2021 Volume 44 Issue 11 Pages 1717-1723
2021 Volume 44 Issue 11 Pages 1717-1723
Acetylcholine (ACh), a quaternary ammonium cation, is known as one of the itch inducer in atopic dermatitis (AD), an inflammatory skin disease with intense itching. Previous research has reported accumulation of ACh in lesional site of AD patients. Generally, ACh is metabolized by cholinesterase (ChE). Therefore, one of the causes of ACh accumulation may be the suppression of ChE activity. Increased levels of the multifunctional bioactive sphingolipid sphingosylphosphorylcholine (SPC) have also been detected in AD. Since SPC possesses a quaternary ammonium cation, like ACh, it is possible that SPC affects the activity of ChE catalyzing ACh metabolization. We investigated whether SPC influences the activity of ChE by performing enzymatic analysis of ChE in the presence of SPC. We found that SPC strongly suppressed acetylcholinesterase (AChE) activity, but the suppression of butyrylcholinesterase by SPC was quite low. The Michaelis constant (Km) of AChE in the presence of SPC increased, and the maximum velocity (Vmax) decreased, indicating that SPC acts as mixed-type inhibitor for AChE. The analysis of SPC analogs clarified the importance of both the quaternary ammonium cation and the carbon chain length of SPC for the AChE inhibitory effect and showed that SPC was unique in AChE inhibition among the sphingolipids in this study. These findings indicate a novel function of SPC on AChE inhibition. Thus, the inhibition activity of SPC may be a factor in the increase of ACh in AD.
Acetylcholine (ACh) is a neurotransmitter that participates in the regulation of cell function and is mainly metabolized by acetylcholinesterase (AChE, EC3.1.1.7) and butyrylcholinesterase (BuChE, EC3.1.1.8). The quaternary ammonium cation and carbonyl groups in ACh are necessary for the binding to the AChE anionic subsite (AS) and catalytic triad subsite (CAS), respectively, resulting in the degradation of ACh to acetic acid and choline.1)
Atopic dermatitis (AD) is the chronic dermatitis disease with itching, and ACh is known as one of the inducers of itching. In fact, injection of ACh to AD patients induce itching.2) Moreover, it has been reported that the ACh-induced itching in AD is alleviated by treatment with botulinum toxin (an ACh release inhibitor), naltrexone (a nicotinic acetylcholine receptor inhibitor), and doxepin (a muscarinic acetylcholine receptor antagonist).3–5)
In previous study, increased level of ACh has been observed in the lesional site of AD.6) The mechanism by which ACh is involved in itching of AD remains unclear, but it is possible that the enzymatic reaction involved in synthesis and/or degradation of ACh may associate with the causes of ACh accumulation.
Elevation of sphingosylphosphorylcholine (SPC) in AD skin has been previously reported.7) SPC is a multifunctional, bioactive sphingolipid and has been implicated in a range of cellular processes, such as cell proliferation, calcium influx, angiogenesis, inflammation, apoptosis, and autophagy.8–13) SPC has the quaternary ammonium cation in the molecular structure similar to ACh. Thus, we hypothesized that the quaternary ammonium cation site of SPC binds to AS in AChE and SPC contributes to ACh accumulation in AD skin via the inhibition of AChE.
In this study, to investigate whether SPC influences the activity of cholinesterase (ChE), we examined the effects of SPC on the action of ChE using enzymatic methods. We found that SPC strongly suppressed the AChE activity, while the inhibition of the BuChE activity by SPC was quite weak. Taken together, our data have unveiled a novel function of SPC: the inhibition of AChE. This inhibition may contribute to the involvement of SPC and ACh in the pathogenic mechanisms of AD.
We purchased sphingosylphosphorylcholine (SPC), sphingomyelin (SM) from chicken egg yolk, and phosphocholine (PC) from Merck KGaA (Darmstadt, Germany); N-acetyl-D-erythro-sphingosylphosphorylcholine (C2-SM) and N-hexanoyl-D-erythro-sphingosylphosphorylcholine (C6-SM) from Avanti Polar Lipids, Inc. (AL, U.S.A.); Fos-choline 10 (FC10) and Fos-choline 12 (FC12) from Anatrace (Maumee, OH, U.S.A.); miltefosine (MF), neostigmine (Neo), and rivastigmine (Riv) from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan); sphingosine-1-phosphate (S1P) from Cayman Chemical Company (Ann Arbor, MI, U.S.A.); glucosylsphingosine (Gluc-Spn) from Abcam (Cambridge, U.K.); cetyltrimethylammonium bromide (C-TAB), hexyltrimethylammonium bromide (Hexyl-TAB), and ATCh from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan); recombinant human AChE and recombinant human BuChE from R&D Systems (Minneapolis, MN, U.S.A.); and 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB) from Dojindo Laboratories (Kumamoto, Japan). General laboratory reagents were obtained from FUJIFILM Wako Pure Chemical Corporation, Merck KGaA, Nacalai Tesque, Inc. (Kyoto, Japan). The structures of compounds used for the experiments are shown in Fig. 1.

Chemical structures of acetylcholine (ACh), sphingosylphosphorylcholine (SPC), sphingosine-1-phosphate (S1P), glucosylsphingosine (Gluc-Spn), sphingomyelin (SM), N-acetyl-D-erythro-sphingosylphosphorylcholine (C2-SM), N-hexanoyl-D-erythro-sphingosylphosphorylcholine (C6-SM), miltefosine (MF), Fos-choline 12 (FC12), Fos-choline 10 (FC10), cetyltrimethylammonium bromide (C-TAB), hexyltrimethylammonium bromide (Hexyl-TAB), phosphocholine (PC), neostigmine (Neo), and rivastigmine (Riv) are shown. Quaternary ammonium cations, phosphate groups, alkyl groups and acyl chain are shown in dotted circles, solid line squares, dotted squares and sold circles, respectively.
SPC and Neo were dissolved in 0.1 M phosphate buffer (pH 8.0) containing 0.01% bovine serum albumin (BSA) (assay buffer) at concentrations of 0.01 to 250 µM, and Riv was dissolved in assay buffer at concentrations of 0.01 to 100 µM. S1P was dissolved in 0.7 M NaOH and the solution was adjusted to pH 9–10 with 1 N HCl, and then S1P solution was diluted in assay buffer with 30% methanol at concentrations of 50 to 250 µM. Gluc-Spn was dissolved in assay buffer with 30% methanol (MeOH) at 90 °C and then diluted at concentrations of 50 to 250 µM. SM was dissolved in assay buffer with 30% MeOH or 30% ethanol (EtOH) at 90 °C and then diluted at concentrations of 50 to 250 µM. C2-SM and C6-SM were dissolved in assay buffer with 30% EtOH and then diluted at concentrations of 50 to 250 µM. Other compounds were dissolved in assay buffer at concentrations of 50 to 250 µM (Supplementary Table 1). AChE was dissolved in assay buffer, assay buffer with 30% MeOH and assay buffer with 30% EtOH at concentrations of 650 pg/mL, 1300 pg/mL and 1950 pg/mL respectively. BuChE was dissolved in assay buffer at a concentration of 90 µg/mL. Both AChE and BuChE were prepared for as cholinesterase (ChE) solution.
Cholinesterase Inhibition AssaysChE inhibition assays were performed using the method published by Ellman.14) Briefly, 75 µL each of ChE solution and test compound solution was mixed in a 96-well plate (Thermo Fisher Scientific, Waltham, MA, U.S.A.). Then, 1 µL of 0.075 mM ATCh in 0.1 M phosphate buffer (pH 7.0), 5 µL of 10 mM DTNB, 0.018 mM NaHCO3 in 0.1 M phosphate buffer (pH 7.0), and 2.5 µL of assay buffer were pre-mixed and added into the each well. After 30 min of incubation at room temperature, the absorbance was measured using Power Scan HT (DS Pharma Biomedical, Osaka, Japan) at 412 nm. A sample blank without ChE, a control without test compounds, and a control blank without either ChE or test compounds were also measured in parallel.
ChE activity was calculated using the following equation: ChE activity (%) = (sample absorbance – absorbance of sample blank)/(control absorbance – absorbance of control blank) × 100. The IC50 value of SPC was calculated by plotting a regression line through the linear portion of the graph at concentrations from 0 to 200 µM.
Statistical AnalysisData were expressed as mean ± standard deviation (S.D.). Statistical analyses were performed using R software version 3.5.3.15) The statistical significance of differences between multiple groups was evaluated using ANOVA, followed by Dunnett’s post hoc test for multiple comparison, and comparisons between two groups were evaluated using unpaired Student’s t-tests.
The quaternary ammonium cation of ACh binds to the AS of AChE and BuChE.1,16) SPC also has a quaternary ammonium cation. To investigate whether SPC affects the activities of AChE and BuChE, we performed ChE inhibition assays, using the AChE inhibitors Neo and Riv as positive controls. As shown in Fig. 2A, both Neo and Riv inhibited the action of AChE protein in a dose-dependent manner, while SPC inhibited AChE at concentration of 10 µM or more. Inhibition of AChE by SPC, Neo, and Riv had IC50 values of 62.2 µM, 0.00459 µM, and 7.7 µM, respectively (Table 1), indicating that the AChE inhibitory activity of SPC was lower than that of Neo and Riv.
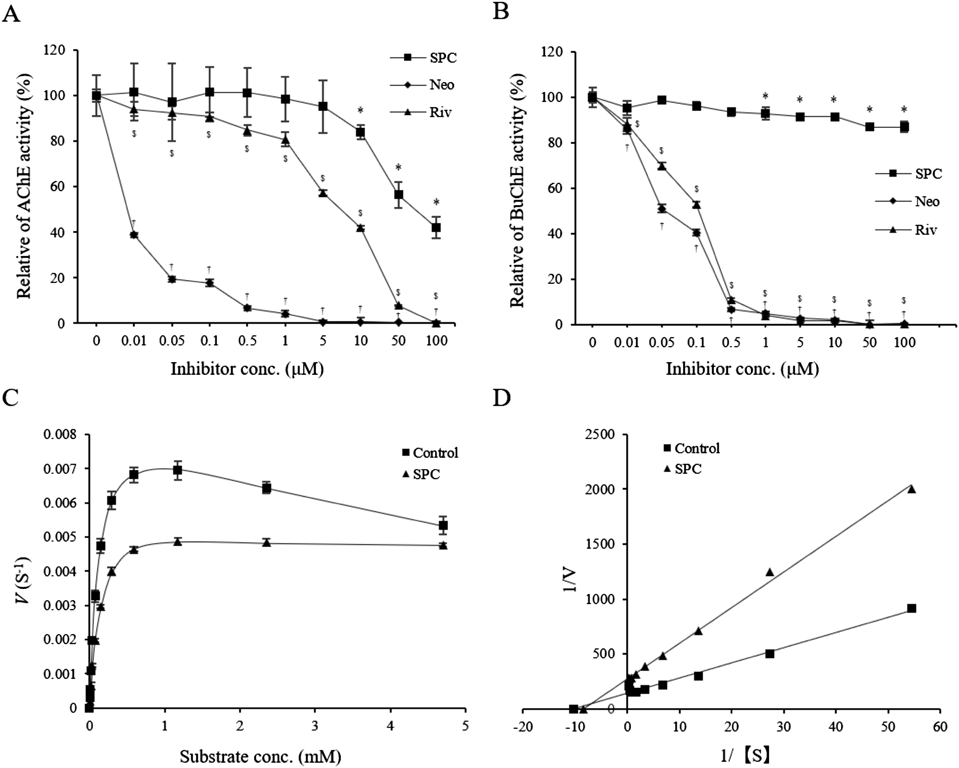
(A, B) Changes in AChE (A) and BuChE (B) activities in the presence of SPC, neostigmine (Neo), or rivastigmine (Riv). AChE or BuChE was incubated with various concentrations of each test compound in the assay buffer containing ATCh for 30 min. AChE and BuChE activities are indicated as relative values when controls not containing SPC were set to 100%. Data are expressed as mean ± S.D. (n = 4). * p < 0.05, # p < 0.05, and † p < 0.05 by ANOVA with Dunnett’s post hoc test compared to control. (C) Michaelis–Menten curve in the absence (control) and presence of SPC (62 µM). (D) Lineweaver–Burk plot converted from Michaelis–Menten curve. The Km and Vmax values of SPC were determined from the Lineweaver–Burk plot.
| SPC | Neo | Riv | |
|---|---|---|---|
| IC50 (µM) | 62.2 ± 5.6 | 0.0046 ± 0.0001 | 7.7 ± 0.18 |
Data are expressed as mean ± standard deviation (S.D.) (n = 4). SPC, sphingosylphosphorylcholine; Neo, neostigmine; Riv, rivastigmine.
We also tested whether SPC suppresses the activity of BuChE. Both AChE inhibitors, Neo and Riv, significantly suppressed BuChE activity in a dose-dependent manner. SPC had a low inhibitory effect on BuChE, with an inhibitory ratio of approximately 10% compared with approximately 60% for AChE at a concentration of 100 µM (Figs. 2A, B). These results indicate that SPC inhibits the activity of AChE with high selectivity.
AChE Inhibition by SPC Is a Type of Mixed InhibitionWe next analyzed the inhibitory mechanism of SPC on AChE, using ATCh as a substrate. The enzymatic reactions were carried out in the absence and presence of SPC, to produce both Michaelis–Menten curve and Lineweaver–Burk plots and to determine the kinetic parameters. The Michaelis–Menten curve showed decreased velocity in the presence of SPC at a substrate concentration of 0.0046 to 4.7 mM when compared with that in the absence of SPC (control; Fig. 2C). From the analysis of the Lineweaver–Burk plot, the values of the Michaelis constant (Km) for the control and SPC were 0.097 ± 0.0042 and 0.12 ± 0.0048 µM, respectively, while those of the maximum velocity (Vmax) were 0.0071 ± 0.00043 and 0.0037 ± 0.00012 S−1, respectively (Fig. 2D, Table 1). Our results indicated an increase in Km and a decrease in Vmax in the presence of SPC, a pattern that is characteristic of mixed inhibition, in which competitive and non-competitive inhibition coexist.
SPC Is a Unique Sphingolipid That Has AChE Inhibition ActivitySphingolipids containing SPC are known as ceramide metabolites due to their molecular structure.17) To verify whether SPC is a unique sphingolipid in AChE inhibition, we selected S1P, Gluc-Spn, and SM, as shown in Fig. 1 as SPC-similar sphingolipid, due to their solubility in assay buffer containing 30% MeOH, and compared their inhibitory effects on AChE. Under these conditions, significant inhibition of AChE was observed only with SPC, not with S1P and Gluc-Spn, which do not have a quaternary ammonium cation structure (Fig. 3A). These results revealed that a quaternary ammonium cation structure was essential for AChE inhibitory effect.

(A) Inhibitory effects among various sphingolipids; SPC, S1P, Gluc-Spn, and SM on AChE activity. AChE was incubated with various concentrations of each sphingolipid in assay buffer with 30% MeOH containing ATCh for 30 min. AChE activity is indicated as a relative value when control not containing sphingolipids was set to 100%. Data are expressed as mean ± S.D. (n = 4). * p < 0.05 by ANOVA with Dunnett’s post hoc test compared to control. (B) Inhibitory effects of sphingomyelin analogs with different lengths of acyl-chains on AChE activity. AChE was incubated with various concentrations of each sphingomyelin analogs in assay buffer with 30% EtOH containing ATCh for 30 min. AChE activity is indicated as a relative value when control not containing sphingomyelin analogs was set to 100%. Data are expressed as mean ± S.D. (n = 4). * p < 0.05, † p < 0.05, $ p < 0.05 by ANOVA with Dunnett’s post hoc test compared to control. (C) Inhibitory effects of SPC analogs with different lengths of alkyl chains on AChE activity. AChE was incubated with various concentrations of each SPC analog in assay buffer containing ATCh for 30 min. AChE activity was indicated as a relative value when the control not containing SPC or SPC analogs was set to 100%. Data are expressed as mean ± S.D. (n = 4). * p < 0.05, † p < 0.05, $ p < 0.05, # p < 0.05 by ANOVA with Dunnett’s post hoc test compared to control.
Unexpectedly, SM, which has a quaternary ammonium cation structure similar to SPC, did not provoke AChE inhibition (Fig. 3A). The structural difference SM and SPC is the presence or absence of acyl-chain (Fig. 1). This prompted us to test whether the presence and length of acyl-chain in SM influences the AChE inhibition. Thus, we next performed ChE inhibition assay using SM analogues of C2-SM and C6-SM with acyl-chain length of 2 and 6 carbons, respectively (Fig. 1). SPC without acyl-chain showed a concentration-dependent AChE inhibitory effect, but no AChE inhibitory activity was detected in C2-SM and C6-SM as well as SM (Fig. 3B). This result showed that AChE inhibitory activity was severely hindered by the presence of the acyl chain regardless of chain lengths even if SM had a quaternary ammonium cation.
Therefore, our results demonstrate that SPC is a unique sphingolipid having AChE inhibition activity.
The Phosphate Group and Long Alkyl Chain of SPC Influence AChE Inhibition ActivityPrevious studies have found that AChE inhibition activity depends on the alkyl chain being more than ten carbons long.18) SPC has an alkyl chain length of 18 carbons. To verify the effect of alkyl chain length on AChE activity inhibition, various compounds with structures similar to SPC were selected. The alkyl chain lengths of the selected PC, FC10, FC12, and MF are 0, 10, 12, and 16 carbons, respectively. The alkyl chain lengths of Hexyl-TAB and C-TAB, which do not have a phosphate group, are 6 and 16 carbons, respectively. The AChE inhibitory activity of these compounds was compared with that of SPC. The AChE inhibition activities of the compounds with short alkyl chains, such as PC, FC10, and Hexyl-TAB, were very weak compared to that of SPC, and no dependence on chain length was observed. Significant AChE inhibition, similar to SPC, was confirmed in FC12, MF and C-TAB with the alkyl chain length of 12 or more carbons (Fig. 3C). In addition, AChE inhibition activities of MF and C-TAB were higher than that of SPC, however, despite the same carbon chain length, C-TAB without phosphate group showed stronger inhibitory effect on AChE than MF with phosphate group (Fig. 3C).
These results show that the alkyl chain length of 12 or more carbons is required to exert the AChE inhibition effect, and the presence of phosphate group is notably involved in diminishing the AChE inhibitory effect.
| Km (µM) | Vmax (S−1) | |
|---|---|---|
| Control | 0.097 ± 0.0042 | 0.0071 ± 0.00043 |
| SPC | 0.12 ± 0.0048* | 0.0037 ± 0.00012* |
Data are expressed as mean ± S.D. (n = 4). * p < 0.05 by unpaired Student’s t-test compared with control.
SPC, which consists of a phosphocholine and a sphingosine, is a well-known lipid mediator implicated in a range of cellular processes.19,20) In this study, we demonstrated that SPC at 10 µM or more markedly suppressed the activity of AChE, which degrades ACh. In the presence of SPC, the kinetic parameters Km and Vmax for AChE increased and decreased, respectively, so it was expected that SPC acted as mixed-type inhibitor against AChE. SPC was the only sphingolipid that inhibited AChE among the sphingolipids used in this study. We demonstrated that the inhibition of BuChE by SPC was extremely low. BuChE is also known to hydrolyze ACh, but BuChE has high affinity for butyrylcholine.21) This result was thought to be related to the different affinities to ACh caused by changes in the aromatic gorge disposition between AChE and BuChE. Thus, we identified a novel function of SPC, wherein SPC inhibited AChE activity with high selectivity.
ACh is a chemical neurotransmitter with a typical quaternary ammonium cation and is metabolized to choline and acetic acid by AChE.1) As SPC also has the quaternary ammonium cation in the molecule structure, it was predicted that SPC would affect AChE activity. In this study, we identified a clear inhibitory effect of SPC on AChE activity. However, other SPC-analog sphingolipids without quaternary ammonium cations, such as S1P and Gluc-Spn, showed almost no inhibition of AChE. These results indicate that the quaternary ammonium cation resident on SPC contributes to the AChE inhibitory effect.
Several lines of evidence have suggested that the alkyl side chain interacts with the peripheral anion site (PAS) of AChE.18,22) Although SM has a quaternary ammonium cation and a sphingosine structure like that of SPC, it did not inhibit AChE activity. Moreover, SM has also acyl-chain in the molecular structure unlike SPC, and not only SM but also SM analogues with different acyl-chain lengths exerted no AChE inhibitory activity (Fig. 3B). These results have suggested the possibility the steric hindrance due to the acyl-chain interfere with interaction between SM/SM analogues and AChE. On the other hand, another study reported that the collagen-like tail of AChE was associated with binding to SM.23) As another possible reason, it was considered that the presence of acyl chain regardless of chain length altered the binding property to AChE and eventually affected AChE inhibition effect. Therefore, we speculated that the interaction between the alkyl chain of SPC and the PAS of AChE played a crucial role in AChE inhibition. It remains unclear which part of AChE interacts with the SPC.
Several studies have reported a relationship between AChE inhibition and the alkyl side-chain length of inhibitors having a quaternary ammonium cation. The AChE activity decreases greatly in compounds with an alkyl side-chain length of ten or more carbons but quite gently in compounds with less than ten carbons.18) SPC has a sphingosine structure consisting of 18 carbons-amino alcohol with a quaternary ammonium cation. In the AChE inhibition reaction of SPC, it is presumed that both the quaternary ammonium cation and the long alkyl chain in SPC are required, and AChE activity should be inhibited in a coordinated manner.
On the other hand, we demonstrated that there was not necessarily proportional relationship between alkyl chain lengths and AChE inhibitory activity when compared to among SPC analogues (Fig. 3C). As the cause of this, we considered that the structural difference of SPC analogues was related to AChE inhibitory effect, and focused on the phosphate group as one of candidates. Based on this, we speculated that the negative charge of the phosphate group approaches the quaternary ammonium cation by electrostatic attraction, and as a result, the interaction with the AChE active center becomes difficult. This led us to the following idea that the binding of SPC to the AChE active center of the anion charge might become extremely weak. The binding of the longer alkyl chains in SPC to the PAS of AChE may stabilize the binding of the phosphocholine portion of SPC to the AChE active center, resulting in blocking ACh traffic into the AChE active center, due to steric hindrance or allosteric change, as it has been previously suggested as a mechanism of action for AChE competitive inhibitors.17) The finding of the mechanism regarding the relationship between the length of this alkyl group and AChE inhibitory activity is consistent with very low inhibitory effects in PC without alkyl groups. However, it appears from the comparison between SPC and SM that an alkyl chain length that is too long is also counterproductive. With respect to the difference in inhibitory effect between SPC and MF, an unsaturated hydrocarbon chain in SPC might also structurally affect the AChE inhibition. The alkyl chain of MF and C-TAB is shorter than that of SPC, but since the alkyl chain of SPC contains an unsaturated bond, the three-dimensional structure of the carbon chain and/or the degree of dissociation of phosphate groups may be different. Unsaturated bonds generally cause the chain to bend, and are not linear, and it can be inferred that the degree of dissociation of adjacent functional groups due to electron attraction by a double bond is affected.
Multifunctional bioactive sphingolipid SPC in cells, tissues, or plasma is generally present at low levels under normal conditions, with the SPC concentration in plasma being approximately 50 nM.19) However, it has been reported that high concentrations of SPC (10 to 20 µM) facilitate the release of Ca2+ from the sarcoplasmic reticulum and levels of SPC higher than 10 µM induce cell death in non-cancer cells and promote apoptosis in vascular endothelial cells.9) These findings suggest that SPC exerts different effects depending on its tissue or cell concentration. In this study, SPC inhibited AChE with an IC50 of 62.2 µM in vitro (Table 1). The level of SPC in the stratum corneum of AD patients (40.08 ± 56.25 ng/mg stratum corneum; approx. 82 µM) is higher than that in healthy controls (13.51 ± 5.16 ng/mg stratum corneum; approx. 26 µM).24) It is therefore possible that SPC strongly inhibits AChE activity in the skin of AD patients. Elevation of ACh levels has also been reported in the skin of AD patients, although the role of ACh in AD pathology is unclear.6) Thus, increased SPC may be a trigger for ACh accumulation in AD.
In conclusion, in this study, we found that quaternary ammonium cation and alkyl groups with a chain length of 12 or more are required for the inhibitory activity of AChE, whereas very long chains of alkyl group and phosphate groups weaken the AChE inhibitory activity. All of these factors are present in SPC. Therefore, SPC has AChE inhibitory activity, but this activity is weak. However, in AD patients, since SM deacylase of SPC synthesis is highly activated,25) it appears that over-synthesis of SPC increases ACh by suppressing the AChE activity even if the AChE inhibitory activity of SPC is weak.
In recent years, SM deacylase has been found to be a β-subunit with divergence by cleavage of the disulfide bond between subunits in acid ceramidase.26) Although the mechanism is unclear, it has been thought that excessive SPC is synthesized by frequent cleavage of the disulfide bond in acid ceramidase in AD patients. Clarification of the cleavage mechanism of the disulfide bond in acid ceramidase will lead to a deeper understanding of the role of SPC in AD patients.
It is important to clarify the mechanisms of the involvement of ACh and SPC in AD. To date, our findings about the role of SPC provide new insights into the mechanism of this compound in the pathology of AD. These insights may lead to the development of novel therapeutic agents for diseases with altered ACh level, such as AD and Alzheimer’s disease.
We gratefully acknowledge the work of past and present members of NANOEGG® Research Laboratories, Inc.
This research was conducted with a research fund from “NANOEGG® Research Laboratories, Inc.,” to which the author belongs. The patent (Japanese Patent Application No. 2016-200979) on this study is being inspected. Y. Yamaguchi is representative director of NANOEGG® Research Laboratories, Inc. K. Kitazawa, K. Tanaka, M. Musashi, Y. Kubota, and T. Nagasawa are employees of NANOEGG® Research Laboratories, Inc. N. Nagasawa-Shimura was an employee of NANOEGG® Research Laboratories, Inc. at the time this research was performed. There is no other conflict of interest to be declared.
The online version of this article contains supplementary materials.