2021 年 44 巻 5 号 p. 627-634
2021 年 44 巻 5 号 p. 627-634
Opioids are widely used for the treatment of moderate/severe pain in cancer and noncancer patients. In this study, we searched for safety signals for a wide variety of opioid-related adverse events (AEs) in elderly patients by disproportionality analysis using the Japanese Adverse Drug Event Report (JADER) database. Data from the JADER database from April 2004 to May 2018 were obtained from the Pharmaceuticals and Medical Devices Agency website. Safety signal detection of opioid-related AEs in elderly patients was defined using the relative elderly reporting odds ratio (ROR). Among the analyzed AEs, opioid-induced neurotoxicity (OIN) was assessed based on the time to onset using the Weibull shape parameter. The following safety signals were detected in elderly patients: respiratory depression, somnolence, hallucinations, akathisia and OIN. Fentanyl, tramadol, oxycodone and morphine exhibited a large relative elderly ROR for OIN. The median time to onset of OIN of transdermal fentanyl, oral tramadol, oral oxycodone and oral morphine was 13.5, 6, 9, and 6 d, respectively. These opioids were classified as early failure types using the Weibull distribution. Our results showed that elderly patients who are administered opioids should be closely monitored for AEs, such as respiratory depression, OIN and akathisia.
Pain strongly affects the QOL of patients. Cancer pain has been reported in 30 to 60% of patients.1) Opioids are widely used for the treatment of moderate/severe pain in cancer and noncancer patients. The most widely accepted algorithm for the treatment of cancer pain was developed by WHO.2,3) It suggests that patients with cancer pain should be treated with acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids. Opioids are effective for treating cancer and noncancer pain; however, adverse events (AEs) frequently occur with opioid therapy. Opioid-induced AEs include constipation, nausea, respiratory depression, delirium and somnolence.4) Furthermore, “opioid-induced neurotoxicity (OIN)” is a distressing symptom in palliative care patients receiving opioids.5) Symptoms of OIN include delirium, drowsiness, hallucinations, myoclonus and seizures.5) OIN was seen in 15% of cancer patients receiving opioids as part of inpatient palliative care.5) However, there have been only a limited number of studies on OIN.
AEs to prescribed medications among elderly patients are frequent causes of hospital admission and death.6,7) AEs were observed in 6 to 15% of hospitalized elderly patients in Japan.8) Polypharmacy and inappropriate prescriptions are known risk factors for AEs in elderly patients. To assess potential inappropriate prescriptions in elderly patients, the Screening Tool of Older People’s Prescriptions (STOPP) and the Screening Tool to Alert to Right Treatment (START) represent important criteria.9) For example, elderly people taking anticholinergic drugs are at increased risk for cognitive decline and dementia.10) The use of benzodiazepines is associated with falls in elderly patients.11) Thus, the possibility of AEs to prescription medications should be kept in mind when evaluating elderly patients.
The Japanese Adverse Drug Event Report (JADER) database of the Pharmaceuticals and Medical Devices Agency (PMDA) is a large spontaneous reporting system that reflects the realities of clinical practice in Japan. The JADER database is utilized to analyze the safety signal detection of AEs. The reporting odds ratio (ROR) is the odds ratio used to analyze safety signals that are important for the detection of AEs.12–15) Previous studies demonstrated that safety signals of respiratory depression were detected for opioids, such as fentanyl, morphine, oxycodone and pentazocine.15) Furthermore, morphine showed a large ROR with statistical significance in elderly (≥70 years old) patients.15) In a secondary analysis of a retrospective cohort study, the risk of respiratory depression increased substantially after 60 years of age.16) In a randomized double-blind placebo-controlled study, older (≥65 years old) participants receiving opioid therapy were more likely than those younger than 65 to report constipation, fatigue and anorexia.17) However, the risk of opioid-related AEs in elderly patients has not been fully investigated. In addition, data are lacking on the incidence of OIN. In this study, we searched for safety signals for the wide variety of opioid-related AEs in elderly patients by disproportionality analysis using the JADER database. Additionally, we analyzed the time to onset of OIN using the JADER database.
Data from the JADER database from April 2004 to May 2018 were obtained from the PMDA website. The database consists of four tables: patient demographic information (DEMO), drug information (DRUG), adverse reactions (REAC), and primary disease (HIST). These tables can be connected using the case ID number. The DEMO table was linked to the DRUG, REAC and HIST tables using the ID number. Elderly people are defined as individuals older than 65 years in general, but the ages of patients registered in the JADER database are provided in the data collected every 10 years. In this study, and similar to previous reports,18,19) we defined “elderly patients” as those ≥60 years old and “younger patients” as those <60 years old. We excluded patients for whom ages were unclear (“unknown,” “adult,” “elderly,” “youth,” “first trimester,” “second trimester,” “third trimester,” “pediatric,” “newborn,” “infant”). To exclude the influence of pediatric populations, we excluded patients who were younger than 20 years old. In the DRUG tables, the contribution of drug-related AEs was categorized into three codes: “suspected drug,” “concomitant drug,” and “interaction.” We only analyzed cases that were categorized as “suspected drug.”
Twelve opioids approved in Japan (fentanyl, tramadol, oxycodone, morphine, buprenorphine, pentazocine, codeine, tapentadol, pethidine, methadone, opium and hydromorphone) were selected as the drugs of interest in this study. “Total cases” was a case of “suspected drug” for any of the twelve opioids.
Data Selection of Opioid-Related AEsThe AEs names were defined using the Medical Dictionary for Regulatory Activities/Japanese version 21.1 (MedDRA/J). As opioid-related AEs, we extracted 286 preferred terms (PTs) and 5 standardized MedDRA queries (SMQs) (Table 1). The analyzed PTs were presented in ≥6 reports among the total cases.
| Category | Adverse event name |
|---|---|
| PT | (>100 reports) |
| “Delirium,” “Nausea,” “Vomiting,” “Respiratory depression,” “Anaphylactic shock,” “Altered state of consciousness,” “Loss of consciousness,” “Hepatic function abnormal,” “Somnolence,” “Depressed level of consciousness,” “Blood pressure decreased,” “Interstitial lung disease,” “Decreased appetite,” “Withdrawal syndrome,” “Drug dependence,” | |
| (10–99 reports) | |
| “Dizziness,” “Urinary retention,” “Liver disorder,” “Seizure,” “Bradycardia,” “Constipation,” “Cardiac arrest,” “Dyspnoea,” “Pneumonia,” “Ileus,” “Hallucination,” “Renal impairment,” “Fall,” “Hypotension,” “Drug eruption,” “Pyrexia,” “Platelet count decreased,” “Drug interaction,” “Malaise,” “Anaphylactic reaction,” “Pneumonia aspiration,” “Diarrhoea,” “Acute kidney injury,” “Heart failures,” “Ileus paralytic,” “Death,” “Oxygen saturation decreased,” “Respiratory arrest,” “White blood cell count decreased,” “Drug-induced liver injury,” “Anemia,” “Blood pressure increased,” “Respiratory failure,” “Hospitalisation,” “Rhabdomyolysis,” “Erythema multiforme,” “Drug withdrawal syndrome,” “Serotonin syndrome,” “Sepsis,” “Dependence,” “Toxic epidermal necrolysis,” “Dementia,” “Cerebral infarction,” “Shock,” “Stevens–Johnson syndrome,” “Neutrophil count decreased,” “Gastric ulcer,” “Intestinal obstruction,” “Atrioventricular block complete,” “Tremor,” “Restlessness,” “Dehydration,” “Rash,” “Anaphylactoid reaction,” “Dysphagia,” “Multiple organ dysfunction syndrome,” “Toxicity to various agents,” “Renal failure,” “Disseminated intravascular coagulation,” “Cardio-respiratory arrest,” “Pain,” “Neoplasm malignant,” “Road traffic accident,” “Hypoxia,” “Delayed recovery from anesthesia,” “Abnormal behavior,” “Blood alkaline phosphatase increased,” “Suicide attempt,” “Condition aggravated,” “Dyskinesia,” “Hallucination, visual,” “Coma,” “Confusional state,” “Renal disorder,” “Neuroleptic malignant syndrome,” “Large intestine perforation,” “Lung neoplasm malignant,” “Pulmonary oedema,” “Erythema,” “Anaphylactoid shock,” “Malignant neoplasm progression,” “Hepatic failure,” “Febrile neutropenia,” “Abdominal pain,” ”Gait disturbance,” “Asthenia,” “Hypertension,” “Asthma,” “Hypoglycemia,” “Hypoxic-ischemic encephalopathy,” “Aspartate aminotransferase increased,” “Liver function test abnormal,” “Muscular weakness,” “Headache,” “Hyperthermia malignant,” “Hyperkalemia,” “Urticaria,” “Electrocardiogram QT prolonged,” “Alanine aminotransferase increased,” “Dysarthria,” “Arrhythmia,” “Arteriospasm coronary,” “Ventricular fibrillation,” “Depression,” “Myoclonus,” “Pleural effusion,” “Hepatitis fulminant,” “Weight decreased,” “Dysuria,” “Pancytopenia,” “Ventricular tachycardia,” “Hypercapnia,” “Rash generalized,” “Pulmonary embolism,” “Gamma-glutamyltransferase increased,” “Feeling abnormal,” “Overdose,” “Thrombocytopenia,” “Hallucination, auditory,” “Insomnia,” “Oedema peripheral,” “Bronchospasm,” “Toxic skin eruption,” “Acute respiratory failure,” “Pancreatitis acute,” “Blood creatinine increased,” “Syncope,” “Sedation complication,“ “Hyponatremia,” “Septic shock,” “Gastric cancer,” “Stomatitis,” “Neutropenia,” “Gastric ulcer hemorrhage,” “Atrioventricular block second degree,” “Leukopenia,” “Generalised erythema,” “Sinus arrest,” “Atrial fibrillation,” “Blood creatine phosphokinase increased,” “Disease progression,” “Heart rate decreased,” “Glossoptosis,” “Hemoglobin decreased,” “Hypoesthesia,” “Hepatic encephalopathy,” “Drug reaction with eosinophilia and systemic symptoms,” “International normalised ratio increased,” ”Laryngeal oedema,“ “Epilepsy,” “Atrioventricular block,” “Aspiration,” “Gastrointestinal hemorrhage,” “Jaundice,” “Blood potassium increased,” “Blood urea increased,” “Duodenal ulcer,” “Hyperhidrosis,” “Weight increased,” “Cognitive disorder,” “Fatigue,” “Delusion,” “Hypoventilation,” “Musculoskeletal stiffness,” “Prinzmetal angina,” “Hypersensitivity,” “Acute myocardial infarction,” “C-reactive protein increased,” “Melena,” ”Liver function test increased,“ “Acute generalised exanthematous pustulosis,” “Inappropriate antidiuretic hormone secretion,” ”Bone marrow failure,“ “Palpitations,” “Pneumonitis,” | |
| (6–9 reports) | |
| “Tachycardia,” “Stress cardiomyopathy,” “Circulatory collapse,” “Akathisia,” “Pruritus,” “Cardiac failure acute,” “Hemophagocytic lymphohistiocytosis,” “Incontinence,” “General physical health deterioration,” “Herpes zoster,” “Cholecystitis,” “White blood cell count increased,” “Hemorrhage subcutaneous,” “Peritonitis,” “Hepatic enzyme increased,” “Myocardial infarction,” “Generalised oedema,” “Adrenal insufficiency,” “Parkinsonism,” “Gastroenteritis,” “Posterior reversible encephalopathy syndrome,” “Cholecystitis acute,” “Chest discomfort,” “Hyperglycemia,” “Fracture,” “Suicidal ideation,” “Disorientation,” “Neurogenic bladder,” “Spinal compression fracture,” “Prostate cancer,” “Cholangitis,” “Enterocolitis,” “Hypokalemia,” “Deafness,” “Urinary tract infection,” “Cerebral hemorrhage,” “Lung disorder,” “Neuropathy peripheral,” “Dysgeusia,” “Agranulocytosis,” “Rib fracture,” “Atelectasis,” “Blood pressure systolic decreased,” “Post procedural complication,” “Arthralgia,” “Cancer pain,” “Pleurisy,” “Colon cancer,” “Thrombotic thrombocytopenic purpura,” “Eosinophilic pneumonia,” “Hypercalcemia,” “Pain in extremity,” “Hemorrhage,” “Abdominal pain upper,” “Deep vein thrombosis,” “Red blood cell count decreased,” “Monoplegia,” “Sudden death,” “Breast cancer,” “Oculomucocutaneous syndrome,” “Paralysis,” “Chills,” ”Apnoea,” “Sinus node dysfunction,” “Pulseless electrical activity,” “Tonic convulsion,” “Sinus bradycardia,” “Irritability,” “Transient ischemic attack,” “Hypophagia,” “Surgery,” “Rheumatoid arthritis,” “Memory impairment,” “Angina pectoris,” “Chest pain,” “Myalgia,” “Angioedema,” “Hematuria,” “Amnesia,” “Respiratory distress,” “Respiratory rate decreased,” “Subdural hematoma,” “Hyperpyrexia,” “Gastrointestinal perforation,” “Neoplasm progression,” “Extrapyramidal disorder,” “Mental disorder,” “Dermatitis contact,” “Secondary hypogonadism,” “Paraplegia,” “Hyperesthesia,” “Pneumatosis intestinalis,” “Tubulointerstitial nephritis,” “Skin necrosis,” “Anxiety,” “Ascites,” “Abdominal distension,” “Gait inability,” “Hemolytic anemia,” | |
| SMQ | “Anaphylactic reaction,” “Hepatic failure, fibrosis and cirrhosis and other liver damage-related conditions,” “Acute central respiratory depression,” “Acute renal failure,” “Interstitial lung disease,” |
| Other | Opioid-induced neurotoxicity (including “Delirium,” “Hallucination,” “Somnolence,” “Hyperesthesia,” “Epilepsy,” “Seizure,” “Myoclonus”) |
In addition, in this study, we defined OIN as being present in reports including “Delirium,” “Hallucination,” “Somnolence,” “Hyperesthesia,” “Epilepsy,” “Seizure” and “Myoclonus” (Table 1).
Definition of Cancer PatientsThe primary diseases in the HIST tables are based on the MedDRA. In this study, and similar to previous reports,15) we extracted 1665 malignant PTs from 19 SMQs and defined them as cancer patients: malignancy-related conditions (SMQ 20000092), tumor markers (SMQ 20000094), biliary malignant tumors (SMQ 20000196), biliary tumors of unspecified malignancy (SMQ 20000197), breast malignant tumors (SMQ 20000198), breast tumors of unspecified malignancy (SMQ 20000199), ovarian malignant tumors (SMQ 20000200), ovarian tumors of unspecified malignancy (SMQ 20000201), prostate malignant tumors (SMQ 20000202), prostate tumors of unspecified malignancy (SMQ 20000203), skin malignant tumors (SMQ 20000204), skin tumors of unspecified malignancy (SMQ 20000205), uterine and fallopian tube malignant tumors (SMQ 20000206), uterine and fallopian tube tumors of unspecified malignancy (SMQ 20000207), liver malignant tumors (SMQ 20000208), liver tumors of unspecified malignancy (SMQ 20000209), malignant lymphomas (SMQ 20000215), hematological malignant tumors (SMQ 20000227), nonhematological malignant tumors (SMQ 20000228).
Safety Signal DetectionThe ROR was calculated using two-by-two contingency tables as follows: a: the number of patients experiencing the AE of interest after using opioids, b: the number of patients experiencing all other AEs after using opioids, c: the number of patients experiencing the AE of interest after using other drugs, and d: the number of patients experiencing all other AEs after using other drugs. The safety signal was considered significant if the lower limit of the 95% confidence interval (CI) exceeded 1:
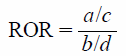 |
Safety signal detection in elderly patients was defined using the relative elderly ROR reported by the European Medicines Agency.20) The relative elderly ROR was calculated as follows:
 |
When the lower limit of the 95% CI of the RORelderly and relative elderly ROR was greater than 1, it was defined as a safety signal in elderly patients. Data analyses were performed using JMP, version 5.0.1 (SAS Institute Inc., Cary, NC, U.S.A.).
Time-to-Onset AnalysisThe median duration, quartiles and Weibull shape parameter were utilized in the evaluations of the time to onset of OIN. The Weibull shape parameter test is used for statistical analysis of time-to-onset data and can describe the nonconstant rate of the incidence of AEs.15) The time to onset from the JADER database was calculated from the time of the patient’s first prescription to the occurrence of the AEs. Patients with complete AE occurrence and prescription start date data were used for the time-to-onset analyses. When the onset time of the AEs was 365 d or longer after the initiation of administration, the calculation was performed using 365 d as the onset time. The scale parameter α of the Weibull distribution determines the scale of the distribution function. A larger scale value stretches the distribution. A smaller scale value shrinks the data distribution. The shape parameter β of the Weibull distribution indicates the hazard without a reference population. When β is equal to 1, the hazard is estimated to be constant over time. When β is greater than 1 and the 95% CI of β excludes 1, the hazard is considered to increase over time. When β is less than 1 and the 95% CI of β excludes 1, the hazard is considered to decrease over time. Time-to-onset analyses were performed using JMP, version 5.0.1 (SAS Institute Inc.).
The JADER database contains 526210 suspected drug cases from April 2004 to May 2018. After patients with unclear ages and patients younger than 20 years were excluded, 445532 cases (5653 total cases and 439879 nonopioid cases) were analyzed (Table 2). Opioid-related AEs were noted in 5653 cases, including 3881 cases involving elderly patients and 1772 cases involving younger patients. Among 445532 cases, cancer patients represented 113182 cases (2165 total cases and 111017 nonopioid cases). Regarding 5653 opioid-related AEs, 2165 (38.3%) cancer patients were included: 1601 elderly patients and 564 younger patients.
| All cases | Cancer patients | |||||
|---|---|---|---|---|---|---|
| Total | Elderly | Younger | Total (proportion*) | Elderly | Younger | |
| Total cases | 5653 | 3881 | 1772 | 2165 (38.3%) | 1601 | 564 |
| Fentanyl | 1761 | 1121 | 640 | 787 (44.7%) | 582 | 205 |
| Tramadol | 1637 | 1275 | 362 | 203 (12.4%) | 167 | 36 |
| Oxycodone | 733 | 537 | 196 | 659 (89.9%) | 485 | 174 |
| Morphine | 507 | 331 | 176 | 355 (70.0%) | 251 | 104 |
| Buprenorphine | 422 | 305 | 117 | 34 (8.1%) | 22 | 12 |
| Pentazocine | 308 | 145 | 163 | 71 (23.1%) | 55 | 16 |
| Codeine | 290 | 138 | 152 | 32 (11.0%) | 18 | 14 |
| Tapentadol | 143 | 109 | 34 | 131 (91.6%) | 99 | 32 |
| Pethidine | 98 | 72 | 26 | 22 (22.4%) | 18 | 4 |
| Methadone | 56 | 27 | 29 | 56 (100%) | 27 | 29 |
| Opium | 12 | 9 | 3 | 8 (66.7%) | 6 | 2 |
| Hydromorphone | 4 | 3 | 1 | 3 (75.0%) | 2 | 1 |
| Nonopioid cases | 439879 | 295128 | 144751 | 111017 (25.2%) | 81926 | 29091 |
*Percentages indicate the number of cancer patients to the number of all cases.
Table 3 shows opioid-related AEs for which safety signals were detected in elderly patients. The following safety signals were detected in elderly patients: “Respiratory depression,” “Somnolence,” “Hallucination,” “Anaphylactoid reaction,” “Suicide attempt,” “Large intestine perforation,” “Liver function test abnormal,” “Overdose,” “Hallucination, auditory,” “Sedation complication,” “Akathisia,” “Acute central respiratory depression” and OIN (Table 3). Three safety signals were detected for fentanyl: “Anaphylactoid reaction” (relative elderly ROR: 3.8, 95% CI: 1.2–12.1), “Overdose” (relative elderly ROR: 8.7, 95% CI: 1.7–45.9) and OIN (relative elderly ROR: 1.7, 95% CI: 1.2–2.4). A safety signal of OIN (relative elderly ROR: 1.6, 95% CI: 1.0–2.4) was detected for tramadol. A safety signal of OIN (relative elderly ROR: 1.8, 95% CI: 1.1–2.8) was detected for oxycodone. Four signals were detected for morphine: “Respiratory depression” (relative elderly ROR: 3.5, 95% CI: 1.5–8.1), “Somnolence” (relative elderly ROR: 5.5, 95% CI: 1.3–24.1), “Acute central respiratory depression” (relative elderly ROR: 2.5, 95% CI: 1.2–5.3) and OIN (relative elderly ROR: 2.6, 95% CI: 1.5–4.7). A safety signal of “Respiratory depression” (relative elderly ROR: 4.8, 95% CI: 1.7–13.4) was detected for pentazocine.
| Elderly patients | Younger patients | Relative elderly ROR | ||||||
|---|---|---|---|---|---|---|---|---|
| ROR | 95% CI | ROR | 95% CI | ROR | 95% CI | |||
| PT | Respiratory depression | Total cases | 78.9 | 65.6–97.1 | 43.0 | 31.6–58.4 | 1.9 | 1.3–2.7 |
| Morphine | 117.5 | 83.7–165.0 | 33.6 | 15.6–72.6 | 3.5 | 1.5–8.1 | ||
| Pentazocine | 122.1 | 75.4–197.9 | 25.4 | 10.3–62.7 | 4.8 | 1.7–13.4 | ||
| Somnolence | Morphine | 29.2 | 18.3–46.8 | 5.3 | 1.3–21.5 | 5.5 | 1.3–24.1 | |
| Hallucination | Total cases | 5.9 | 4.5–7.7 | 2.7 | 1.3–5.4 | 2.2 | 1.0–4.7 | |
| Anaphylactoid reaction | Total cases | 1.6 | 1.0–2.6 | 0.6 | 0.3–1.2 | 2.9 | 1.2–6.9 | |
| Fentanyl | 3.5 | 1.9–6.3 | 0.9 | 0.3–2.4 | 3.8 | 1.2–12.1 | ||
| Suicide attempt | Total cases | 2.9 | 1.5–5.6 | 1.0 | 0.6–1.8 | 2.7 | 1.2–6.5 | |
| Large intestine perforation | Total cases | 1.7 | 1.1–2.7 | 0.2 | 0.03–1.5 | 8.4 | 1.1–62.8 | |
| Liver function test abnormal | Total cases | 4.8 | 2.9–7.9 | 0.9 | 0.2–3.8 | 5.0 | 1.1–22.2 | |
| Overdose | Total cases | 7.7 | 4.0–14.8 | 1.3 | 0.6–3.2 | 5.8 | 1.9–17.4 | |
| Fentanyl | 12.8 | 5.2–31.5 | 1.5 | 0.4–5.9 | 8.7 | 1.7–45.9 | ||
| Hallucination, auditory | Total cases | 6.6 | 3.8–11.7 | 1.0 | 0.2–4.0 | 6.8 | 1.5–30.5 | |
| Sedation complication | Total cases | 15.7 | 8.7–28.6 | 1.8 | 0.2–12.9 | 8.9 | 1.1–70.2 | |
| Akathisia | Total cases | 8.2 | 3.5–19.0 | 1.9 | 0.6–6.1 | 4.2 | 1.0–17.5 | |
| SMQ | Acute central respiratory depression | Morphine | 24.3 | 18.0–32.9 | 9.6 | 4.9–18.9 | 2.5 | 1.2–5.3 |
| Other | OIN | Total cases | 9.3 | 8.5–10.3 | 4.7 | 4.0–5.6 | 2.0 | 1.6–2.4 |
| Fentanyl | 8.0 | 6.7–9.6 | 4.6 | 3.5–6.1 | 1.7 | 1.2–2.4 | ||
| Tramadol | 6.6 | 5.5–7.9 | 4.3 | 2.9–6.2 | 1.6 | 1.0–2.4 | ||
| Oxycodone | 15.0 | 12.1–18.5 | 8.5 | 5.7–12.6 | 1.8 | 1.1–2.8 | ||
| Morphine | 12.8 | 9.7–17.0 | 4.9 | 2.9–8.2 | 2.6 | 1.5–4.7 | ||
95% CI: 95% confidence interval, OIN: opioid-induced neurotoxicity.
The number of OIN cases and the proportion of cancer patients for each opioid were as follows: total cases (663 cases and 56.1%), fentanyl (192 cases and 62.0%), tramadol (160 cases and 15.0%), oxycodone (140 cases and 93.6%), morphine (77 cases and 83.1%), buprenorphine (37 cases and 5.4%), pentazocine (21 cases and 14.3%), codeine (13 cases and 0%), tapentadol (48 cases and 95.8%), pethidine (4 cases and 50.0%), methadone (13 cases and 100%), opium (0 case and 0%), and hydromorphone (0 case and 0%).
OIN was exclusively analyzed in cancer patients (Table 4). In the total cases, oxycodone and morphine produced a safety signal of OIN in elderly cancer patients. The relative elderly ROR values of total cases, oxycodone and morphine were 2.0 (95% CI: 1.5–2.7), 1.9 (95% CI: 1.1–3.1) and 2.7 (95% CI: 1.3–5.5), respectively.
| Elderly patients | Younger patients | Relative elderly ROR | ||||
|---|---|---|---|---|---|---|
| ROR | 95% CI | ROR | 95% CI | ROR | 95% CI | |
| Total cases | 25.0 | 21.6–28.8 | 12.6 | 9.6–16.6 | 2.0 | 1.5–2.7 |
| Fentanyl | 15.8 | 12.5–19.9 | 12.5 | 8.2–18.9 | 1.3 | 0.8–2.0 |
| Tramadol | 12.0 | 7.6–18.8 | 4.5 | 1.1–19.0 | 2.6 | 0.6–11.8 |
| Oxycodone | 24.2 | 19.4–30.3 | 13.1 | 8.4–20.3 | 1.9 | 1.1–3.1 |
| Morphine | 22.3 | 16.4–30.3 | 8.4 | 4.3–16.2 | 2.7 | 1.3–5.5 |
| Buprenorphine | —* | —* | — | |||
| Pentazocine | 4.5 | 1.4–14.3 | —* | — | ||
| Codeine | —* | —* | — | |||
| Tapentadol | 47.8 | 31.7–72.1 | 30.8 | 14.1–67.0 | 1.6 | 0.6–3.7 |
| Pethidine | 9.7 | 2.2–42.2 | —* | — | ||
| Methadone | 27.3 | 11.5–64.6 | 20.4 | 8.2–50.3 | 1.3 | 0.4–4.7 |
| Opium | —* | —* | — | |||
| Hydromorphone | —* | —* | — | |||
*Number of cases <2. 95% CI: 95% confidence interval, OIN: opioid-induced neurotoxicity.
Many of the symptoms of OIN included “Delirium,” “Somnolence” and “Hallucination” (Fig. 1a). The most common route of administration for fentanyl was transdermal. Tramadol, oxycodone and morphine were commonly administered orally (Fig. 1b). The time to onset of OIN was profiled using the Weibull distribution. For the time-to-onset analysis, we extracted combinations for which complete information regarding the date of treatment initiation and the date of AE onset were available. Younger patients had few cases of complete information and thus were not analyzed. The median time to onset of OIN for transdermal fentanyl, oral tramadol, oral oxycodone and oral morphine was 13.5, 6, 9, and 6 d, respectively (Table 5). The β values of transdermal fentanyl, oral tramadol, oral oxycodone and oral morphine were 0.6 (95% CI: 0.5–0.8), 0.6 (95% CI: 0.5–0.7), 0.6 (95% CI: 0.5–0.7) and 0.6 (95% CI: 0.5–0.8), respectively. Figure 2 presents a histogram of the number of cases of OIN between 0 and 100 d. The peaks of reports for any opioids were all within 5 d.

(a) Number of individual adverse event of OIN. (b) Number of route of opioid administration.
| Drugs | Case reports | Median (d) | Lower quartile (d) | Upper quartile (d) | Minimum (d) | Maximum (d) | Scale parameter | Shape parameter | Pattern | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| α | 95% CI | β | 95% CI | ||||||||
| Transdermal fentanyl | 60 | 13.5 | 4 | 31 | 1 | 858 | 28.5 | 18.5–43.4 | 0.6 | 0.5–0.8 | Early failure |
| Oral tramadol | 69 | 6 | 3 | 15 | 1 | 474 | 18.6 | 11.8–28.9 | 0.6 | 0.5–0.7 | Early failure |
| Oral oxycodone | 50 | 9 | 4 | 33.5 | 1 | 1268 | 26.4 | 15.8–43.2 | 0.6 | 0.5–0.7 | Early failure |
| Oral morphine | 29 | 6 | 3 | 14 | 1 | 208 | 19.2 | 9.8–36.7 | 0.6 | 0.5–0.8 | Early failure |
OIN: opioid-induced neurotoxicity.

(a) Transdermal fentanyl. (b) Oral tramadol. (c) Oral oxycodone. (d) Oral morphine.
In this study, we searched for safety signals of the wide variety of opioid-related AEs in elderly patients by disproportionality analysis using the JADER database. Additionally, we analyzed the time to onset of OIN using the JADER database. Our results suggest that several safety signals were detected for opioids in elderly patients. A safety signal of OIN was detected for total cases, fentanyl, tramadol, oxycodone and morphine in elderly patients. Among these drugs, a safety signal of OIN was detected for total cases, oxycodone and morphine in elderly cancer patients. Furthermore, we demonstrated that OIN tended to occur early after the initiation of opioid administration to elderly patients.
In general, the risk of AEs is higher in elderly patients than in younger patients.21) Previous reports showed that AEs are frequent in patients ≥65 years old.22,23) Elderly patients exhibit reduced hepatic and renal function, which can affect drug pharmacokinetics and pharmacodynamics.24) Furthermore, the risk of AEs in elderly patients increases with comorbidity, polypharmacy, and inappropriate prescribing.21) The package inserts of opioids describe risks for respiratory depression in elderly patients, but information regarding other AEs is unclear. The risk of opioid-related AEs in elderly patients is not defined by the STOPP criteria. Opioids are needed to control moderate/severe pain in cancer and noncancer elderly patients. Consequently, our results have important implications for elderly patient management.
Recent reports have described the risk of AEs using the JADER database in elderly patients. Sugawara et al. reported that the safety signal of respiratory depression was detected for opioids in elderly patients.15) Chisaki et al. reported 27 combinations of drugs and AEs with an increased risk in elderly patients over 70 years old compared with younger patients.25) Hatahira et al. reported that several drugs (such as calcium channel blockers, benzodiazepines, and drugs for herpes zoster virus infection) and increased patient age are both associated with fall-related AEs.26)
This is the first study to detect a wide variety of safety signals for opioids in elderly patients using the JADER database and RORs. From a disproportionality analysis using the Food and Drug Administration Adverse Event Reporting System (FAERS) database, Andreaggi et al. reported that opioid related-depression and suicide self-injury were more likely to be reported for ages ≥65 compared to age group 18 to 64.27) In our analysis using relative elderly ROR, the safety signal of suicide attempt was detected in the total cases, while depression was not detected. However, the RORelderly and RORyounger values for depression were 2.8 (95% CI: 1.6–5.0) and 0.9 (95% CI: 0.4–2.5), respectively. Furthermore, the relative elderly ROR of depression was not significant but was high at 3.0 (95% CI: 0.96–9.4). Depression in older adults is associated with an increased risk of morbidity and suicide.28) On the other hand, depression is common in patients with pain, especially cancer patients. It is unclear whether depression and suicide attempts are associated with AEs of opioids. However, we need to monitor the mental health of elderly patients who are administered opioids.
Notably, several central nervous system (CNS) safety signals were detected, such as OIN and akathisia. Zedler et al. reported that a risk factor for serious opioid-related respiratory or CNS depression was age ≥55 years old.29) Albrecht et al. reported increased sensitivity of the CNS to midazolam in elderly subjects.30) Similarly, increased sensitivity of the CNS to opioids is also assumed in elderly patients.
In this study, four safety signals were detected for morphine. Morphine is converted to the morphine-6-glucuronide (M6G) metabolite, which has analgesic activity. After conversion, M6G is excreted in the urine. Renal function is reduced with age, and M6G can accumulate to higher levels in elderly patients.31) Three safety signals were detected for fentanyl. Previous studies demonstrated that serum fentanyl concentrations were higher in elderly patients than in younger patients.32) Decreased fentanyl clearance in elderly patients might be a result of several factors, including decreases in hepatic blood flow or hepatic microsomal enzyme activity, or increases in drug protein binding.32) Similarly, oxycodone and tramadol clearance decrease in elderly patients and patients with renal dysfunction.31) In addition to pharmacokinetic changes, enhanced pharmacodynamic sensitivity is seen with opioids in elderly patients.31) In this study, safety signals of an overdose in elderly patients were detected for total cases and fentanyl. Therefore, the use of these opioids for elderly patients may increase the risk of AE occurrence due to changes in their pharmacokinetics and pharmacodynamics.
OIN is a distressing condition seen in palliative care patients receiving opioids. Symptoms of OIN include delirium, hallucinations, somnolence, hyperesthesia, epilepsy, seizures and myoclonus. Risk factors for OIN include escalating doses of opioids, dehydration, renal failure, end-stage disease and advanced age.33) On the other hand, the risk of OIN in elderly patients has not previously been assessed in an observational or database study. The primary treatment for OIN is hydration, dose reduction or discontinuation of opioids and opioid rotation. Failure to appropriately diagnose and treat OIN will lead to poor symptom control and the potential for seizures.34) We found that the safety signal of OIN was detected in elderly patients. The most common symptoms of OIN cases were delirium, somnolence and hallucination. This result is consistent with a previous report.5) The most common routes of administration of fentanyl and the other three drugs (tramadol, morphine and oxycodone) in the OIN cases were transdermal and oral, respectively. This result was considered to reflect the typical dosage form of each drug used in Japan.
Furthermore, we evaluated the time to onset of OIN using Weibull distribution parameters. The usefulness of the Weibull distribution for profiling the time to the onset of AEs has been reported.12,15,35) Sugawara et al. reported that the time to onset of respiratory depression associated with opioids was classified as the early failure type.15) Similarly, our results indicated that transdermal fentanyl, oral tramadol, oral oxycodone and oral morphine-induced neurotoxicity were early failure types. Opioid receptors (mu-, delta-, kappa-opioid receptors) exist throughout the central and peripheral nervous systems and are linked to a variety of neurotransmitters. Opioids have immediate clinical effects by directly stimulating those receptors. Similarly, opioid-related AEs, such as OIN, may develop almost immediately by directly stimulating those receptors. Oral tramadol and oral morphine-induced neurotoxicity were reported in 75% of patients within approximately 2 weeks after initiating their use. Transdermal fentanyl and oral oxycodone-induced neurotoxicity were reported in 75% of patients within approximately one month and exhibited delayed onset compared to oral tramadol and oral morphine neurotoxicity. Our results suggest that neurotoxicity, including delirium, hallucinations and somnolence, should be carefully monitored for the first month and especially the first 5 d, among patients who are administered opioids.
Furthermore, we defined cancer patients based on MedDRA considering the confounding nature of classification by indications. The results showed that a safety signal of OIN was detected in elderly cancer patients prescribed oxycodone and morphine. When these drugs are administered for the management of cancer pain in elderly patients, the potential for neurotoxicity should be carefully assessed. On the other hand, a safety signal of OIN was not detected in elderly cancer patients administered fentanyl and tramadol. The number of AEs for these two drugs was reduced based on an exclusive analysis of cancer patients. Subgroup analyses showed benefits in both sensitivity and precision over crude analyses for the larger databases; however, for the smaller databases, a gain in precision tended to result in some loss of sensitivity.36) We believe that the fentanyl and tramadol results were affected by the decreased number of patients based on the exclusive analysis of cancer patients. Delirium is the most common neuropsychiatric complication observed in patients with cancer.37) The influence of disease on OIN requires further investigation.
Our study has several limitations. Spontaneous reporting systems, such as the JADER database, are associated with various biases, including overreporting, underreporting, missing data, and the lack of a denominator.12–15) In addition, we did not evaluate drug–drug interactions and opioid dose. The time to onset of OIN in young patients has not been investigated and was not compared with elderly patients. In this study, “total cases” referred to a collection of 12 opioids. However, it is likely that each opioid has different patterns of AEs in elderly patients. We have not been able to consider why some safety signals were detected, such as anaphylactoid reaction and large intestine perforation. Finally, the ROR indicates an increased risk of AE reporting and not a risk of AE occurrence.15) Further studies, such as observational studies, are needed to evaluate the risk of opioid-related AEs in elderly patients.
In summary, this study was the first to evaluate the association between opioids and a wide variety of AEs in elderly patients using the JADER database and the relative elderly ROR. We demonstrated that opioid-related AEs, such as respiratory depression, OIN and akathisia, in patients ≥60 years old, are potentially increased compared to those in patients <60 years old. Our results showed that elderly patients who are administered opioids should be closely monitored for AEs.
The authors declare no conflict of interest.