2021 Volume 44 Issue 11 Pages 1670-1680
2021 Volume 44 Issue 11 Pages 1670-1680
Bisphosphonates (BPs) are major anti-bone-resorptive drugs. Among them, the nitrogen-containing BPs (NBPs) exhibit much stronger anti-bone-resorptive activities than non-nitrogen-containing BPs (non-NBPs). However, BP-related osteonecrosis of the jaw (BRONJ) has been increasing without effective strategies for its prevention or treatment. The release of NBPs (but not non-NBPs) from NBP-accumulated jawbones has been supposed to cause BRONJ, even though non-NBPs (such as etidronate (Eti) and clodronate (Clo)) are given at very high doses because of their low anti-bone-resorptive activities. Our murine experiments have demonstrated that NBPs cause inflammation/necrosis at the injection site, and that Eti and Clo can reduce or prevent the inflammatory/necrotic effects of NBPs by inhibiting their entry into soft-tissue cells. In addition, our preliminary clinical studies suggest that Eti may be useful for treating BRONJ. Notably, Eti, when administered together with an NBP, reduces the latter’s anti-bone-resorptive effect. Here, on the basis of the above background, we examined and compared in vitro interactions of NBPs, non-NBPs, and related substances with hydroxyapatite (HA), and obtained the following results. (i) NBPs bind rapidly to HA under pH-neutral conditions. (ii) At high concentrations, Eti and Clo inhibit NBP-binding to HA and rapidly expel HA-bound NBPs (potency Eti>>Clo). (iii) Pyrophosphate also inhibits NBP-binding to HA and expels HA-bound NBPs. Based on these results and those reported previously, we discuss (i) possible anti-BRONJ strategies involving the use of Eti and/or Clo to reduce jawbone-accumulated NBPs, and (ii) a possible involvement of pyrophosphate-mediated release of NBPs as a cause of BRONJ.
Bisphosphonates (BPs) are important anti-bone-resorptive drugs.1–3) BPs bind strongly to bone, especially to bones exhibiting rapid turnover or inflammation. Due to their high affinity for the hydroxyapatite (HA) of bones, BPs accumulate within bones upon repeated administration. Among the BPs, the nitrogen-containing BPs (NBPs) have anti-bone-resorptive activities that are far stronger than those of the non-nitrogen-containing BPs (non-NBPs)2,4) (Supplementary Fig. 1). However, NBPs are known to have inflammatory/necrotic side effects after intravenous administration, as well as various gastrointestinal side effects after oral administration. In particular, since the first reports in 2003,5,6) NBP-related osteonecrosis of the jaw (BRONJ) has been increasing without effective clinical strategies.7) In Japan, 263 BRONJ patients were reported in the period 2006–2008, but as many as 4797 during 2011–2013.8)
Only two non-NBPs (etidronate (Eti) and clodronate (Clo)) are currently used for osteoporosis. To compensate for their weak anti-bone-resorptive activities, they are given to patients at doses much higher than those of NBPs.1,9) Unlike NBPs, Eti and Clo have no severe inflammatory/necrotic side effects. It is notable that Clo is a BP that can be given intramuscularly as well as orally and intravenously.10)
A single injection of a BP into young mice produces a clear sclerotic band in their tibias (we call this the “BP-band”) that is detectable by radiography a few weeks after the injection, reflecting an inhibition of bone resorption. By examining the BP-band, we previously obtained the following results. Eti, when administered to mice together with an NBP reduces the anti-bone-resorptive effect of the NBP,11) while such an effect of Clo, if any, is minimal.12) Eti, when administered after an NBP, also reduces the anti-bone-resorptive effect of the NBP.13) Before obtaining those findings, we had reported that Clo can inhibit the inflammatory/necrotic effects of NBPs in mice.14) Our later pharmacological studies indicated that Eti and Clo inhibit the entry of NBPs into soft-tissue cells via inhibition of phosphate transporters.15–17) Taken together, these results suggest that (i) Eti inhibits the binding of an NBP when the two are co-administered, (ii) Eti can expel NBPs that have already accumulated within bones, and (c) Eti may inhibit the entry of the expelled NBPs into soft-tissue cells around the bones, thus preventing the inflammatory/necrotic effects of the expelled NBPs and reducing the anti-bone-resorptive activities of NBPs. Our clinical trials to examine the use of Eti as a substitution drug for NBPs suggest that Eti may be useful for reducing the signs of BRONJ.18,19) Here, based on the background described above, we examined and compared the in vitro effects of Eti and Clo on the binding of NBPs to HA and on the release of HA-bound NBPs. We focused particularly on the latter because the NBPs released from NBP-accumulated jawbones may play critical roles in the development of BRONJ.
Minodronate-H2O (Min) and pamidronate-2Na·anhydrous (Pam) were from LKT Laboratories, Inc. (St. Paul, MN, U.S.A.). 2,4,6-Trinitrobenzene sulfonic acid hydrate (TNBS), alendronate-Na·3H2O (Ale), zoledronate-2Na·4H2O (Zol), risedronate-Na·2.5H2O (Ris), and etidronate-2Na·anhydrous (Eti) were purchased from Tokyo Kasei (Tokyo, Japan). Phosphate-Na·2H2O (Pi), dimethylsulfoxide (DMSO), CaCl2·2H2O, and MgCl2·6H2O were from Nacalai Tesque (Kyoto, Japan). Clodronate-2Na·anhydrous (Clo) and hydroxyapatite (HA) (#677418: particle size ≤200 nm, specific surface area ≥9.4 m2/g) were from Sigma-Aldrich (St. Louis, MO, U.S.A.). Pyrophosphate-4Na·anhydrous (PPi) and phosphonoformic acid (PFA) were from Alfa Aesar (Tewksbury, MA, U.S.A.). Phosphonoacetic acid (PAA) was from Wako (Osaka, Japan).
Preparation of BP SolutionsBPs were dissolved in distilled water. Unless otherwise indicated, solutions were adjusted to pH 7 with NaOH solution. In some experiments, solutions were adjusted to the indicated pH values with NaOH or HCl solutions. Then, the concentration of the BP solutions was adjusted to that required with distilled water.
Preparation of HAHA was suspended in distilled water, with the pH of the suspension being adjusted to 7 with NaOH solution or, in some experiments, adjusted to the indicated pH values with NaOH or HCl solutions. Then, the concentration of the HA suspensions was adjusted to 100 mg/mL with distilled water and used as indicated. The required amount of the HA suspension (100 µL; i.e., 10 mg HA, in most experiments) was transferred to a test tube. Then, the tube was centrifuged at 10000 × g for 5 min, and the supernatant was removed. The resultant precipitate was used as HA.
Binding of NBPs to HATo a tube containing HA, 100 µL solution containing an NBP alone or NBP plus a test material(s) was added, and the contents of the tube mixed by pipetting. Then, the tube was left standing at room temperature for approx. 60 min before being centrifuged at 10000 × g for 5 min. The resultant supernatant was subjected to measurement of the amount of the NBP. The amount of the NBP bound to the HA was expressed as a percentage of the NBP added.
Release of an NBP from NBP-Bound HANBP-bound HA (abbreviated “NBP-HA”) was prepared by mixing 10 mg HA and 100 µL NBP (concentration indicated) and removal of the supernatant following centrifugation. To an NBP-HA, a solution of a test material(s) (100 µL) was added, and the contents of the tube were mixed by pipetting. Then, the tube was left standing at room temperature for approx. 60 min before being centrifuged at 10000 × g for 5 min. The resultant supernatant was subjected to measurement of the amount of the NBP, and the amount of the NBP released from the NBP-HA was calculated and expressed as a percentage of the NBP bound to HA.
Measurement of Pam and AlePam and Ale were measured spectrophotometrically (absorbance at 450 nm) after reaction of their amino group with 2,4,6-trinitrobenzene sulfonate (TNBS).20) To each well of a 96-well plate, 200 µL borate buffer (0.2 M, pH 8.5), 100 µL TNBS solution (1 mg/mL DMSO), and 10 µL of the solution of Pam or Ale were added. After completing the TNBS reaction for 30 min at 37 °C, the absorbance of the reaction mixture at 450 nm was measured.
Measurement of Zol, Ris, and MinZol, Ris, and Min were measured spectrophotometrically by measuring their native UV absorbance (at 220 nm for Zol and Ris and at 290 nm for Min). However, in the presence of a large amount of Eti and/or Clo, Zol and Ris could not be measured because Eti and Clo, at high concentrations, also exhibit UV absorption at around 220 nm and therefore disturb the measurement of Zol and Ris (e.g., the absorbances at 220 nm of 4 mM Zol, 4 mM Ris, 150 mM Eti, and 150 mM Clo are 1.75, 1.9, 0.5, and 1.5, respectively). Fortunately, Min could be measured even in the presence of a large amount of Eti and/or Clo. The UV absorbance of a given solution was measured by using a UV-star 96-well plate (Greiner, Frickenhausen, Germany) and a FlexStation 3 microplate reader (Molecular Devices, CA, U.S.A.).
Statistical AnalysisExperimental values are given as the mean ± standard deviation (S.D.) from 4 tubes. Sample size was based on an α error of 0.05 and a β error of 0.2 using power analysis. The statistical significance of differences was analyzed using a Bonferroni multiple comparison test after ANOVA with the aid of InStat software (InStat, Scottsdale, AZ, U.S.A.). The results shown for a given experiment were confirmed by repeating the experiment at least once more.
Figure 1A shows that in spite of structural differences, with each NBP almost 100% of 100 µL of a 4 mM solution of the NBP binds to about 10 mg HA. This means that (i) the 10 mg HA used in the present study is almost completely saturated with 400 nmols of an NBP, and thus (ii) when 10 mg HA was mixed with 100 µL of 4 mM of an NBP solution, almost no free NBP (i.e., not attached to HA) is present in the mixture. Figure 1B shows that most of each NBP became bound to HA during the mixing (pipetting), although Min and Zol take a somewhat longer time than Ale and Pam for complete binding. Figure 2A shows that at pH values from 3-11, all the tested NBPs bind almost completely to HA within 60 min, indicating that NBPs exhibit potent affinities for HA over a wide pH range. Figure 2B shows that the non-NBPs Eti and Clo each concentration-dependently (16–128 mM) reduce NBP-binding to HA for all of the N-BPs tested, indicating that NBPs and non-NBPs bind competitively to HA. In this experiment, Zol was not tested because Eti and Clo disturb the measurement of Zol (see Materials and Methods).
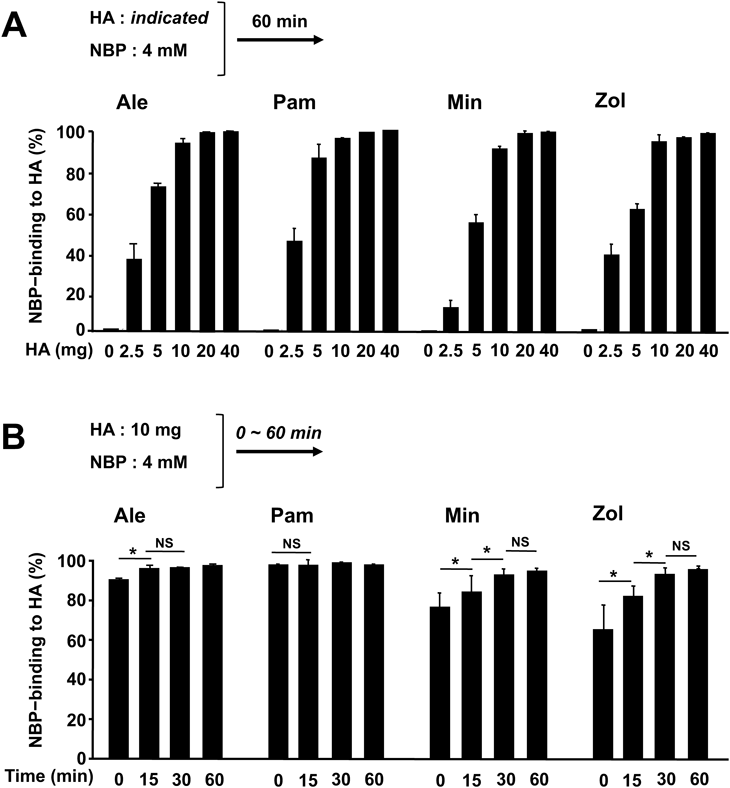
(a) NBP-binding capacity of HA. An indicated amount of HA and 4 mM of a given NBP (100 µL) were mixed by pipetting, and the mixture was left standing for 60 min and then centrifuged. Then, the NBP in the resultant supernatant was measured. (b) NBP-binding velocity to HA. HA 10 mg and 4 mM of a given N-BP (100 µL) were mixed by pipetting, and the mixture was left standing for the indicated time before being centrifuged. Finally, the NBP in the resultant supernatant was measured. Each value is the mean ± S.D. from 4 tubes. * p < 0.05 vs. indicated group.

(a) Effects of pH on the binding of NBPs to HA. HA 10 mg and 4 mM of a given NBP (100 µL) (pH of each having been adjusted as indicated) were mixed by pipetting, and the mixture was left standing for 60 min before being centrifuged. Finally, the NBP in the resultant supernatant was measured. (b) Effects of Eti and Clo on the binding of NBPs to HA. HA 10 mg and 100 µL of a solution containing 4 mM of an NBP and the indicated concentration of Eti or Clo were mixed by pipetting, and the mixture was left standing for 60 min before being centrifuged. Finally, the NBP in the resultant supernatant was measured. Each value is the mean ± S.D. from 4 tubes. * p < 0.05 vs. indicated group.
If an NBP-bound HA is not saturated with an NBP, then when the NBP is released from the HA, the released NBP might immediately reattach to the HA. In the following experiments, to avoid that happening, we used HA saturated with an NBP, i.e., each NBP-bound HA was prepared from 10 mg HA and 100 µL of 4 mM NBP (abbreviated “NBP(4mM)-HA”). Thus, the HA is nearly saturated with the relevant NBP. Figure 3A shows that Eti and Clo concentration-dependently release each NBP. For each NBP, about 80% of the amount bound to HA was released by 300 mM Eti, while the release induced by 300 mM Clo was smaller. As shown in Fig. 3B, a combination of Eti and Clo (Eti + Clo), each 150 mM, produced no detectable additive effect on the release of N-BPs from HA, indicating affinities for HA of Eti>>Clo. Figure 3C shows that 30–40% of each NBP is released during mixing, although the release of Min is slightly slower than those of Ale and Pam. In these experiments, the mixing was carried out 20–30 times during 15–20 s until each NBP-HA in the tube was completely dispersed. Such mixing with 300 mM Eti two or three times in total resulted in complete release or removal of each NBP from HA (Fig. 3D).

In these experiments, NBP(4 mM)-HA was used. (a, b) Effects of concentrations of Eti and Clo. To each NBP-HA, 100 µL of the indicated concentration of Eti, Clo, or both was added and they were mixed by pipetting, left standing for 60 min, and the mixture was centrifuged and the resultant supernatant subjected to measurement of the N-BP. (c) NBP-releasing velocity from HA. NBP(4 mM)-HA and 100 mM Eti (100 µL) were mixed by pipetting. Then, the mixture was left standing for the indicate time and centrifuged, and the resultant supernatant was subjected to measurement of the NBP. (d) Effect of repeated treatment with Eti. NBP(4 mM)-HA was mixed with Eti (concentration as indicated) by pipetting without standing, and the supernatant obtained by centrifugation immediately after the mixing was subjected to measurement of the NBP. This treatment was performed three times. Each value is the mean ± S.D. from 4 tubes. * p < 0.05 vs. indicated group.
As shown in Fig. 2A, the binding of NBPs to HA was not modified by pH under the conditions tested. However, Fig. 4A shows that the release of NBPs bound to HA by Eti is markedly affected by pH; i.e., the release of Ale and Pam increased with the increase in pH, while the release of Min was maximal at pH 5.

In these experiments, NBP(4 mM)-HA was used. (a) Effects of pH on Eti-induced NBP-release from HA. HA-NBP and 50 mM Eti (100 µL), with the pH adjusted as indicated, were mixed by pipetting and left standing for 60 min, and the mixture was then centrifuged and the resultant supernatant subjected to measurement of the NBP. (b) Effects of Eti or Clo bound to HA (Eti-HA or Clo-HA) on NBP-binding to HA. HA-Eti or HA-Clo was prepared from HA 10 mg and 4 mM Eti or Clo (100 µL). They were mixed with 4 mM of a given NBP (100 µL) by pipetting and the mixture left standing for 60 min. Then, the mixture was centrifuged and the resultant supernatant subjected to measurement of the NBP. (c) Effects of BP-related substances on NBP-release from NBP-HA. NBP(4 mM)-HA and the indicated test substance (100 µL) were mixed by pipetting and the mixture left standing for 60 min. Then, the mixture was centrifuged and the resultant supernatant subjected to measurement of the NBP. Eti, Clo, PFA, and PAA exhibited a UV spectrum at 220 nm. So, their effects on Zol could not be measured in the present study. Data were compared vs. those obtained from distilled water (data not shown). Each value is the mean ± S.D. from 4 tubes. * p < 0.05 vs. distilled water group.
As shown in Fig. 3A, for NBP-release from NBP-bound HA, a high concentration (approx. 300 mM) of non-NBP (Eti or Clo) was required. However, Fig. 4B shows that the NBPs tested, even at 4 mM, almost entirely bind to non-NBP(4 mM)-bound HA, indicating that the affinities for HA are NBPs (Ale, Pam, Min)>>non-NBPs (Eti and Clo).
Effects of BP- and HA-Related Substances on NBP-ReleaseBPs are analogs of PPi, and the BPs used in the present study are sodium salts, except Min. So, we examined whether NaCl, PPi, and PPi-related substances (Pi, PFA, and PAA) can release NBPs from NBP(4 mM)-HA. Figure 4C shows that although effects of 300 and 450 mM NaCl are absent, 300 mM Pi, 150 mM PFA, and 150 mM PAA slightly but significantly release the NBPs. In this experiment, we could measure Zol, too, because PPi does not disturb the measurement of Zol. Interestingly, 150 mM PPi markedly releases all the NBPs tested. For Ale and Pam, the releasing effects are Eti > Clo > PPi. However, for Min, the releasing effects are Eti ≥ PPi. Ca2+ is a component of HA, and Mg2+ easily substitutes for the Ca2+. However, even at 300 mM, they exhibit no significant effects on NBP-release from HA. Histamine, putrescine, and spermidine are basic substances, and their productions are known to be increased at inflammatory sites. So, they might interact with the PO43- in HA in vivo. However, none of them, at 150 mM, exhibited a detectable effect (data not shown).
Effects of pH on the PPi-Induced NBP-ReleaseFigure 5A shows that the release of Ale and Pam from HA increases as pH increases. However, the release of Min was maximal at pH 5, and the release of Zol increased with the increase in pH and reached near maximum at pH 7.

(a) Effects of pH. NBP(4 mM)-HA and 150 mM PPi (100 µL), with the pH adjusted as indicated, were mixed by pipetting and left standing for 60 min, and the mixture was the centrifuged and the resultant supernatant subjected to measurement of the NBP. * p < 0.05 vs. pH 7. (b) Effects of histamine (H), putrescine (Put), and spermidine (Spd). Min(4 mM)-HA and 100 µL of a solution of a test substance(s) were mixed by pipetting and left standing for 60 min before the mixture was centrifuged and the resultant supernatant subjected to measurement of the NBP.
As shown in Fig. 4C, Ca2+, Mg2+, and amines, by themselves, exhibited no detectable effects on NBP-release from HA. In the present experiments, however, we could not study the effects of Ca2+ and Mg2+ on PPi-induced NBP-release from HA because CaCl2 and MgCl2 each formed insoluble product when mixed with PPi. Concerning histamine, putrescine, and spermidine, none of them modified the PPi-induced release of Min from HA (Fig. 5B).
Experiments Using 2 mM NBPs (a Concentration That Does Not Saturate HA (10 mg))As shown in Fig. 1B, for complete binding of 4 mM NBPs to HA, Min and Zol required a longer time than Ale and Pam. In addition, the release of Min from HA induced by Eti was slower than those of Ale and Pam (Fig. 3C). We suspected these differences might be due to steric hindrance, because the molecular sizes of Min and Zol are greater than those of Ale and Pam, and 4 mM is the concentration that nearly saturates 10 mg HA with each NBP (Fig. 1A). So, in the following experiments, 2 mM NBPs was used. At this concentration, NBPs bind rapidly to HA (Fig. 6A) and are released rapidly from HA (Fig. 6B). In the experiments using 4 mM NBPs, the order of NBP-releasing effects was Eti > Clo > PPi for Ale and Pam, but Eti ≥ PPi ≥ Clo for Min (Fig. 4C). Figure 6C shows that these orders are the same in the experiments using 2 mM NBPs, too. In this experiment, like Min and Zol, Ris too is released by PPi with the magnitude of the effect being similar to those on Min and Zol; i.e., the potency to release Min, Zol, and Ris may be Eti ≥ PPi > Clo.

(a) NBP-binding velocity to HA. HA 10 mg and 2 mM of a given N-BP (100 µL) were mixed by pipetting, and the mixture was left standing for the indicated time, and then centrifuged. Finally, the NBP in the resultant supernatant was measured. (b) NBP-releasing velocity. NBP(2 mM)-HA and 100 mM Eti (100 µL) were mixed by pipetting. Then, the mixture was left standing for the indicated time and centrifuged, and the resultant supernatant was subjected to measurement of the NBP. Each value is the mean ± S.D. from 4 tubes. * p < 0.05 vs. indicated group. (c) Effects of Eti, Clo, and PPi on NBP-release from NBP-HA. NBP(2 mM)-HA and the indicated test substance or distilled water (100 µL) were mixed by pipetting and the mixture left standing for 60 min. Then, the mixture was centrifuged and the resultant supernatant was subjected to measurement of the NBP. Each value is the mean ± S.D. from 4 tubes. * p < 0.05 vs. distilled water group (data not shown).
As shown in Fig. 7, PPi reduced the binding of all the NBPs tested, with its effects on Min, Zol, and Ris being greater than those on Ale and Pam. Pi also reduced the binding of all the NBPs tested, but the magnitude of the effects was very small. Although CaCl2 exhibited no effects, MgCl2 slightly reduced the binding of Min, Zol, and Ris. Concerning the effects of amines, we could measure only Min, because the amines reacted with TNBS and exhibited UV absorbance at 220 nm (used for measuring Zol and Ris). None of the amines tested reduced the binding of Min to HA.

HA 10 mg and 100 µL of the indicated test sample(s) were mixed by pipetting, and the mixture was left standing for 60 min before being centrifuged. Then, the NBP in the resultant supernatant was measured. Each value is the mean ± S.D. from 4 tubes. * p < 0.05 vs. NBP alone.
As shown in Fig. 6C, the NBP-releasing effects of PPi were greater for Min, Zol, and Ris than for Ale and Pam. Thus, it was of interest how PPi might inhibit the binding of each NBP to HA. So, finally, we compared the inhibitory effects of various concentrations of PPi among all the NBPs used in the present study (Fig. 8). Interestingly, again we found that the effects of PPi were greater for Min, Zol, and Ris than for Ale and Pam.

HA 10 mg and 100 µL of a solution containing 4 mM of an NBP and the indicated concentration of PPi were mixed by pipetting, and the mixture was left standing for 60 min before being centrifuged. Then, the NBP in the resultant supernatant was measured. Each value is the mean ± S.D. from 4 tubes. * p < 0.05 vs. 0 (no PPi).
Finally, we examined whether the NBP released from HA retains its ability to bind HA. Although the P–C–P bond of BPs is a structure that is stable against hydrolytic destruction, we cannot rule out the possibility that some unknown mechanisms might alter the ability of the released NBPs to bind to HA. However, the result shown in Supplementary Fig. 2 demonstrates that the NBP Ale released from HA has an unchanged ability to bind to HA (versus Ale not released from HA).
In the present study, by employing the TNBS-reaction or UV spectrometry, we were able to measure NBPs in the presence of high concentrations of Eti, Clo, or PPi and to evaluate NBP-binding to HA and NBP-release from NBP-bound HA. The notable findings are summarized in Table 1.
| Test substances | Effects of test substances on | |
|---|---|---|
| NBP-binding to HA | NBP-release from HA-bound NBPs | |
| Eti, Clo, PPi | Inhibition | Promotion |
| Eti > Clo (Fig. 2B) | Eti>>Clo (Fig. 3A) | |
| Eti > Clo>PPi for Ale and Pam (Figs. 4C, 6C) | ||
| Eti ≥ PPi > Clo for Min, Zol, Ris (Figs. 4C, 6C) | ||
| Pi, PFA, PAA | Inhibition | Promotion |
| Very weak, if any (Fig. 7) | PPi > PFA > Pi > PAA (Fig. 4C) | |
| Ca2+, Mg2+ | Inhibition | Promotion |
| Very weak, if any (Fig. 7) | Very weak, if any (Fig. 4C) | |
| Amines* | No detectable effect (Fig. 7) | No detectable effects (Fig. 5B) |
NBPs used in the present study were Ale, Pam, Min, Zol, and Ris. *Histamine, putrescine, and spermidine Note: 1. NBP-releasing effects and NBP-binding inhibitory effects of PPi are greater for Min, Zol, and Ris than for Ale and Pam (Figs. 6C, 8). 2. The effects of Pi, PFA, PAA, Ca2+, and Mg2+, and amines on NBP-binding to HA and NBP-release from NBP-bound HA are minimal, if any. 3. Under neutral conditions, NBPs bind to HA and the binding is saturable (1 mg HA is nearly saturated with 40 nmols of each NBP) (Figs. 1A, B). The velocities of both NBP-binding to HA and NBP-release from NBP-bound HA are very rapid at an unsaturated concentration of each NBP (Figs. 6A, B). 4. For complete binding to HA and for complete release from NBP-bound HA, Min and Zol require a somewhat longer standing time than Ale and Pam (Figs. 1B, 3C). 5. Although NBP-binding to HA was not modulated by pH under the conditions used (Fig. 2A), NBP-release from HA by Eti or PPi was modulated by pH (Figs. 4A, 5A). 6. The affinities for HA are NBPs (Ale, Pam, Min)>>non-NBPs (Eti and Clo) (Figs. 3A, 4B).
NBPs possess both negative and positive charges, and HA is also composed of cationic and anionic ions (Supplementary Fig. 3). Thus, interactions between NBP and HA might be modified by pH and/or the presence of ionic substances. Indeed, Eti, Clo, and PPi markedly modified them, and the NBP-release from HA induced by Eti or PPi was markedly modified by pH. In contrast, Ca2+ and basic biogenic amines (histamine, putrescine, and spermidine) exhibited no detectable effects, while Mg2+ and Pi were only slightly inhibitory on NBP-binding to HA.
The velocities of both NBP-binding to HA and NBP-release from NBP-bound HA were very rapid under an unsaturated concentration of NBPs, although for complete binding to HA and for complete release from HA, Min and Zol required a somewhat longer standing time than did Ale and Pam. In addition, the effects of PPi on both NBP-binding to HA and NBP-release from HA were greater for Min, Zol, and Ris than for Ale and Pam, while the effects of Pi were minimal. Unlike those of PPi, the effects of PFA and PAA were also very small. These results suggest that, in addition to positive and negative charges, steric hindrance may also modify the interactions between NBPs and HA. The fact that Eti, Clo, and PPi (but not Pi) with a P–C–P or P–O–P structure profoundly affect the interactions between NBPs and HA indicates that the sterically oriented negative charges of the two phosphate residues are critically important in both NBP-binding to HA and NBP-removal from HA.
In the present in vitro experiments, NBPs bound to HA were demonstrated to be released by Eti and Clo (both non-BPs) and by PPi. As described in Introduction, NBPs are supposed to enter soft-tissue cells via phosphate transporters (SLC20 and/or SLC34).15–17) In contrast, the non-NBPs Eti and Clo have been shown to be not retained within soft tissues after their intravenous injection into mice.21) Thus, if we consider the in vivo environment, a number of possibilities for the fate of the released NBPs suggest themselves: (a) rebinding or reattachment to bones, (b) entry into soft-tissue cells around the bone that released the NBP, and (c) excretion. When the bones are not saturated with an NBP, the released NBP might rebind to the non-saturated sites in the bones. In that case, the entry of the released NBP into soft-tissue cells might be minor, if any. However, if all bones are saturated with an NBP, entry of the released NBP into soft-tissue cells and/or its excretion might occur to a significant extent. The ratio between these two events would differ depending on the degree of saturation with NBPs as well as on the doses of administered non-NBPs or the amount of endogenous PPi present. It should be noted that bone scintigraphy using non-NBPs (the non-NBPs Med or Oxi) (see Supplementary Fig. 1) labeled with 99mTc is a useful method for detecting the sites in HA (i.e., in bones) to which 99mTc-BP attaches, including when tumors, fractures, infection, and/or inflammation are present. Oral tissues are frequently exposed to bacteria, and bacteria are thought to trigger or promote BRONJ.22,23) Notably, NBPs can be detected in the saliva of BRONJ patients,24) indicating that jawbones are susceptible to the accumulation of BPs and may become saturated with a BP after its repeated administration. Therefore, the following scenarios need to be considered. (i) The NBPs released by PPi may enter soft-tissue cells, leading to BRONJ. (ii) The NBPs released by Eti and Clo may not enter soft-tissue cells because the co-presence of Eti and/or Clo inhibits SLC20 and/or SLC34. We discuss these ideas in the following paragraphs.
A reduction in alkaline phosphatase has been shown to result in an increase in PPi in the urine in both humans and mice.25,26) Alkaline phosphatase is expressed on the cell membrane of osteoblasts, and NBPs are toxic to osteoblasts at 10−5 M.27) Thus, if NBPs reach 10−5 M around jawbones, the number of osteoblasts might be reduced, resulting in an increase in PPi. It is also known that PPi is extracellularly produced by the hydrolysis of nucleotide triphosphates, including ATP, and ATP is released from nerve termini, immune cells, injured cells, and even from bacteria.28) Interestingly, platelets store PPi in dense granules together with ATP.29) A cell-wall component of Gram-negative bacteria, namely lipopolysaccharide (LPS), and the inflammatory cytokines interleukin (IL)-1 and tumor necrosis factor α (TNF-α) are potent stimulators of platelets.30) Thus, ATP and PPi may be increased at inflammatory, necrotic, and/or infected sites in the oral tissues of NBP-treated patients. Collectively, the above makes it seem likely that PPi-induced NBP-release occurs in NBP-accumulated jawbones, contributing to the development of BRONJ (Supplementary Fig. 4).
As shown in Supplementary Table 1, the non-NBPs Eti and Clo have a variety of properties in addition to their anti-bone-resorptive effects. Taking these properties and the present findings into account, we now discuss the clinical applications of Eti and Clo.
(i) Eti as a Substitution Drug for NBPs to Treat and Prevent BRONJ: Once taken into cells, NBPs (dependent on their amounts) are cytotoxic for all cell types31) because they inhibit the mevalonate pathway (common to all eukaryotic cells).32) Here, we demonstrated that Eti releases NBPs from NBP-bound HA. In addition, we previously demonstrated that NBPs enter cells via the phosphate transporters SLC20 and/or 34, and that Eti and Clo can inhibit this entry.15–17) The anti-bone-resorptive effect of Eti is far less powerful than those of NBPs (Supplementary Fig. 1). To compensate for this weak effect, Eti is used clinically at much larger doses (200–1000 mg) than NBPs (used at 1–50 mg). Thus, such a large dose of Eti would expel bone-bound NBPs from the bone and inhibit the entry of the released NBPs into soft-tissue cells around the jawbones. Consequently, Eti would be expected to be effective as a substitution drug for an NBP in patients with BRONJ or at risk of BRONJ. Indeed, our clinical attempts support this idea.18,19) Eti might also be effective for preventing BRONJ if it is given orally or intravenously just prior to invasive dental treatments.
(ii) Eti as an Irrigating Agent during Invasive Dental Treatments in NBP-Treated Patients: Invasive dental treatments (e.g., tooth extraction, dental implants, apical/periodontal surgery) are thought to be risk or causal factors for BRONJ.8) Interestingly, Eti has appeared recently on the market as a root canal-irrigating agent (brand name “Dual Rinse® HEDP”),33,34) with the Eti being used at a very high concentration [0.9 g/10 mL (360 mM)]. When injected into mouse ear-pinnas (20 µL/ear), Eti does not exhibit inflammatory/necrotic effects even at 100 mM.35) On the contrary, Eti, when injected together with an NBP, inhibits the NBP-induced inflammation by inhibiting its entry into cells. For example, 16–50 mM Eti completely prevents the inflammatory/necrotic effects of 2–4 mM Zol.13,17) Thus, irrigation with a solution including a high concentration of Eti might be a safe and effective method for reducing jawbone-accumulated NBPs. As shown in Figs. 4A and 5A, release of an NBP (Ale, Pam, or Zol, but not Min) from NBP-HA is greater at higher pH. Thus, an Eti solution with a higher pH (possibly around pH 9) may be very suitable as an irrigating solution for removing jawbone-bound NBPs (Ale, Pam, or Zol). Interestingly, Hokugo et al. recently reported that in Zol-treated mice, injections of Eti around a tooth one day before its extraction reduced the development of jawbone necrosis.36) The same authors previously (2013) showed by in vitro experiments using fluorophore-conjugated NBPs that a BP and HA would establish an equilibrium, and thus an additional application of a BP may partially replace the BP while maintaining the equilibrium.37)
(iii) Clo as a Preventive Drug against BRONJ: The affinity of Clo for HA is the lowest among BPs and lower than that of PPi (Supplementary Table 2), and its ability to release NBPs from HA is much weaker than that of Eti. In our in vivo studies in mice, although Eti reduces the anti-bone-resorptive activities of NBPs,12,35) Clo does not reduce them.11,13) Clo does, however, prevent or reduce the inflammatory/necrotic effects of NBPs by inhibiting NBPs’ entry into cells via the phosphate transporters SLC20 and/or SLC34, and this effect is stronger than that of Eti.14,15,38,39) Thus, a combination of Clo and an NBP might be effective for preventing BRONJ while preserving the powerful anti-bone-resorptive effect of the NBP. Moreover, Clo and Eti have analgesic effects that are independent of their anti-bone-resorptive effects and are derived from inhibition of the phosphate transporter SLC17.15,40–45) The analgesic effect of Clo is also greater than that of Eti.40,43)
The affinities for HA are NBPs (Ale, Pam, Min)>>non-NBPs (Eti and Clo) (Figs. 3A, 4B). So, the results shown in Fig. 4B suggest that if these NBPs were given to patients who had previously been treated with non-NBPs for the removal of NBPs (to prevent or treat BRONJ), the non-NBPs may then be replaced with NBPs, i.e., restoring the potent anti-bone-resorptive effects of NBPs.
Eti and Clo were the first BPs reported as possible drugs following collaborations between Fleisch and Francis.46) Eti (synthesized as early as 1897) has recently undergone reappraisal because it has various properties that appear unique vs. NBPs and it has a host of “off-label” uses.47) Clo has also been reappraised because of its variety of effects (including its analgesic effect) and because of the availability of various administration routes (including intramuscular injection) and its tolerability or safety.10,48,49)
Within the last few years, NBPs have been studied as drugs for preventing the non-gravity-induced osteoporosis that occurs during a prolonged stay in space, supporting NBPs being very important drugs available for preventing osteoporosis. Therefore, establishing methods for their safe use is critical. In conclusion, our findings may contribute to this. Although NBPs have been thought to undergo stable binding to bone, and thus to have a long half-life in bone, the present results suggest that NBPs might be released from NBP-accumulated jawbones by PPi produced in the environment of the jawbones, leading to the development of BRONJ.
Research funding provided by Japan Society for Promotion of Science (18K17240 and 21K10157 to KB). The authors are grateful to Dr. Robert Timms, a former language-editor of J. Physiol. (Lond.), who edited our manuscripts throughout our studies and provided many instructive suggestions. We also thank the Biomedical Research Core of Tohoku University Graduate School of Medicine for the use of its equipment.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.