2021 年 44 巻 10 号 p. 1530-1535
2021 年 44 巻 10 号 p. 1530-1535
We investigated the vascular response to nucleobase adenine using freshly isolated superior mesenteric arteries of spontaneously hypertensive rats (SHR) and its control, Wistar Kyoto (WKY) rats. Endothelium-dependent and endothelium-independent relaxations were assessed in isolated segments in an organ bath. The releases of the metabolites of thromboxane A2 and prostaglandin I2 were also detected. Adenine induced vasorelaxation in both the endothelium-intact and endothelium-denuded arteries in a concentration-dependent manner. In the SHR group, the adenine-induced relaxation was slightly but significantly reduced in the endothelium-intact rings when compared with that in the WKY group. However, the relaxation in the endothelium-denuded rings were similar between the two groups. The difference in the adenine-mediated relaxation in the superior mesenteric arteries between the SHR and WKY groups was eliminated by endothelial denudation and a nitric oxide (NO) synthase inhibitor. In the absence and presence of adenine, SHR tended to have higher levels of metabolites of thromboxane A2 and prostaglandin I2 compared with WKY. However, adenine did not induce the release of these substances in the arteries in both the SHR and WKY groups. These results suggest that the reduced adenine-mediated relaxation in the superior mesenteric arteries in SHR is due to a lack of contribution from the endothelium-derived NO and not from the release of prostanoids.
Adenine-containing extracellular nucleotides, such as ATP and ADP, are well-known to possess various biological properties via the activation of purinergic P2 receptors in various tissues and cells, including the blood vessels.1,2) Extracellular ATP and ADP are hydrolyzed by ecto-nucleotides to AMP, which is then converted by ecto-5′-nucleotidase to adenosine.1,3) Extracellular adenosine induces biological functions through the purinergic P1 receptors, which are considered as potential therapeutic targets in many diseases, including hypertension.1,2) In mammals, extracellular adenosine is converted to inosine, but not adenine, by adenosine deaminase. Therefore, the nucleobase adenine is not generated by the extracellular degradation of purine nucleotides but through the degradation of nucleic acids within the cells, which is a part of the uric acid pathway. This involves the sequential degradation of AMP to adenosine, adenine, hypoxanthine, xanthine, and finally to uric acid.4) Since the relationship between uric acid and hypertension is well established,4) it is important to understand the role of adenine in hypertension.
The amount of evidence that suggests adenine can induce various physiological properties is growing. For example, adenine has anti-inflammatory properties,5) proalgesic effects,6) and modulative effects on diuresis.7) In addition, adenine causes vasorelaxation in isolated rat aorta.8,9) It was reported that the adenine receptors, which are characterized as G protein-coupled receptors, are expressed in various organs including the kidneys, liver, brain, lungs, and arteries.7,10–12) In the vascular system, adenine could induce the relaxation in the aorta of a rat in both endothelium-independent and adenosine receptor-independent mechanisms.8) Moreover, adenine-induced vasorelaxation was mediated by the suppression of the calcium-contraction signaling pathway through adenine receptor/protein kinase A activation in the aortic vascular smooth muscle cells.9) However, little attention has been given to adenine-mediated vasomotion in hypertension. Therefore, this study examined the vasorelaxant effects of adenine in the superior mesenteric arteries obtained from spontaneously hypertensive rats (SHR), a representative hypertensive animal model,13–15) and its control, Wistar Kyoto (WKY) rats.
Four-week-old male rats (WKY and SHR) were obtained from Hoshino Laboratory Animals, Inc. (Ibaraki, Japan) and allowed to age. Rats aged 7 to 10 months were used in the study. SHR had higher blood pressures (189 ± 3 mmHg, n = 24, p < 0.001) compared to WKY (124 ± 2 mmHg, n = 24), as confirmed by tail-cuff methods14) before the experiments. The body weights were significantly lower in SHR (386.2 ± 8.2 g, n = 24, p < 0.05) than in WKY (413.0 ± 7.7 g, n = 24). All experiments were conducted in accordance with the Guiding Principles for the Care and Use of Laboratory Animals from the Committee for the Care and Use of Laboratory Animals of Hoshi University (Tokyo, Japan) and approved by the committee (Permission Code: 19-076).
The concentration–relaxation responses of the vascular function in the superior mesenteric arteries were determined by measuring the isometric force, as previously reported.14,16) The concentration–response curves for adenine (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) (10−8–10−3 M) in the superior mesenteric arteries precontracted with phenylephrine (PE) (Sigma Chemical Co., St. Louis, MO, U.S.A.) (0.5–9 µM) were calculated in endothelium-intact and endothelium-denuded conditions as well as under inhibition of nitric oxide (NO) synthase (NOS) by Nω-nitro-L-arginine (L-NNA) (Sigma Chemical Co.) (10−4 M), cyclooxygenase (COX) inhibition by indomethacin (Sigma Chemical Co.) (10−5 M), NOS and COX inhibition by L-NNA (10−4 M) and indomethacin (10−5 M), and inhibition of COX, small-conductance calcium-activated potassium channel (SKCa), and intermediate-conductance calcium-activated potassium channel (IKCa) by indomethacin (10−5 M), apamin (PEPTIDE Institute Inc., Osaka, Japan) (10−7 M), and TRAM-34 (Sigma Chemical Co.) (10−5 M), respectively.
Measurement of Thromboxane A2 and Prostaglandin I2 ReleaseThe release of thromboxane A2 and prostaglandin I2 by the superior mesenteric arteries of SHR and WKY rat was detected as their metabolites thromboxane B2 and 6-keto prostaglandin F1α, respectively, by using an enzyme immunoassay (Cayman Chemical, Ann Arbor, MI, U.S.A.), as previously reported.16) The arterial rings (approximately 5 mm long) were incubated in a modified 1.0 mL Krebs–Henseleit solution (KHS; composition in mmol/L: NaCl, 118.0; KCl, 4.7; NaHCO3, 25.0; CaCl2, 1.8; NaH2PO4, 1.2; MgSO4, 1.2; and glucose, 11.0) at 37 °C for 30 min. The rings were then rapidly transferred to KHS containing phenylephrine (10−6 M) at 37 °C for 10 min. The rings were then divided into two groups, with one group treated with adenine (10−4 M) at 37 °C for 15 min and the other group left untreated. After the rings were removed and weighed, the tubes containing the released substances were flash-frozen in liquid nitrogen and stored at −80 °C for later analysis. the levels of the released substances were presented as pg/mg or ng/mg wet weight of tissue.
Data AnalysisData were expressed as mean ± standard error of the mean (S.E.M.), with n representing the number of animals used in the experiments. For the determination of pD2, the individual concentration–response curves were fitted using a non-linear regression-fitting program with a standard slope with GraphPad Prism ver. 8.0 (GraphPad Software Inc., San Diego, CA, U.S.A.). Significant differences were calculated using Student’s t-test for comparisons between two groups. One-way ANOVA followed by Tukey’s testing were used for comparisons among three or more groups. In the concentration–response curves, the differences between values were evaluated using a two-way repeated measures of ANOVA followed by Sidak’s multiple comparison test. p Values <0.05 were considered significant.
Whether the relaxation induced by adenine in the superior mesenteric arteries obtained from WKY rats and SHR rats that had contracted in response to PE (0.5–3 µM) would be altered was first determined (Figs. 1A–C). The tension developed in response to phenylephrine was similar between the endothelium-intact superior mesenteric arteries from SHR and those from WKY rats (Supplementary Table S1). As shown in Figs. 1A and B, adenine-induced relaxation in a concentration-dependent manner. In the endothelium-intact superior mesenteric arteries from SHR rats, the relaxation induced by adenine was slightly but significantly reduced compared with those from WKY rats (Fig. 1C, Table 1).

(A, B) The representative trace of adenine-mediated relaxation in endothelium-intact superior mesenteric arteries of WKY (A) and SHR (B). (C–E) The relaxation induced by adenine in endothelium-intact (C), endothelium-denuded (D), and in the presence of nitric oxide synthase inhibitor 10−4 M Nω-nitro-L-arginine (L-NNA). The relaxations were evaluated as a percentage of precontraction induced by phenylephrine. n number is shown in parentheses. * p < 0.05, SHR vs. WKY.
| Emax | pD2 | |||
|---|---|---|---|---|
| WKY | SHR | WKY | SHR | |
| Fig. 1C | 99.8 ± 0.2 (8) | 94.7 ± 1.6 (7)* | 3.53 ± 0.08 (8) | 3.25 ± 0.08 (7)* |
| Fig. 1D | 97.9 ± 1.1 (5) | 96.6 ± 2.5 (6) | 3.00 ± 0.08 (5) | 3.00 ± 0.10 (6) |
| Fig. 1E | 97.3 ± 1.0 (4) | 91.5 ± 2.9 (4) | NC | NC |
| Fig. 3A | 96.0 ± 2.3 (4) | 89.7 ± 2.4 (4) | 3.19 ± 0.10 (4) | NC |
| Fig. 3B | 98.3 ± 1.7 (4) | 97.6 ± 1.2 (4) | 3.15 ± 0.13 (4) | 3.00 ± 0.14 (4) |
| Fig. 3C | 96.4 ± 3.3 (4) | 95.5 ± 2.4 (4) | 3.53 ± 0.13 (4) | 3.15 ± 0.02 (4)* |
| Fig. 3D | 96.2 ± 3.7 (4) | 98.0 ± 2.0 (4) | 3.54 ± 0.12 (4) | 3.54 ± 0.17 (4) |
Values are expressed as means ± S.E. The number of experiments is shown in parentheses. Emax = maximal adenine-induced relaxation expressed as % of PE-induced contraction; pD2=−log (adenine) required to produce 50% of the maximal relaxation; NC, not calculated. * p < 0.05 vs. WKY.
Next, the contribution of the endothelium in the reduced adenine-mediated relaxation in the arteries of SHR was then investigated. In the endothelium-removed superior mesenteric arteries, the contractile forces induced by PE were similar between the SHR and WKY groups (Supplementary Table S1). Under the endothelium-denuded condition, the relaxation induced by adenine was similar between the SHR and WKY groups, and the difference in the adenine-mediated relaxation between the SHR and WKY groups was completely eliminated (Fig. 1D, Table 1).
To investigate whether the contribution of NO in adenine-mediated relaxation would be altered in hypertensive arteries, the concentration–response curves for adenine in the presence of a representative NO synthase inhibitor, L-NNA, which could attenuate endothelium-dependent relaxation in the superior mesenteric artery, have been calculated next.17,18) In the L-NNA-treated superior mesenteric arteries, the contractile forces induced by PE were similar between the SHR and WKY groups (Supplementary Table S1). The relaxation induced by adenine in the presence of L-NNA was similar between the SHR and WKY groups (Fig. 1E, Table 1). Under the NO synthase-inhibited condition, the difference of adenine-mediated relaxation between the SHR and WKY groups was completely eliminated (Fig. 1E, Table 1).
Prostanoid ReleaseProstanoids play an important role in controlling the vascular tone; however, in hypertensive arteries, their signals are altered.19,20) Therefore, we investigated whether the release of thromboxane A2 and prostaglandin I2 would be caused by adenine and if their amounts would be altered in the superior mesenteric arteries of SHR and WKY. As shown in Fig. 2, adenine did not cause the release of thromboxane A2 (measured as its metabolite thromboxane B2) nor of prostaglandin I2 (measured as its metabolite 6-keto prostaglandin F1α) in the superior mesenteric arteries of SHR and WKY, although the released metabolites were higher in the SHR arteries compared with the WKY arteries.

The released substances were determined to be their metabolites, thromboxane B2 (A), and 6-keto-prostaglandin F1α (B), respectively. Data are shown as means ± standard error of the mean (S.E.M.); n = 8.
Three major factors including NO, prostaglandin I2, and endothelium-derived hyperpolarizing factor (EDHF) are known.19) Besides NOS inhibition as shown in Fig. 1E, adenine-induced relaxation under various conditions in the superior mesenteric arteries of SHR and WKY rats at 10 months old has been investigated. In an endothelium-intact condition, the adenine-induced relaxation was impaired in the superior mesenteric arteries of SHR at 10 months old than in those from age-matched WKY rats (Fig. 3A, Table 1). At the preservation of EDHF signaling by inhibitions of NOS and COX, the adenine-induced relaxation was similar between the WKY and SHR groups (Fig. 3B, Table 1). Conversely, at the preservation of NO signaling21) by inhibitions of COX and SKCa/IKCa, as a source of EDHF,19) the impaired adenine-induced relaxation was seen in the superior mesenteric arteries of SHR (vs. WKY) (Fig. 3C, Table 1). Under COX inhibition, the adenine-induced relaxation was tended to increase in the SHR artery (vs. intact preparation shown in Fig. 3A), and the relaxant responses in the presence of indomethacin were similar between the SHR and WKY groups (Fig. 3D, Table 1).
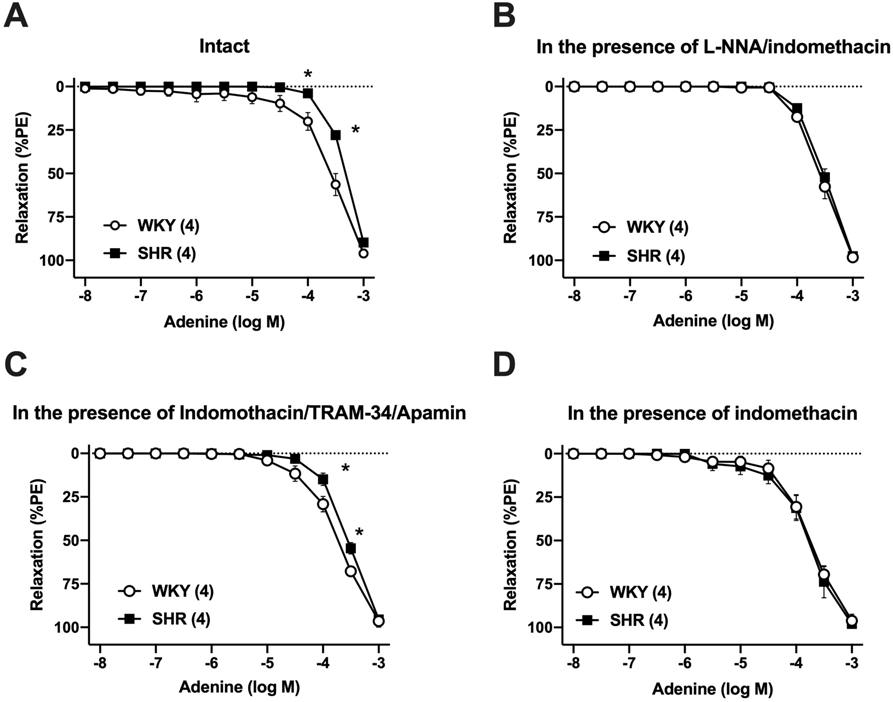
The relaxation induced by adenine in endothelium-intact (A), in the presence of (B) nitric oxide synthase inhibitor 10−4 M Nω-nitro-L-arginine (L-NNA) and cyclooxygenase inhibitor 10−5 M indomethacin, (C) 10−5 M indomethacin, 10−5 M TRAM-34, and 10−7 M apamin, and (D) 10−5 M indomethacin. The relaxations were evaluated as a percentage of precontraction induced by phenylephrine. n number is shown in parentheses. * p < 0.05, SHR vs. WKY.
The major findings of this study are that adenine induces vasorelaxation in rat superior mesenteric arteries, and such relaxation is reduced in the artery of SHR (vs. WKY). Thus far, few studies have investigated the vasorelaxation response induced by adenine using isolated rat aorta.8,9) To the best of our knowledge, this study is the first to demonstrate that adenine could induce relaxation in the superior mesenteric arteries of rats, and such responses are reduced in hypertensive arteries.
Endothelial and vascular smooth muscle functions play an important role in vascular tone, whereas their functions are impaired with hypertension.15,19) In this study, under the endothelium-denuded condition, the difference in the adenine-mediated relaxation between the SHR and WKY groups was completely eliminated. These results suggest that the impaired adenine-mediated relaxation was due to the reduction of endothelium-derived relaxant components, and adenine-mediated endothelium-independent relaxation (viz. smooth muscle relaxant signaling activated by adenine) was not impaired in the hypertensive arteries. This is supported by evidence suggesting that endothelium-dependent relaxations induced by various endothelial stimulators were impaired in the superior mesenteric arteries of SHR.13–15)
The endothelium-derived factors, including endothelium-derived relaxing factors and contracting factors were reported to be altered in hypertensive arteries.19,20) To investigate whether the contribution of NO in adenine-mediated relaxation would be altered in hypertensive arteries, the concentration–response curves have been calculated for adenine in the presence of a representative NO synthase inhibitor, L-NNA, which could attenuate endothelium-dependent relaxation in the superior mesenteric artery.17,18) The relaxation induced by adenine in the presence of L-NNA was similar between the SHR and WKY groups, and under the NO synthase-inhibited condition, the difference of adenine-mediated relaxation between the SHR and WKY groups was completely eliminated. EDHF is one of the endothelium-derived relaxing factors (EDRFs) in rat superior mesenteric artery and EDHF-mediated responses were impaired in the artery of SHR.15) As shown in Fig. 3B, in the situation of preservation of EDHF (i.e., NOS and COX inhibitions), adenine-induced relaxation was similar between SHR and WKY. Conversely, in the situation of preservation of NO [i.e., COX inhibitions and source of EDHF (SKCa/IKCa inhibitions)22)], the relaxation induced by adenine was reduced in the superior mesenteric arteries of SHR than in those of WKY rats. These results suggest that the reduction of endothelium-derived NO signaling rather than EDHF may underlie the mechanisms that reduce the adenine-mediated relaxation in the superior mesenteric arteries of SHR rats. Currently, there is no report suggesting that adenine could generate NO in vessels. Future investigation will be required on the production of NO and endothelial nitric oxide synthase (eNOS) activation by adenine in hypertensive arteries.
Prostanoids play an important role in controlling the vascular tone; however, in hypertensive arteries, their signals are altered.19,20) Therefore, we investigated whether the release of thromboxane A2 and prostaglandin I2 would be caused by adenine and if their amounts would be altered in the superior mesenteric arteries of SHR and WKY. As shown in Fig. 2, adenine did not cause the release of thromboxane A2 (measured as its metabolite thromboxane B2) nor of prostaglandin I2 (measured as its metabolite 6-keto prostaglandin F1α) in the superior mesenteric arteries of SHR and WKY, although the released metabolites were higher in the SHR arteries compared with the WKY arteries. These results suggest that adenine is not able to induce the production of thromboxane A2 and prostaglandin I2 in the superior mesenteric arteries of rats. Since the production of these prostanoids tended to increase at baseline in the SHR arteries and indomethacin treatment promoted a slight increase in adenine-induced relaxation in the SHR arteries and abolished the difference in adenine-induced relaxation between SHR and WKY rats (Fig. 3D, Table 1), they may have partly contributed to the reduced adenine-mediated relaxation in the SHR arteries. This notion is supported by several reports suggested that the overproduction of cyclooxygenase-derived prostanoids including thromboxane A2 (a potent constrictor) and prostaglandin I2 (exert as a constrictor via activated thromboxane-prostanoid receptor)19,20) was attributable to the impaired endothelium-dependent relaxation in some hypertensive arteries, and inhibitors of cyclooxygenase are able to normalize endothelium-dependent relaxation in SHR arteries.14,19,23,24)
In a previous study, Fukuda et al.9) have observed that the adenine-induced relaxation in the aorta was due to calcium signaling suppression in vascular smooth muscle cells through the adenine receptor-mediated protein kinase A activation. In the present study, we observed that the adenine-mediated relaxation was observed to be partly reduced by endothelial denudation in the superior mesenteric arteries in the WKY group. This difference in the adenine-induced relaxation between the aorta and the superior mesenteric artery may be dependent on the vessel type. Further investigations using the arteries obtained from different regions are required to elucidate this phenomenon.
This study had several limitations. Although a report has suggested that adenine receptors are present in the vascular smooth muscle,9) there is little research regarding the expression of adenine receptors in endothelial cells. The expressions of adenine receptors in the endothelial and vascular smooth muscle cells in the arteries of SHR and WKY rats remain unclear. Additionally, the extent of endothelial cell activation in the superior mesenteric arteries of SHR and WKY rats that is induced by adenine, NO, and/or endothelium-dependent hyperpolarization remains unclear. In our previous unpublished observation, sodium nitroprusside-, a donor of NO, induced relaxation was similar in the superior mesenteric arteries between SHR and WKY rats at 7 months old. These data suggest that impaired adenine-induced relaxation may be due to the reduction of NO bioavailability (viz. decreased production or disruption of NO) rather than impaired NO sensitivity in the superior mesenteric arteries of SHR. Further investigations will be required to determine these points.
In summary, we have revealed that adenine induces relaxation in the superior mesenteric arteries of rats, but this is impaired in the arteries of SHR, which may be due to the reduction of NO signaling. Since the understanding of adenine as a signaling molecule is important in gaining insight into its pathophysiological roles in the vascular system, we believe that our observations should stimulate further interest in adenine as a signaling molecule in the vascular system.
We would like to thank Yuna Tanaka, Keisuke Ozawa, Shiho Kakihana, Aiko Yamada, Sena Nagai, Mai Kato, Yuta Sato, Suzuha Shinya, and Rui Shimoyama for the excellent technical assistance. This work was supported in part by grants JSPS KAKENHI Grant Numbers JP21K06811 (to Kumiko Taguchi) and JP21K06878 (to Tsuneo Kobayashi).
Takayuki Matsumoto designed research. Takayuki Matsumoto, Keisuke Takayanagi, Tomoki Katome, Mihoka Kojima, and Kumiko Taguchi conducted experiments and analyzed data. Takayuki Matsumoto and Tsuneo Kobayashi wrote the manuscript. All authors have read and approved the manuscript.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.