increased radical elimination with increased EGCG concentration (Fig. 1B). In order to determine whether EGCG suppresses cellular ROS production, the effect of EGCG on cell survival was tested. The results suggested that 1, 10, and 20 µM of EGCG did not affect cell survival (Fig. 1C). When fibroblast was treated with TNF-α alone, ROS production was confirmed using DCF-DA fluorescence, which was reduced when treated with EGCG 20 µM (Figs. 1D, E).
Inhibitory Effect of EGCG on MMP-1 mRNA Expression and Secretion in TNF-α-Stimulated Hs68 Dermal FibroblastEffects of EGCG on suppressing MMP-1 gene expression and secretion were confirmed using TNF-α treated Hs68 cells. Hs68 cells were treated with EGCG (1, 10, 20 µM) and TNF-α (20 ng/mL) was added after 45 min. After 24 h, MMP-1 quantity and intracellular mRNA expression were measured from the supernatant with ELISA and qRT-PCR. When treated with TNF-α alone, MMP-1 quantity in the supernatant was 2.5 times greater. When treated with EGCG, increased MMP-1 quantity in the supernatant was reduced dose-dependently, and EGCG treatment at 20 µM reduced the quantity similar to that in supernatant for cells not treated with TNF-α. Forskolin, a positive control known to reduce MMP-1, was used at 10 µM and it also reduced MMP-1 quantity (Fig. 2A). In order to identify the effects of EGCG on increased MMP-1 expression in fibroblast in response to TNF-α, MMP-1 mRNA expression was measured using qRT-PCR. When treated with TNF-α only, the expression increased by 17.5 times compared to the control, as expected. When treated with EGCG 1, 10, and 20 µM, MMP-1 mRNA expressions increased only by 6.7, 3.6, and 4.2 times, respectively, which were greatly reduced compared to when treated with TNF-α only (Fig. 2B). There have been reports of forskolin, which increases cAMP by activating adenylyl cyclase, in suppressing MMP-1 expression in TNF-α-stimulated fibroblast. Therefore, it was used as a positive control.17) In this study, forskolin suppressed MMP-1 mRNA expression that was increased in response to TNF-α as well (Fig. 2B).

(A) EGCG inhibits TNF-α-induced MMP-1 mRNA expression. (B) EGCG inhibits TNF-α-induced MMP-1 secretion. Hs68 cells were pre-treated with EGCG (1–20 µM) for 45 min, and then stimulated with TNF-α (20 ng/mL) for 15 min. Forskolin was positive control. # p < 0.05 compared with the vehicle control; * p < 0.05 compared with the TNF-α. ** p < 0.01 compared with the TNF-α.
The effects of EGCG on the activation of MAPK in Hs68 cells stimulated with TNF-α were investigated, as there have been reports of MAPK important role in MMP-1 gene expression and secretion. EGCG (1, 10, and 20 µM) was treated 45 min before TNF-α (20 ng/mL) treatment, which was treated for 15 min. EGCG dose-dependently suppressed ERK phosphorylation but did not affect the phosphorylation of JNK and p38 (Figs. 3A, B). These results suggest that EGCG suppresses TNF-α-induced ERK signaling pathway and therefore affects MMP-1 expression and secretion. For this reason, MEK and Src phosphorylation, which are upstream signaling pathways of ERK, were investigated, and it was found that MEK and Src phosphorylation increased after TNF-α treatment but EGCG 10 and 20 µM treatments suppressed their phosphorylation (Figs. 3C, D).

Respective western blots presenting the effects of EGCG on phosphorylation of (A and, B) ERK, JNK and p38. (C and, D) The effects of EGCG on phosphorylation of Src and MEK. Inhibitory effect of EGCG on MMP-1 secretion through extracellular signal-regulated kinase MMP-1 was secreted through extracellular signal-regulated kinase (ERK) inactivation in TNF-α stimulated Hs68 cells. Hs68 cells were pre-treated with EGCG (1–20 µM) for 45 min, and then stimulated with TNF-α (20 ng/mL) for 15 min. Forskolin was positive control. # p < 0.05 compared with the vehicle control; * p < 0.05 compared with the TNF-α; ** p < 0.01 compared with the TNF-α.
There have been reports of 7,8-Dihydroxyflavone and Compound K, which are known to suppress TNF-α-induced MMP-1 increase in fibroblast, suppressing Akt activation. Therefore, the effects of Akt on phosphorylation were investigated.6,7) In this study, there was no increase in Akt phosphorylation as a result of TNF-α treatment. Interestingly, Akt phosphorylation after EGCG 10 and 20 µM treatments increase by approximately 3 times, compared to the control group, and the positive control suppressed Akt phosphorylation to a great extent (Figs. 4A, B).

Hs68 cells were pre-treated with EGCG (1–20 µM) for 45 min, and then stimulated with TNF-α (20 ng/mL) for 15 min. Forskolin was positive control. # p < 0.05 compared with the vehicle control; * p < 0.05 compared with the TNF-α. ** p < 0.01 compared with the control.
Collagen is the main component of ECM and it contributes to the elasticity of the skin. Collagen exists in the dermis in a great amount. As it has a close relationship with skin wrinkles, collagen deficiency can induce skin wrinkles. MMP-1 is an important enzyme in collagen breakdown, and it is a known cause of wrinkle formation by increasing collagen breakdown in the dermis. Therefore, substances that suppress the increase of MMP-1 expression or secretion in fibroblasts have gained attention as a cosmetic material. There are several reasons that increase MMP-1 expression and secretion in fibroblasts. UVA and heat are direct causes while cytokines are indirect causes.4,5,12) Keratinocytes of epidermis exposed to UVB are known to secrete inflammatory cytokines, including TNF-α, which stimulate epidermal fibroblasts, thus increasing ROS and MMP-1 expression and secretion. There are also reports of ROS playing a role in MMP-1 expression as well as increased ROS production in response to TNF-α stimulation. Since there may be an association between increased MMP-1 and ROS in response to TNF-α, suppressive effects of EGCG on ROS production was first investigated. In this study, it was found that EGCG has a direct effect on radical elimination using DPPH assay. Existing studies report IC50 of 13 µM for DPPH elimination with EGCG, suggesting that the results of the current study are similar to previous results.18) It was found that EGCG suppressed ROS production in fibroblasts treated with TNF-α. EGCG has been reported to suppress ROS production in damaged brain tissues.19) Antioxidant effects of EGCG may involve direct elimination of radicals, but there are also reports that EGCG increases the expression of enzymes that resist oxidative stress in various cell types. Therefore, the effects of EGCG by increasing antioxidant enzymes in dermal fibroblast may not be excluded.20,21) EGCG effects on MMP-1 in response to TNF-α was measured by quantifying MMP-1 and mRNA expression in the supernatant using ELISA and qRT-PCR. Interestingly, EGCG 1, 10, and 20 µM treatments clearly suppressed mRNA production. But MMP-1 amount in the supernatant that was decreased dose-dependently at EGCG 10 and 20 µM was not observed at 1 µM. The effects of TNF-α on MMP-1 of dermal fibroblast can be classified as increased activation of MMP-1 transcription factors and increased extracellular secretion. In this study, EGCG appears to suppress the activation of transcription factors that participate in MMP-1 gene expression at low concentrations, but higher concentrations may be required to suppress signaling pathways that play a role in secretion. Moreover, in order to ECM remodeling to occur, increased extracellular MMP-1 activation is required and it is thought that signaling pathways that intensity secretion plays more role than increased MMP-1 expression in fibroblasts, but more studies are needed to confirm this. MAPK is a well-known signaling protein to participate in MMP-1 expression and secretion.22,23)
ERK, in particular, increases MMP-1 activation by enhancing AP-1 activation, and p38 has been reported to increase MMP-1 mRNA stability.24) Effects of EGCG on MAPK phosphorylation in response to TNF-α were investigated, which showed suppressed ERK phosphorylation but not suppression of JNK and p38 phosphorylation. Phosphorylation of MEK, which is an upstream signaling protein of ERK, was measured and similar results as ERK phosphorylation were shown. This indicates that EGCG may partially interrupt the MEK–ERK signaling pathway. There have been reports of Compound K, found in ginseng, suppresses MMP-1 secretion and Src phosphorylation in dermal fibroblasts treated with TNF-α, therefore Src phosphorylation after EGCG was measured.7) At EGCG 20 µM, Src phosphorylation was greatly suppressed but phosphorylation increased at 1 µM and it was greater than when treated with TNF-α alone. More studies will be needed but it can be considered that the effects of EGCG on Src phosphorylation in response to TNF-α may vary based on the dose. As previously shown, EGCG at 1 µM suppressed MMP-1 mRNA expression but without changes in MMP-1 quantity in the supernatant may be related to Src phosphorylation.
There are studies reporting conflicting results of the effects of Akt on MMP-1 expression and secretion. 7,8-Dihydroxyflavone and Compound K are reported to suppress increased MMP-1 expression and secretion in response to TNF-α and phosphorylation of Akt,6,7) but there are also reports of suppressed Akt activation increasing the expression and secretion of MMP-1.25,26) The effects of IL-13 in suppressing MMP-1 in TNF-α treated fibroblasts are reversed when treated with Akt suppressants.27)
In this study, EGCG at 10 and 20 µM improved Akt phosphorylation. Forskolin, which was used as a positive control for suppressed MMP-1 expression and secretion, suppressed Akt phosphorylation. This suggests that the direction of MMP-1 expression and secretion pathway cannot be estimated with Akt phosphorylation alone, and various signaling proteins for MMP-1 expression and secretion are related to Akt phosphorylation. Taken all together, EGCG suppressed MMP-1 production and secretion, in response to the pro-inflammatory cytokine TNF-α, in fibroblasts and can be used as an anti-aging substance for the skin. However, further studies will be conducted as more reports are needed for understanding the effects of different EGCG concentrations on phosphorylation of signaling proteins and Akt activation.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.