2021 年 44 巻 10 号 p. 1514-1523
2021 年 44 巻 10 号 p. 1514-1523
Drug-induced liver injury (DILI) is a common adverse drug event. Spontaneous reporting systems such as the Japanese Adverse Event Report Database (JADER) have been used to evaluate the association between drugs and adverse drug events. However, the association of drugs with adverse drug events may be overestimated due to reporting biases. Therefore, it is important to objectively evaluate the association using liver function test values. The aim of the present study was to predict potential hepatotoxic drugs using real-world data including electronic medical records and the JADER database. A total of 70009 (2779 with DILI and 67230 without DILI) and 438515 (10235 with DILI and 428280 without DILI) Japanese adult patients were extracted from electronic medical records and the JADER database, respectively. Drugs with ≥100 DILI patients in both of the two databases were regarded as suspected drugs for DILI. We used multivariate logistic regression to evaluate the association between the suspected drugs and increased risk of DILI. Among the suspected drugs, broad-spectrum antibiotics such as meropenem, tazobactam/piperacillin and ceftriaxone were significantly associated with an increased risk of DILI, and meropenem had a greater risk of DILI in both of the two databases. Additionally, there were significant associations of mosapride and L-carbocisteine with increased risk of DILI. In addition to well-known associations between antibiotic drugs and DILI, mosapride and L-carbocisteine were found to be new potential signals of drugs causing hepatotoxicity. This study indicates potential hepatotoxic drugs that require further causality assessment.
Drug-induced liver injury (DILI) is a common adverse drug reaction and a cause of acute liver failure.1,2) Worldwide, the estimated annual incidence of DILI is 139.0–240.0 per 1 million persons.3) However, the incidence of DILI may be underestimated because a large number of DILI cases are missed in the diagnoses made by physicians and the content of discharge letters.4) In addition, approximately 1 in 100 patients develops DILI during hospitalization, but more than 50% of cases are missed when they occur in non-hepatology departments. It is reported that the classes of drugs most frequently associated with liver injury are anti-inflammatory, antimicrobial, and anti-epileptic agents.5,6) Furthermore, various drugs can cause liver injury irrespective of the class.7) It is therefore important to evaluate the risk of DILI in individual drugs that are used clinically.
Spontaneous reporting systems (SRSs) such as the Food and Drug Administration Adverse Event Reporting System (FAERS), the Japanese Adverse Drug Event Report (JADER) database, and the database of the WHO Programme for International Drug Monitoring (VigiBase™) are used to evaluate the risk of adverse drug events.8–11) Most cases of adverse drug reactions are reported to these systems by patients themselves or healthcare professionals. However, few studies have investigated the associations between drugs and the risk of DILI with adjustment for potential confounding factors including age and sex. Moreover, it is known that there are reporting biases in SRSs, and these biases affect the risk of adverse drug events.12,13) For these reasons, it is important to 1) adjust for these potential confounding factors and 2) use not only spontaneous reporting systems but also electronic medical records including liver function test values to objectively evaluate the association between drugs and the risk of DILI. Hence, the aim of the present study was to detect potential hepatotoxic drugs using both a spontaneous reporting system and electronic medical records.
The JADER database and the Nihon University School of Medicine’s Clinical Data Warehouse (NUSM’s CDW), in which approximately 2.4 million electronic medical records have accumulated, were used in the present study. The NUSM’s CDW is a centralized data repository that integrates separate databases, including patient demographics, diagnoses, and laboratory data, from the hospital information systems at three hospitals affiliated with the NUSM; Nihon University Itabashi Hospital, Nerima Hikarigaoka Hospital, and Surugadai Nihon University Hospital. To protect patient privacy, patient identifiers are replaced by anonymous identifiers in all databases of the CDW. The JADER database is published by the Pharmaceuticals and Medical Devices Agency of Japan and is comprised of four types of tables (patient demographic and administrative information, DEMO; drug information, DRUG; adverse events, REAC; and medical history, HIST). Quarterly data files (Q1 2004 to Q1 2020) of the JADER database (downloaded on May 26, 2020) were used for the present study.
The experimental protocol was approved by the Ethics Committee of the NUSM (Approval No. P20-20-0), and the study was conducted in compliance with the Ethical Guidelines for Medical and Health Research Involving Human Subjects of the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare, Japan.
Study SubjectsNUSM’s CDWThis study was based on a case-control study including 203744 Japanese patients aged 18 and older who underwent liver function tests (i.e., alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP)) at least two times during the period from April 2004 to March 2020. First, patients whose ALT and AST values reached >3 times the upper limit of normal (ULN) were regarded as DILI patients, and the date of the first abnormal test result was regarded as the event date. Of the DILI patients, those whose ALT and AST values measured within 90 d before the event date were within the normal range were classified into the DILI group (case group, Fig. 1). On the other hand, patients who had undergone liver function tests at least twice within 90 d and whose ALT and AST were within the normal range on all measurement days were regarded as non-DILI patients, and were classified into the non-DILI group (control group). In addition, the latest date of liver function measurement was regarded as the reference date (Fig. 1). Next, among the patients in the two groups, patients who met the following exclusion criteria were excluded. Finally, the remaining 70009 patients (2779 patients in DILI group, 67230 patients in non-DILI group) were included in the statistical analysis.

Event date is the date when patients’ ALT and AST values reached >3 times the upper limit of normal. Reference date is the latest date of measurement of liver function in patients who underwent liver function tests at least 2 times within 90 d and whose ALT and AST values were within the normal range on all measurement days. Patients with pre-existing liver disease and those who had taken any drugs for liver disease before the event date were excluded. Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; DILI, drug-induced liver injury; NUSM’s CDW, Nihon University School of Medicine’s Clinical Data Warehouse; ULN, upper limit of normal.
JADER contained 632409 patients in the DEMO table. In this database, the terminology of adverse events and medical history have been unified with preferred terms according to the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Medical Dictionary for Regulatory Activities (MedDRA, version 23.0). In addition, age in the DEMO table is grouped by decade. First, patients in their 20 s and over were extracted from this database. Among the extracted patients, patients with the following reported DILI-related adverse events were classified into the DILI group (case group): “drug-induced liver injury,” “hepatitis,” “hepatitis fulminant,” “hepatic function abnormal,” “hepatocellular injury,” “hepatic failure,” or “liver injury.” The date when these patients developed these DILI-related adverse events was regarded as the event date. On the other hand, patients who did not report these events were classified into the non-DILI group (control group). Next, among these patients in the two groups, patients who met the following exclusion criteria were excluded.
Finally, the remaining 438515 patients (10235 patients in DILI group, 428280 patients in non-DILI group) were included in the statistical analysis.
Suspected Drugs for DILIIn 70009 patients extracted from NUSM’s CDW, 1162 drugs were newly started within 90 d before the event date. Among these drugs, 60 drugs had more than 100 DILI patients. Thus, these 60 drugs were regarded as suspected drugs for DILI to statistically evaluate the association between the suspected drugs and DILI (Fig. 1). In 438515 patients extracted from JADER, 1730 drugs were newly started within 90 d before the event date. Among these drugs, 40 drugs with ≥100 DILI patients were regarded as suspected drugs for DILI. Of the suspected drugs, 23 suspected drugs with ≥100 DILI patients in both of the two databases were assessed to detect potential hepatotoxic drugs.
Covariates to Assess Association with DILIIn the analysis using NUSM’s CDW, age, sex, number of concomitant drugs, and medical history were considered as potential confounding factors. The number of concomitant drugs was defined as the number of drugs that had been used within 90 d before the event date. Medical history included the following eight diagnoses: 1) diabetes (ICD-10 codes; E10-E14), 2) dyslipidemia (ICD-10 code; E78), 3) hypertension (ICD-10 codes; I10-I15), 4) ischemic heart disease (ICD-10 codes; I20-I25), 5) cerebrovascular disease (ICD-10 codes; I60-I69), 6) thyroid disease (ICD-10 codes; E00-E07), 7) kidney disease (ICD-10 codes; N00-N29), and 8) inflammatory bowel disease (ICD-10 codes; K50, K51). In the analysis using JADER, age, sex, number of concomitant drugs, and medical history were considered as potential confounding factors. The number of concomitant drugs was defined as the number of drugs that had been used within 90 d before the event date. MedDRA terms were used to extract the following eight medical conditions from the HIST table (see Supplementary Table S2): 1) diabetes, 2) dyslipidemia, 3) hypertension, 4) ischemic heart disease, 5) cerebrovascular disease, 6) thyroid disease, 7) kidney disease, and 8) inflammatory bowel disease.
Statistical AnalysisIn the analysis using NUSM’s CDW, to compare differences in patient background between the DILI group and non-DILI group, two-tailed Student’s t-test or Wilcoxon rank-sum test was performed for continuous data including age, number of concomitant drugs, and liver function test values. Chi-squared test was performed for differences in categorical data including sex and medical history. Associations between the use of each suspected drug and the risk of DILI were assessed by means of multivariate logistic regression, with adjustment for age, sex, number of concomitant drugs, and medical history. Adjusted odds ratio (OR) and corresponding 95% confidence interval (95%CI) were calculated. In the analysis using JADER, to compare differences in patient background between the DILI group and non-DILI group, two-tailed Student’s t-test was performed for the number of concomitant drugs. Chi-squared test for independence was performed for differences in categorical data including age and sex. Associations between the suspected drugs and DILI were assessed by multivariate logistic regression, with adjustment for age, sex, number of concomitant drugs, and medical history. The level of significance was set at 5.0% for all statistical analyses. All statistical analyses were conducted with R software (version 3.5.1; R Foundation for Statistical Computing, Vienna, Austria).
A total of 70009 patients were extracted from NUSM’s CDW (2779 patients in DILI group, 67230 patients in non-DILI group). Differences in patients’ background are shown in Table 1. Mean age in the DILI group was 61.4 ± 18.3 (standard deviation) and in the non-DILI group was 53.9 ± 20.4, with a significant difference between the two groups (p < 0.001). In addition, the male percentage and number of concomitant drugs in the DILI group were significantly higher than those in the non-DILI group (p < 0.001, respectively). The percentages of patients with six medical conditions except for dyslipidemia and kidney disease were significantly higher in the DILI group than in the non-DILI group. The period from the initiation of medication until the onset of liver injury is shown in Supplementary Fig. S1.
| Characteristics | DILI group (N = 2779) | Non-DILI group (N = 67230) | p-Value |
|---|---|---|---|
| Age (years), mean ± S.D. | 61.4 ± 18.3 | 53.9 ± 20.4 | <0.001 |
| Male, N (%) | 1691 (60.8) | 28795 (42.8) | <0.001 |
| Number of concomitant drugs, mean ± S.D. | 11.5 ± 7.8 | 7.8 ± 5.7 | <0.001 |
| Laboratory parameters (units/L), median (IQR) | |||
| ALT | 184.0 (151.0–290.2) | 15.0 (11.0–21.0) | <0.001 |
| AST | 175.0 (121.0–356.0) | 19.0 (15.0–23.0) | <0.001 |
| ALP | 342.0 (232.5–542.0) | 212.0 (169.0–268.0) | <0.001 |
| Medical history, N (%) | |||
| Diabetes | 850 (30.6) | 24790 (36.9) | <0.001 |
| Dyslipidemia | 364 (13.1) | 9829 (14.6) | 0.028 |
| Hypertension | 709 (25.5) | 13388 (19.9) | <0.001 |
| Ischemic heart disease | 658 (23.7) | 10388 (15.5) | <0.001 |
| Cerebrovascular disease | 770 (27.7) | 8853 (13.2) | <0.001 |
| Thyroid disease | 296 (10.7) | 8501 (12.6) | 0.002 |
| Kidney disease | 630 (22.7) | 15832 (23.5) | 0.295 |
| Inflammatory bowel disease | 21 (0.8) | 270 (0.4) | 0.007 |
Student’s t-test was performed for differences in age and number of concomitant drugs. Wilcoxon rank-sum test was performed for differences in laboratory parameters. Chi-squared test was performed for differences in categorical variables such as sex and medical history. Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DILI, drug-induced liver injury; IQR, interquartile range; NUSM’s CDW, Nihon University School of Medicine’s Clinical Data Warehouse; S.D., standard deviation.
A total of 438,515 patients were extracted from the JADER database (10235 patients in DILI group, 428280 patients in non-DILI group). The differences in patients’ characteristics between the two groups are shown in Table 2. There was a significant difference in the frequency distribution of age grouped by decade between the two groups (p < 0.001). There was no significant difference in the male percentage between the DILI group and non-DILI group (51.6 vs. 51.2%, p = 0.480). The number of concomitant drugs was significantly lower in the DILI group than in the non-DILI group (p < 0.001). The percentages of patients with dyslipidemia, cerebrovascular disease, thyroid disease, and kidney disease were significantly higher in the DILI group than in the non-DILI group.
| Characteristics | DILI group (N = 10235) | Non-DILI group (N = 428280) | p-Value |
|---|---|---|---|
| Male, N (%) | 5280 (51.6) | 219406 (51.2) | 0.480 |
| Age (years), N (%) | <0.001 | ||
| 20–29 | 480 (4.7) | 15732 (3.7) | |
| 30–39 | 828 (8.1) | 25790 (6.0) | |
| 40–49 | 1001 (9.8) | 34841 (8.1) | |
| 50–59 | 1652 (16.1) | 56527 (13.2) | |
| 60–69 | 2391 (23.4) | 103667 (24.2) | |
| 70–79 | 2492 (24.3) | 120022 (28.0) | |
| 80–89 | 1185 (11.6) | 62936 (14.7) | |
| 90–99 | 205 (2.0) | 8605 (2.0) | |
| 100– | 1 (0.0) | 160 (0.0) | |
| Number of concomitant drugs, mean (S.D.) | 2.4 (2.3) | 4.4 (4.1) | <0.001 |
| Medical history, N (%) | |||
| Diabetes | 1134 (11.1) | 47173 (11.0) | 0.848 |
| Dyslipidemia | 899 (8.8) | 29648 (6.9) | <0.001 |
| Hypertension | 1767 (17.3) | 71315 (16.7) | 0.103 |
| Ischemic heart disease | 352 (3.4) | 14076 (3.3) | 0.408 |
| Cerebrovascular disease | 537 (5.2) | 14918 (3.5) | <0.001 |
| Thyroid disease | 245 (2.4) | 7942 (1.9) | <0.001 |
| Kidney disease | 634 (6.2) | 30037 (7.0) | 0.001 |
| Inflammatory bowel disease | 88 (0.9) | 3880 (0.9) | 0.664 |
Student’s t-test was performed for differences in number of concomitant drugs. Chi-squared test was performed for differences in categorical variables such as age, sex, and medical history. Abbreviations: DILI, drug-induced liver injury; JADER, Japanese Adverse Drug Event Report database; S.D., standard deviation.
There were 60 suspected drugs with ≥100 patients with DILI in NUSM’s CDW. Of these drugs, 23 had ≥100 patients with DILI also in JADER: 6 antibiotic drugs, 5 anti-inflammatory drugs, 7 digestive tract drugs, and 5 drugs for other diseases. The effect of the 23 suspected drugs on the risk of DILI is shown in Table 3, and that of all 60 suspected drugs on the risk of DILI is shown in Supplementary Table S3. Among antibiotic drugs, while meropenem (adjusted OR, 4.65; 95% confidence interval (95%CI), 3.71–5.84; p < 0.001), tazobactam/piperacillin (adjusted OR, 2.31; 95%CI, 1.85–2.90; p < 0.001), ceftriaxone (adjusted OR, 1.63; 95%CI, 1.37–1.94; p < 0.001), and sulbactam/ampicillin (adjusted OR, 1.44; 95%CI, 1.26–1.64; p < 0.001) were significantly associated with an increased risk of DILI, cefazolin was significantly associated with a decreased risk of DILI (adjusted OR, 0.35; 95%CI, 0.31–0.40; p < 0.001). Of the anti-inflammatory drugs, methylprednisolone showed an association with a high risk of DILI (adjusted OR, 2.25; 95%CI, 1.85–2.75; p < 0.001). With regard to digestive tract drugs, mosapride (adjusted OR, 2.83; 95%CI, 2.44–3.28; p < 0.001), lansoprazole (adjusted OR, 1.40; 95%CI, 1.25–1.57; p < 0.001), and famotidine (adjusted OR, 1.36; 95%CI, 1.25–1.49; p < 0.001) were associated with a high risk of DILI. Among drugs for other diseases, furosemide (adjusted OR, 1.34; 95%CI, 1.18–1.53; p < 0.001) and L-carbocisteine (adjusted OR, 2.16; 95%CI, 1.94–2.40; p < 0.001) were significantly associated with an increased risk of DILI. Differences in patients’ characteristics between users and non-users of all 60 suspected drugs are presented in Supplementary Table S4. The association between the covariates and the risk of DILI in NUSM’s CDW is shown in Supplementary Table S5.
| Class | Drug name | N | Adjusted OR [95%CI] | p-Value | |
|---|---|---|---|---|---|
| DILI group | Control group | ||||
| Antibiotic drugs | Meropenem | 129 | 362 | 4.65 [3.71–5.84] | <0.001 |
| Tazobactam/piperacillin | 123 | 457 | 2.31 [1.85–2.90] | <0.001 | |
| Ceftriaxone | 176 | 1703 | 1.63 [1.37–1.94] | <0.001 | |
| Sulbactam/ampicillin | 320 | 3080 | 1.44 [1.26–1.64] | <0.001 | |
| Levofloxacin | 159 | 3889 | 0.84 [0.71–1.00] | 0.051 | |
| Cefazolin | 345 | 12672 | 0.35 [0.31–0.40] | <0.001 | |
| Anti-inflammatory drugs | Methylprednisolone sodium succinate | 135 | 941 | 2.25 [1.85–2.75] | <0.001 |
| Prednisolone | 133 | 2678 | 1.12 [0.93–1.35] | 0.222 | |
| Acetaminophen | 693 | 11920 | 1.03 [0.93–1.14] | 0.574 | |
| Diclofenac | 297 | 11555 | 0.52 [0.46–0.59] | <0.001 | |
| Loxoprofen | 481 | 17470 | 0.46 [0.41–0.51] | <0.001 | |
| Digestive tract drugs | Mosapride | 283 | 1298 | 2.83 [2.44–3.28] | <0.001 |
| Lansoprazole | 457 | 4614 | 1.40 [1.25–1.57] | <0.001 | |
| Famotidine | 903 | 13009 | 1.36 [1.25–1.49] | <0.001 | |
| Magnesium oxide | 294 | 6490 | 0.76 [0.67–0.87] | <0.001 | |
| Rabeprazole | 207 | 4628 | 0.63 [0.54–0.73] | <0.001 | |
| Rebamipide | 241 | 10611 | 0.42 [0.37–0.48] | <0.001 | |
| Teprenone | 166 | 10692 | 0.36 [0.30–0.42] | <0.001 | |
| Blood coagulation system drugs | Heparin sodium | 760 | 20874 | 0.51 [0.47–0.57] | <0.001 |
| Tranexamic acid | 200 | 8957 | 0.39 [0.34–0.46] | <0.001 | |
| Diuretics | Furosemide | 420 | 3757 | 1.34 [1.18–1.53] | <0.001 |
| Antihypertensive drugs | Amlodipine | 324 | 5635 | 0.74 [0.65–0.85] | <0.001 |
| Expectorants | L-Carbocisteine | 539 | 4413 | 2.16 [1.94–2.40] | <0.001 |
Data are presented as therapeutic class and risk magnitude. Adjusted ORs of DILI for all 60 suspected drugs are shown in Supplementary Table S3. Abbreviations: CI, confidence interval; DILI, drug-induced liver injury; NUSM’s CDW, Nihon University School of Medicine; OR, odds ratio.
There were 40 suspected drugs with ≥100 patients with DILI in JADER. Of these drugs, 23 suspected drugs had ≥100 patients with DILI also in NUSM’s CDW. The association between the 23 suspected drugs and the risk of DILI is shown in Table 4, and that between all 40 suspected drugs and the risk of DILI is shown in Table S6. All 6 antibiotic drugs were significantly associated with an increased risk of DILI: meropenem (adjusted OR, 5.47; 95%CI, 4.79–6.25; p < 0.001), sulbactam/ampicillin (adjusted OR, 4.63; 95%CI, 3.90–5.50; p < 0.001), tazobactam/piperacillin (adjusted OR, 4.30; 95%CI, 3.63–5.09; p < 0.001), ceftriaxone (adjusted OR, 2.89; 95%CI, 2.47–3.39; p < 0.001), levofloxacin (adjusted OR, 2.88; 95%CI, 2.58–3.21; p < 0.001), and cefazolin (adjusted OR, 2.58; 95%CI, 2.12–3.15; p < 0.001). Of the 5 anti-inflammatory drugs, an association with a high risk of DILI was observed with acetaminophen (adjusted OR, 3.13; 95%CI, 2.78–3.52; p < 0.001), methylprednisolone sodium succinate (adjusted OR, 2.04; 95%CI, 1.69–2.47; p < 0.001), loxoprofen (adjusted OR, 1.83; 95%CI, 1.66–2.01; p < 0.001), and diclofenac (adjusted OR, 1.72; 95%CI, 1.48–1.99; p < 0.001). With regard to digestive tract drugs, mosapride (adjusted OR, 2.38; 95%CI, 1.95–2.91; p < 0.001), rebamipide (adjusted OR, 1.91; 95%CI, 1.69–2.15; p < 0.001), teprenone (adjusted OR, 1.85; 95%CI, 1.55–2.21; p < 0.001), and famotidine (adjusted OR, 1.83; 95%CI, 1.65–2.04; p < 0.001) were associated with a high risk of DILI. Among drugs for other diseases, tranexamic acid (adjusted OR, 3.36; 95%CI, 2.75–4.10; p < 0.001), L-carbocisteine (adjusted OR, 2.39; 95%CI, 2.02–2.82; p < 0.001), and furosemide (adjusted OR, 1.53; 95%CI, 1.34–1.74; p < 0.001) were significantly associated with an increased risk of DILI. The differences in patients’ characteristics between users and non-users of all 40 suspected drugs are presented in Supplementary Table S7. The association between the covariates and the risk of DILI in JADER is shown in Supplementary Table S8.
| Class | Drug name | N | Adjusted OR [95%CI] | p Value | |
|---|---|---|---|---|---|
| DILI group | Control group | ||||
| Antibiotic drugs | Meropenem | 255 | 5663 | 5.47 [4.79–6.25] | <0.001 |
| Sulbactam/ampicillin | 149 | 2875 | 4.63 [3.90–5.50] | <0.001 | |
| Tazobactam/piperacillin | 153 | 3293 | 4.30 [3.63–5.09] | <0.001 | |
| Ceftriaxone | 171 | 3818 | 2.89 [2.47–3.39] | <0.001 | |
| Levofloxacin | 362 | 10081 | 2.88 [2.58–3.21] | <0.001 | |
| Cefazolin | 105 | 3496 | 2.58 [2.12–3.15] | <0.001 | |
| Anti-inflammatory drugs | Acetaminophen | 317 | 10950 | 3.13 [2.78–3.52] | <0.001 |
| Methylprednisolone sodium succinate | 115 | 5431 | 2.04 [1.69–2.47] | <0.001 | |
| Loxoprofen | 485 | 24332 | 1.83 [1.66–2.01] | <0.001 | |
| Diclofenac | 191 | 8828 | 1.72 [1.48–1.99] | <0.001 | |
| Prednisolone | 264 | 41556 | 0.40 [0.36–0.46] | <0.001 | |
| Digestive tract drugs | Mosapride | 103 | 5757 | 2.38 [1.95–2.91] | <0.001 |
| Rebamipide | 314 | 18162 | 1.91 [1.69–2.15] | <0.001 | |
| Teprenone | 133 | 8457 | 1.85 [1.55–2.21] | <0.001 | |
| Famotidine | 405 | 22972 | 1.83 [1.65–2.04] | <0.001 | |
| Lansoprazole | 280 | 25765 | 1.05 [0.93–1.20] | 0.413 | |
| Rabeprazole | 109 | 11682 | 0.95 [0.78–1.16] | 0.624 | |
| Magnesium oxide | 172 | 28668 | 0.80 [0.68–0.93] | 0.005 | |
| Blood coagulation system drugs | Tranexamic acid | 106 | 3255 | 3.36 [2.75–4.10] | <0.001 |
| Heparin sodium | 120 | 5630 | 1.15 [0.96–1.39] | 0.137 | |
| Diuretics | Furosemide | 252 | 21755 | 1.53 [1.34–1.74] | <0.001 |
| Antihypertensives | Amlodipine | 107 | 29641 | 0.24 [0.20–0.29] | <0.001 |
| Expectorants | L-Carbocisteine | 154 | 7325 | 2.39 [2.02–2.82] | <0.001 |
Data are presented as therapeutic class and risk magnitude. Adjusted ORs of DILI for all 40 suspected drugs are shown in Supplementary Table S6. Abbreviations: CI, confidence interval; DILI, drug-induced liver injury; JADER, Japanese Adverse Event Report database; OR, odds ratio.
Figure 2 shows a two-dimensional scatter plot of the adjusted OR of DILI and corresponding 95%CI for each of the 23 suspected drugs. The x-axis represents the adjusted OR of DILI calculated from JADER, and the y-axis represents that calculated from NUSM’s CDW. In the present study, drugs with a lower limit of the adjusted OR 95%CI >1.0 in both of the databases were considered to be associated with an increased risk of DILI in clinical settings. Among the 23 suspected drugs, meropenem was most strongly associated with an increased risk of DILI. Additionally, another 3 antibiotic drugs (tazobactam/piperacillin, sulbactam/ampicillin, and ceftriaxone), 2 digestive tract drugs (mosapride and famotidine), L-carbocisteine, methylprednisolone, and furosemide were associated with an increased risk of DILI in clinical settings. In only JADER, there were significant associations between the following 8 suspected drugs and DILI: 3 anti-inflammatory drugs (acetaminophen, diclofenac, and loxoprofen), 2 antibiotic drugs (levofloxacin and cefazolin), 2 digestive tract drugs (rebamipide and teprenone), and tranexamic acid. On the other hand, the upper limit of the adjusted OR 95%CI for amlodipine and magnesium oxide was <1.0.
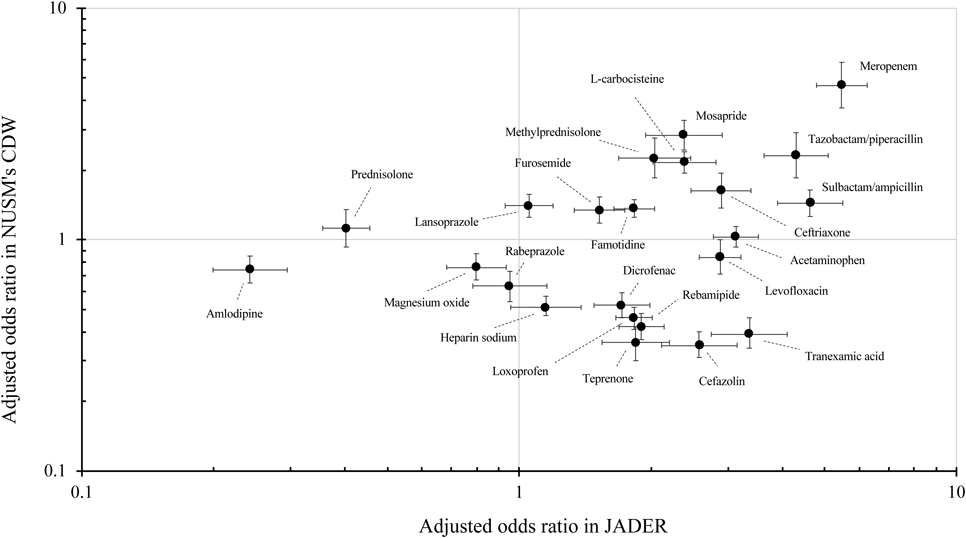
The x and y axes represent odds ratio calculated from JADER and NUSM’s CDW, respectively. Odds ratios were adjusted for age, sex, number of concomitant drugs, and medical history. Error bar represents 95% confidence interval for the odds ratio. A logarithmic scale is used on the x and y axes.
In this study, we investigated drugs associated with the risk of DILI in clinical settings using both a spontaneous reporting system and electronic medical records. Our results showed that patients treated with 23 suspected drugs frequently developed DILI, and that meropenem was strongly associated with the risk of DILI. Adjusted ORs for most of the suspected drugs calculated from JADER tended to be high. The potential for DILI of 1036 drugs was classified into the following four classes based on the hepatotoxic description presented in U.S. Food and Drug Administration (FDA)-approved drug labeling documents and assessment of causality evidence in the literature: Most-, Less-, No-, and Ambiguous-DILI-concern. The list is provided as DILIrank Dataset by the FDA. The potential for DILI of acetaminophen, diclofenac, and levofloxacin is classified into Most-DILI-concern drugs in DILIrank (Table 5). According to the package insert of diclofenac, meaningful elevations of ALT and/or AST occurred in about 4% of patients in a large, open-label, controlled trial of 3700 patients treated with oral diclofenac sodium.14) However, a national survey in Japan performed by the Ministry of Health, Labour and Welfare, Japan showed that there were no cases of diclofenac-induced acute liver failure from 2010 to 2016.15) Furthermore, drugs implicated in causing DILI may differ between Western countries16–18) and East Asian countries such as Japan and China.19,20) While the number of DILI cases caused by antibiotic drugs is large irrespective of region, liver injury caused by non-steroidal anti-inflammatory drugs (NSAIDs) is more common in Western countries than in East Asia. Therefore, the concern about DILI for each drug may be different by region.
| Class | Compound name | Label section | DILI concern |
|---|---|---|---|
| Antibiotic drugs | Meropenem | Warnings and precautions | Less |
| Piperacillin | Warnings and precautions | Ambiguous | |
| Ceftriaxone | Adverse reactions | Less | |
| Ampicillin | Warnings and precautions | Less | |
| Levofloxacin | Warnings and precautions | Most | |
| Cefazolin | Adverse reactions | Less | |
| Anti-inflammatory drugs | Methylprednisolone | Adverse reactions | Less |
| Prednisolone | Adverse reactions | Less | |
| Acetaminophen | Warnings and precautions | Most | |
| Diclofenac | Warnings and precautions | Most | |
| Loxoprofen | NA | NA | |
| Digestive tract drugs | Mosapride | NA | NA |
| Lansoprazole | Adverse reactions | Less | |
| Famotidine | Adverse reactions | Less | |
| Magnesium oxide | NA | NA | |
| Rabeprazole | Adverse reactions | Less | |
| Rebamipide | NA | NA | |
| Teprenone | NA | NA | |
| Blood coagulation system drugs | Heparin sodium | Adverse reactions | Less |
| Tranexamic acid | No match | No | |
| Diuretics | Furosemide | Adverse reactions | Ambiguous |
| Antihypertensive drugs | Amlodipine | Adverse reactions | Less |
| Expectorants | L-Carbocisteine | NA | NA |
DILIrank dataset is available on the Food and Drug Administration website (https://www.fda.gov/). DILI concern consists of three groups (Most, Less, and No) based on confirmed causal evidence, and one additional group (Ambiguous) with undetermined causality. Abbreviations: DILI, drug-induced liver injury; NA, not available.
Four antibiotic drugs (meropenem, tazobactam/piperacillin, sulbactam/ampicillin, and ceftriaxone), which are β-lactam antibiotic drugs, were clinically associated with an increased risk of DILI, and the use of meropenem appeared to have the greater risk of DILI rather than other suspected drugs observed in this study. A high risk of DILI with meropenem has reported in China.21) Furthermore, meropenem, tazobactam/piperacillin, and ceftriaxone are broad-spectrum antibiotics that are used during initial empiric antibiotic therapy to treat suspected bacterial infections.22) According to WHO, antimicrobial resistance has been detected around the world and its spread has been accelerating in recent years.23) It is known that multidrug-resistant strains have higher mortality and morbidity than susceptible strains, and delay the start of effective treatment against infections. For this reason, broad-spectrum antibiotics are overused to mitigate these adverse outcomes.24,25) Therefore, the occurrence of increased drug-resistant strains leads to overuse of these broad-spectrum antibiotics, and may result in an increased number of DILI reports.
The association of glucocorticoids such as prednisolone and methylprednisolone with the risk of DILI was assessed in the present study. Of these glucocorticoids, while methylprednisolone was significantly associated with an increased risk of DILI, prednisolone was not. Corticosteroids are considered to be safe in terms of hepatotoxicity due to their immunosuppressive properties, and are often empirically used to treat idiosyncratic hepatotoxicity with severe features.26) However, it was suggested that methylprednisolone, a synthetic glucocorticoid drug, induces acute liver injury in Japan and Europe.27,28) Furthermore, as DILI occurred in 8.6% of patients with multiple sclerosis who used this drug, methylprednisolone-induced liver injury is not infrequent.29) Therefore, it is important to evaluate the risk of DILI with individual drugs, rather than in each therapeutic class such as anti-inflammatory drugs, antibiotic drugs, and corticosteroids.
Mosapride, a gastroprokinetic agent, enhances gastrointestinal motility by stimulating the 5-hydroxytryptamine 4 receptor. In Japan, there are some case reports of liver injury suspected to be caused by mosapride.30,31) However, to our knowledge, there are no studies that have assessed the risk of DILI with mosapride. L-Carbocisteine, a widely used expectorant, is used as an OTC drug as well as a prescription drug in Japan. As with mosapride, there are no studies on the association between L-carbocisteine and the risk of DILI. However, as point estimates of the adjusted ORs for the two drugs were more than 2.0 in both of the databases,32) it was suggested that attention should be paid to mosapride- and L-carbocisteine-related liver injury in clinical settings.
Famotidine is an antacid drug that neutralizes acid in the stomach and is used as an OTC drug. Thus, famotidine-induced liver injury may occur in not only patients who take this as a prescription drug, but also patients who self-medicate to treat self-recognized conditions or symptoms. However, there are few reports about the association between famotidine and the risk of DILI. Furosemide, a loop diuretic, has been used as a hepatotoxicant in animal experiments.33,34) While it is also reported that furosemide showed toxicity in a human liver cell line,35) there are few clinical studies that have investigated the association between this drug and the risk of hepatotoxicity.36) From our results, although the magnitude of the adjusted ORs of DILI for famotidine and furosemide was small, the association of these drugs with the risk of DILI was statistically significant. Therefore, it is necessary to be vigilant about the development of DILI during use of these drugs.
Inconsistency in Odd Ratios Calculated from NUSM’s CDW and JADERThere were 8 suspected drugs that were significantly associated with increased risk of DILI in JADER, but not in NUSM’s CDW: 3 anti-inflammatory drugs (acetaminophen, diclofenac, and loxoprofen), 2 antibiotic drugs (cefazolin and levofloxacin), tranexamic acid, rebamipide, and teprenone. It is reported that the number of DILI cases caused by anti-inflammatory drugs and antibiotic drugs was higher than that caused by drugs in other therapeutic classes in the U.S.A.6) However, there is reporting bias such as notoriety bias and a tendency for serious events to be more likely to be reported than non-serious events in SRSs.37–39) Therefore, the risk of adverse drug events tends to be overestimated due to the existence of bias.40) In fact, for most of the suspected drugs, point estimates of the adjusted ORs on the x-axis (JADER-axis) were greater than 1.0. Furthermore, the lower limits of the adjusted OR 95%CI for drugs well known to be associated with DILI, such as NSAIDs and antibiotic drugs, were greater than 1.0, whereas the adjusted ORs on the y-axis for these well-known drugs were widely distributed. It is reported that although the number of DILI cases in patients who use anti-inflammatory drugs is high, the incidence of DILI is lower than that with other hepatotoxic drugs.41,42) In addition, the risk of acute liver injury in patients who used NSAIDs was the same as that in control patients.43) Thus, the adjusted ORs of DILI calculated from JADER for individual drugs, especially those well known to be associated with liver injury, may be overestimated due to the existence of reporting bias.
It is known that although administration of unfractionated heparin sodium to healthy volunteers is associated with elevation of serum aminotransferases, hepatotoxicity of this drug is low-grade.44) Furthermore, some studies suggested that heparin sodium mitigates liver injury in rodent models.45) With regard to tranexamic acid, rebamipide, and teprenone, to our knowledge, there are no clinical studies on the association between these drugs and the risk of DILI. However, all these drugs have a potential curative effect on liver injury in experimental animal models.46–48) Thus, the association of these 4 drugs with an increased risk of DILI may be weak in clinical settings.
The adjusted ORs of DILI for cefazolin and levofloxacin were lower than those for other antibiotic drugs in this study. There are several case reports of cefazolin-induced liver injury.49,50) In addition, it is also reported that the number of DILI cases attributed to cefazolin is higher than that with other cephalosporins.51,52) However, there are few studies with comparison between patients with liver injury who used cefazolin and patients without liver injury who used this drug (i.e., risk evaluation). Levofloxacin, a fluoroquinolone drug, was shown to augment the risk of DILI related to other antibiotic drugs in Canada.53) Furthermore, levofloxacin showed the highest relative risk among other antibiotic drugs that were not evaluated in this study.54) However, in Japan, it is reported that the incidence of DILI for levofloxacin itself was very low in clinical trials.55) Therefore, the association of the two drugs with an increased risk of DILI may be relatively lower than that of other antibiotic drugs that were evaluated in this study. The above-mentioned findings suggest that the adjusted ORs of DILI were overestimated in the JADER database, and some drugs have an effect to mitigate liver injury in animal experiments. Therefore, although the number of DILI reports for these 8 suspected drugs was ≥100, the association between these suspected drugs and an increased risk of DILI may be relatively low in clinical settings.
The present study has some limitations. Firstly, this study was a case-control study with potential for selection bias and confounding factors. This study controlled potential confounding factors that were available and measurable, but failed to adjust for nonobserved risk factors. Secondly, this study did not perform causality assessment. Because information on other host-dependent risk factors (e.g., alcohol consumption) is not available in both the NUSM’s CDW and JADER database, it was not possible to use well-known causality scales such as the Roussel Uclaf Causality Assessment Method in this study.56,57) Thirdly, in NUSM’s CDW, patients in whom transaminases reached >3 × ULN were regarded as DILI patients. However, these DILI patients have not been definitively diagnosed by hepatologists. Therefore, these limitations may lead to incorrect detection of potential hepatotoxic drugs.
In the present study, the associations of individual drugs with an increased risk of DILI were evaluated using both an adverse event reporting system and electronic medical records. The use of broad-spectrum antibiotic drugs tended to have a greater risk of DILI than other suspected drugs; especially, the use of meropenem was associated with a high risk of DILI. Additionally, a small but new signal of mosapride- and L-carbocisteine-related liver injury was detected in this study.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.