2021 Volume 44 Issue 11 Pages 1732-1737
2021 Volume 44 Issue 11 Pages 1732-1737
Doxorubicin (DOX) is an effective anticancer anthracycline drug; however, the cardiotoxicity limits its application. The aim of the present study was to investigate the potential protective effect of taurine against DOX-induced chronic cardiotoxicity in mice. We found that exogenous supplementation of taurine can inhibit the weight loss of mice caused by DOX. The increased activity of myocardial enzymes creatine kinase (CK) and lactate dehydrogenase (LDH) in response to DOX treatment were significantly hampered. In addition, taurine supplementation alleviated the decrease in superoxide dismutase (SOD) activity, glutathione (GSH) content, glutathione peroxidase 4 (Gpx4) expression, and the increase in malondialdehyde (MDA) content caused by DOX. Besides, taurine alleviated myocardial myofibrillar disruption and mitochondrial edema. Furthermore, our results showed that taurine decreased the expressions of cleaved caspase-3 and Bax/Bcl2, thereby inhibiting apoptosis. These collective data demonstrated that exogenous taurine supplementation has a potentially protective effect against the myocardial damage caused by doxorubicin in mice by enhancing antioxidant capacity and reducing oxidative damage and apoptosis.
Doxorubicin (DOX) is an anthracycline anti-tumor drug. It has been reported that DOX is cytotoxic to various types of cells1) and thus has a broad anti-tumor spectrum, such as acute leukemia, malignant lymphoma,2) and breast cancer.3) However, DOX has a strong affinity for the myocardium and can specifically accumulate in the heart, thus causing cardiomyopathy and congestive heart failure.4) The obvious cardiomyopathy always leads to a poor prognosis, which limits the clinical application of DOX.5) Previous studies on the mechanism of DOX myocardial toxicity have shown that the actions of DOX produce the free radicals, which generates a large amount of reactive oxygen species to attack myocardial cells and cause myocardial damage.6,7) Moreover, the antioxidant enzymes and antioxidants in the antioxidant system in the myocardium are inhibited by DOX.8) In the process of injury, the production of reactive oxygen species initiates a series of apoptotic programs, which enhances the myocardial demage.9)
Taurine is a widely distributed free amino acid, usually rich in electrically excited tissues.10) The taurine level in the animal body mainly depends on the dietary intake of sulfur-containing amino acids and enzymatic biosynthesis.11) Moreover, most animals and humans are able to synthesize taurine by cysteine sulfinic acid decarboxylase (CSD) using cysteine or methionine as a substrate.12) The entry of taurine into cells mainly relies on taurine transporters TauT.13,14) Taurine exerts its physiological roles by regulating the level of intracellular calcium ions, the activity of ion channels, the stability of cell membrane and protein, energy metabolism, immunity, and antioxidants.15,16) In addition, it has been shown that taurine can inhibit reactive oxygen species (ROS) generation17,18) and the mitochondrial-dependent cell apoptosis caused by various toxins or pathological stimulants.19) There have been reports on the inhibitory effect of taurine on the toxicity of DOX, such as the protection of taurine on the kidney and the treatment on melanoma cell during the administration of DOX.20,21) Since most study used acute intraperitoneal injection of DOX and taurine,20,22,23) the study adopted a longer period of medication that was closer to the chronic injury caused by DOX and the lower concentration of taurine supplementation to reflect the toxicity inhibitory effect from several aspects.
ICR mice, 8 weeks age, weighing about 32g were obtained from Yangzhou University Comparative Medical Center. All the animals were housed in an environment with a temperature of 22 ± 1 °C, a relative humidity of 50 ± 1%, and a light/dark cycle of 14/10 h. All animal studies (including the mice euthanasia procedure) were done in compliance with Yangzhou University institutional animal care regulations and guidelines and conducted according to the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and the Institutional Animal Care and Use Committee (IACUC) guidelines.
The mice were divided into four groups (8 mice per group): DOX, taurine-DOX, taurine, and control groups. DOX group was injected with DOX (BOSF, China) dissolved with physiological saline weekly for a four-week cumulative dose of 20 mg/kg24) by intraperitoneal injection. In the taurine-DOX group, mice were treated with DOX in the same way as the DOX group, while 0.3% taurine (Sigma, U.S.A.) was continuously added to the drinking water from when the mice were injected with the DOX (Fig. 1A). The taurine group was fed with the taurine added to the drinking water. The control group was given normal drinking water and injected the same amount of physiological saline as the DOX treatment. Mice in four groups were simultaneously treated and analyzed two weeks after the last dose of DOX. The serum taurine levels in different groups were determined by HPLC system (Fig. 1B).
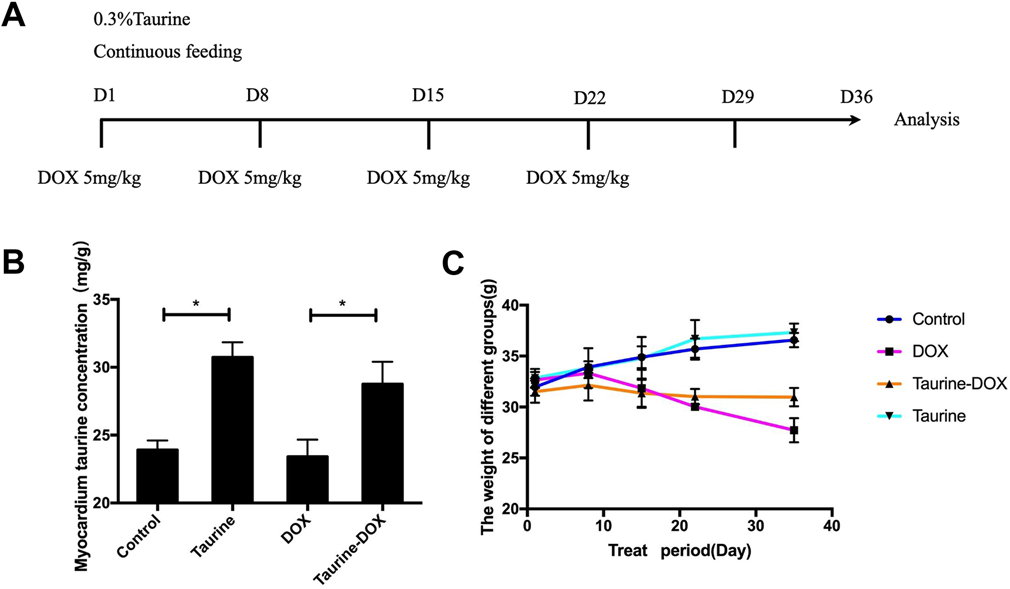
(A) The way mice were treated. (B) The myocardium taurine concentration detected by HPLC. (C) Trends in body weight of mice during the treatment. (Color figure can be accessed in the online version.)
Myocardium tissues were immediately dissected and stored at −80 °C before assays. They were homogenized in ice-cold saline and centrifuged at low temperatures to take the supernatant. The activities of creatine kinase (CK), lactate dehydrogenase (LDH), superoxide dismutase (SOD), reduced glutathione (GSH), and malondialdehyde (MDA) were measured by enzyme-based colorimetric methods using commercial reagent kits run on a biochemistry analyzer (Nanjing Jiancheng Bioengineering Institute, China).
Western BlottingProteins were extracted from myocardium tissues using radioimmunoprecipitation assay (RIPA) buffer (Cell Signaling, U.S.A.) containing 1 mmol/L phenylmethylsulfonyl fluoride (PMSF, Cell Signaling). The protein concentrations were determined using a bicinchoninic acid (BCA) assay reagent (Beyotime Biotechnology, China) according to the manufacturer’s instructions. Proteins lysates were electrophoresed on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories, U.S.A.). The PVDF membranes were blocked with 5% (w/v) nonfat dry milk for 1 h and incubated with either: anti-CSD antibody (1 : 5000; Abcam, U.S.A.), anti-TauT antibody (1 : 5000; Abcam), anti-cleaved caspase-3 (1 : 1000; Abcam), anti-BAX (1 : 2000; Abcam), anti-Bcl-2 (1 : 2000; Abcam) and internal control anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (1 : 10000; Abcam) overnight at 4 °C. The membranes were then washed three times in Tris-buffered saline with Tween-20 (TBST) and incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse immunoglobulin G (IgG) (1 : 10000, Abcam), or HRP-conjugated goat anti-rabbit IgG (1 : 10000; Abcam) for 1 h at room temperature. After washing, the membrane was treated with the enhanced chemiluminescence (ECL) reagent (Vazyme, China) to visualize the immunoreactive bands. The expressed amount of target protein was normalized in relation to the expression of GAPDH.
Real-Time PCRTotal RNA was extracted from mouse myocardial tissues using the TRIzol reagent (TaKaRa, Dalian, China) according to the manufacturer’s instructions. cDNAs were synthesized using M-MLV reverse transcription reagents (Promega, Madison, WI, U.S.A.). Gene expression levels were measured using SYBR Green master mix (TaKaRa) in the ABI PRISM 7500 Sequence Detection System (Applied Biosystems, Foster City, CA, U.S.A.). The levels of all transcripts were normalized to the endogenous expression of GAPDH. The primer sequence of GAPDH was as follows: 5′-AGC AAT GCC TCC TGC ACC ACC A-3′, reverse: 5′-TGA GTC CCT CCA CGA TGC CGA A-3′. The primer sequence of Gpx4 is as follows forward: 5′-TGT GGT TTA CGG ATT CTG G-3′, reverse: 5′-CCT TGG GCT GGA CTT TCA-3′.
Transmission Electron (TEM) and Hematoxylin–Eosin (H&E)-StainedThe left ventricular myocardium was fixed in 2.5% glutaraldehyde (Sigma) in 0.1 M phosphate buffer and then sliced into 1 mm3 pieces. Ultra-thin sections of the pruned tissue were cut and stained to observe images of mitochondrial ultrastructure (Hitachi, Japan). Some left ventricular tissues were fixed in 4% paraformaldehyde (Macklin, Shanghai, China) in 0.1 M phosphate buffer overnight and then embedded in paraffin wax (Leica, Germany). Sections were cut and stained with H&E.
HPLCFill the ion exchange column with a cation and an anion exchange resin with a pH close to 7.0. The taurine concentration of 0, 2.5, 5, 10, 20, and 40 µM were used as a standard while the glutamine concentration of 40 µM was used as an internal standard. The taurine in mouse serum or tissues were extracted with sulfosalicylic acid. The standard products and samples passed through the ion exchange column were filtered through a 0.22 µm filter. They were derivatized and detected by HPLC. Count the peak area at 340 nm and then calculate the concentration of taurine in the serum based on the ratio of the peak area of the standard product to the internal standard and the concentration of the standard product.
Statistical AnalysisData and statistical analyses were performed using GraphPad Prism 7.0 software. The results are expressed as means ± standard error of the mean (S.E.M.) of at least three independent experiments. The differences among groups were determined using a Student’s t-test or a one-way ANOVA. p < .05 was considered to be statistically significant.
No significant differences were observed in mice’s body weights at the time of assignment to each experimental group. Taurine levels in the myocardium were detected to increase by 28.6% after taurine supplementation (Fig. 1B). The body weight in mice from the taurine treatment group and the control group steadily increased (Fig. 1C), but the body weight of the mice in the DOX treatment group kept decreasing, while the body weight in the taurine-DOX mice did not have significant change throughout the duration of the experiment (Fig. 1C). This showed that taurine feeding could restrain the weight loss caused by DOX.
Taurine Reduces the Activities of Myocardial Enzymes in the SerumThe CK and LDH activities were assayed in the serum of the DOX, taurine, taurine-DOX, and control mice, respectively. The results showed that DOX treatment increased the CK and LDH activities by 9.8 and 40.3%, respectively, compared to the control, but there were no significant differences among the control, taurine alone and taurine-DOX groups (Figs. 2A, B). These data suggested that taurine supplementation can reduce the degree of myocardial injury caused by doxorubicin, and that taurine treatment alone did not cause injury to the myocardium.
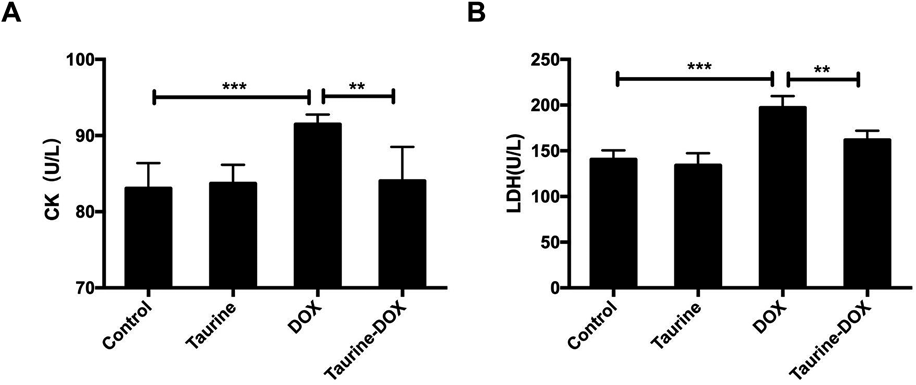
(A) The activity of CK in the mice serum. (B) The activity of LDH in the mice serum. Results are shown as means ± standard deviation (S.D.) of six mice per group. * p < .05, ** p < .01, *** p < .001.
In order to assay the antioxidant capacity of taurine in the DOX-damaged myocardium, the antioxidant and oxidative damage levels were detected. The results showed that the SOD activity, GSH content, and the expression of glutathione peroxidase coding gene Gpx4 decreased by 33.2, 37.0, and 28.5%, respectively, whereas MDA increased by 30.4% in the myocardium treated with DOX, compared with the control group. In contrast, these indicators in the taurine and DOX treatment group myocardium were significantly rescued (Fig. 3), which indicated that taurine could reduce the DOX cardiotoxicity by improving the antioxidant capacity.

(A) The activity of superoxide dismutase (SOD) in the myocardium. (B) The content of reduced glutathione (GSH) in the myocardium. (C) The relative expression of glutathione peroxidase 4 (Gpx4) in the myocardium. (D) The content of malondialdehyde (MDA) in the myocardium. Results are shown as means ± S.D. of six mice per group. * p < .05, ** p < .01, *** p < .001.
H&E staining and TEM experiments were performed to reveal the histological damage in mouse myocardium. DOX treatment caused the disorder of myocardial fibers and cell nuclei with some edema of cells. Less abnormalities in the cardiomyocyte structure were observed in the DOX and taurine treatment group (Fig. 4A). In addition, the myocardial fiber arrangement disorder, myofilament dissolution, and mitochondria swelling (73.5%) were observed in the myocardium treated with DOX, whereas the myocardial fibers had less myofilament dissolution and swollen mitochondria (33.3%) in the taurine and DOX group (Fig. 4B).

Bax, Bcl2, and cleaved caspase-3 protein levels were analyzed by scanning and normalized to GAPDH. (A) H&E staining of myocardium, Bar: 50 µm. (B) Electron micrographs of left ventricular myocardium, Bar: 20 µm. (C) Bax, Bcl2, and cleaved caspase-3 protein levels were assayed by Western blot and then analyzed by scanning and normalized to GAPDH. Results are shown as means ± S.D. of six mice per group. * p < .05, ** p < .01. (Color figure can be accessed in the online version.)
The further Western blot analysis showed that DOX increased cleaved caspase-3 level by 98.4% compared with the control group, but it decreased by 29.2% in mice treated with taurine. The Bax/Bcl2 ratio in the DOX treatment group was significantly increased by 67.2% compared with the control group, while it was decreased by 18.9% compared to the control after taurine supplementation (Fig. 4C, all p < 0.05). These data suggested that taurine reduces DOX-induced cardiotoxicity by inhibiting myocardial apoptosis and protecting the structure of the myocardium.
DOX is a kind of anticancer drug with cardiotoxicity, which causes myocardial damage by producing a large amount of free radicals.25) Excessive free radicals activate a series of apoptotic pathways and destroy the structure of mitochondria,26) thereby affecting the normal function of myocardium. To improve the safety of DOX in clinical application, continuous efforts have been made to overcome cardiotoxicity.27,28) Our results confirmed that taurine has potential protective effects on myocardium injury induced by DOX.23,28,29) The difference from the previous studies was that we examined the effects of exogenous taurine on myocardial damage induced by long-term accumulation of DOX, which made the experimental conditions in line with the clinically chronic DOX-induced cardiomyopathy patients.24) In addition, the study sought to investigate the connection of taurine and DOX in oxidative damage.
HPLC detected increased concentration of myocardial taurine after taurine feeding. Weight loss, which was evaluated after each dose of DOX, was significantly lower after the last dose, suggesting that DOX caused toxic damage. However, taurine supplementation had an inhibitory effect on weight loss in mice, which suggested the inhibitory effect of taurine on DOX toxicity.
Cardiomyopathy induced by DOX was demonstrated by an increase in serum levels of CK and LDH. The activities of CK and LDH are considered to be the indicators of injuries to myocardium.30,31) Changes in myocardial enzymes indicated that exogenous taurine could reduce myocardial injury induced by DOX.
The anti-oxidation system, which defends against oxidative stress, consists of enzymatic antioxidants and non-enzymatic antioxidants.32) This report reflected the body’s antioxidant capacity from these two aspects and measured oxidation products’ content to indicate the degree of oxidative damage. This study indicated that taurine acted on both enzymatic antioxidants and non-enzymatic antioxidants to enhance antioxidant capacity. In addition, taurine also had a role in scavenging the oxidation product MDA. This may be the main mechanism for taurine to recover the cardiotoxicity of DOX and this study provides more experimental data for clinical DOX medication.
Since tissue integrity is fundamental to cardiac function, HE staining and TEM of pathological sections were performed to show the destruction of the myocardial tissue after DOX-treated, while the pathological changes were significantly alleviated by taurine. According to our results, the main damage caused by DOX was the destruction of mitochondria and myofibrils, while taurine had a protective role in cardiomyocytes’ structure. Previous studies indicated that DOX produces reactive oxygen species in the cell, which causes the sarcoplasmic reticulum in the myocardial cell to release calcium ions, whose levels sharply increase, in turn activating the P53 protein in the myocardium and initiating a series of apoptosis programs.33) Therefore, from the aspect of apoptosis, the mechanism of cardiotoxicity caused by DOX in the taurine treatment was explored.24,28) As the results indicated, DOX led to an increase in the expression of caspase-3 in the apoptosis pathway and an increase in the Bax/Bcl-2 ratio, while exogenous supplementation of taurine effectively inhibited apoptosis. It showed that taurine also reduced the cardiotoxicity caused by doxorubicin by inhibiting apoptosis.
According to existing reports, taurine has been applied in various ways, including pretreatment,34) co-mixing, and post-drug application, and each has a different degree of DOX toxicity resistance.28,35) According to the non-toxic, heart-enriched, and cell-protective characteristics, taurine feeding and chronic DOX modeling were first used to investigate the protective effect against cardiotoxicity caused by DOX. In conclusion, taurine supplementation effectively alleviated the degree of myocardial injury, improved the antioxidant capacity of the myocardium, and reduced apoptosis during the DOX chronic myocardial injury. Although there are some shortcomings in the current study, such as the unknown optimal dose of taurine and the lack of experimental verification in cells, we advanced the understanding of the mechanism of taurine action and provided a more theoretical basis for the application of DOX and taurine.
The authors would like to thank all study participants who were enrolled in this study.
This work was supported by the Natural Science Foundation of China (31772692) and the Project of the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
The authors declare no conflict of interest.