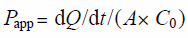Abstract
In vitro transport studies across cells grown on culture inserts are widely used for evaluating pharmacokinetic characteristics such as intestinal membrane permeability. However, measurements of the apparent permeability coefficient of highly lipophilic compounds are often limited by transport across the membrane filters, not by transport across the cultured cells. To overcome this concern, we have investigated the utility of a high-porosity membrane honeycomb film (HCF) for transcellular transport studies. Using the HCF inserts, the apparent permeability coefficient (Papp) of the drugs tested in LLC-PK1 and Caco-2 cells tended to increase with an increase in lipophilicity, reaching a maximum Papp value at Log D higher than 2. In contrast, using the commercially available Track-Etched membrane (TEM) inserts, a maximum value was observed at Log D higher than 1. The basolateral to apical transport permeability Papp(BL→AP) of rhodamine 123 across LLC-PK1 cells that express P-glycoprotein (P-gp) cultured on HCF inserts and TEM inserts was 2.33 and 2.39 times higher than the reverse directional Papp(AP→BL) permeability, respectively. The efflux ratio (Papp(B-A)/Papp(A-B)) of rhodamine 123 in LLC-PK1 expressing P-gp cells using HCF inserts was comparable to that obtained using TEM inserts, whereas the transported amount in both directions was significantly higher when using the HCF inserts. Accordingly, due to the higher permeability and high porosity of HCF membranes, it is expected that transcellular transport of high lipophilic as well as hydrophilic compounds and substrate recognition of transporters can be evaluated more accurately by using HCF inserts.
INTRODUCTION
In vitro transcellular transport studies evaluate drug transport across cell monolayers cultured on cell culture inserts and are widely used in the drug discovery process for the prediction of intestinal drug absorption,1) renal clearance,2) and drug distribution into the brain.3) In particular, intestinal Caco-2 cells are used in high throughput transcellular transport assays to evaluate the intestinal permeability of orally active drug candidates, mainly because membrane permeability in this system is well correlated with intestinal absorption in human.1) In addition, carrier-mediated transport of drugs is often evaluated using cells expressing uptake and efflux transporters. For example, transport assays have been used to determine the substrates of the membrane transporter P-glycoprotein (P-gp, ABCB1), which is localized at the apical membrane of enterocytes and restricts intestinal absorption of a wide range of structurally diverse compounds by excreting drugs out of the cells.4) Likewise, canine kidney MDCKII cells co-expressing both uptake and efflux transporters were used to evaluate transcellular transport across epithelial cells that mimic renal reabsorption and hepatobiliary excretion.5–7)
As detailed above, in vitro transcellular transport assays are invaluable for the evaluation of pharmacokinetic characteristics. However, transport assays which use inserts to culture such cells have their limitations. Cell membrane permeability of drugs is dependent on lipophilicity, exhibiting a maximum when lipophilicity of the drug is high, over Log D = 0.1) Use of the maximum permeability coefficient may lead to an inadequate evaluation of drug absorption in physiologically-based pharmacokinetic model, where the absorption phase is evaluated using the apparent permeability coefficient (Papp) value obtained by an in vitro study, resulting in gaps from drug plasma concentration observed in vivo.8) In addition, the maximum permeability coefficient may affect substrate recognition of membrane transporters.
During a transcellular transport study, transfer of test compounds from the donor side to the receiver side involves not only transfer through the cell monolayer, but also transfer through an unstirred water layer. Accordingly, when cell membrane permeability is high enough as is often the case with lipophilic drugs, permeability across unstirred water layer potentially become a rate-determining step.9) In addition, it may be necessary to consider the permeability across the filter membrane when assessing the membrane permeability of highly lipophilic compounds.
Many cell culture inserts currently utilize a Track-Etched membrane (TEM) made of polycarbonate or polyethylene terephthalate. The advantages of these membranes include toughness, stiffness, and transparency with outstanding impact resistance. However, the porosity of commercially available products is approximately 0.8% for a 0.4 µm pore size membrane and 8.8% for a 3.0 µm pore size membrane.10) Thus, the apparent permeation rate of highly permeable compounds may be a poor estimation. A more accurate estimation of permeability may only be obtained by substitution with a membrane with a higher porosity. To address this issue, cell culture inserts can be fabricated from a honeycomb film (HCF) made of amphiphilic polymers using a simple solution-casting process.11–13) An HCF is characterized by honeycomb-formation pores of uniform diameter and high porosity. Moreover, the pore size and thickness can be easily regulated. Culture fibroblasts, vascular endothelial cells, HeLa cells, and hepatocytes are all easily cultured on HCF.14–17) In addition, HCF may promote tissue maturation by cell–cell interaction when co-cultured with cells on the other side of the film. In cell-based assays, the superior porosity and thickness of HCF can be expected to reduce any rate-determining effects of the filter membrane compared to conventional TEM such as Transwell®. In the present study, the utility of the HCF inserts for a transcellular study was investigated.
MATERIALS AND METHODS
MaterialsTranswell® permeable inserts made of polyethylene terephthalate (pore size 3.0 µm) were purchased from Corning (Corning, NY, U.S.A.). Rhodamine 123 and lucifer yellow CH di-lithium salt were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.) and FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan), respectively. LLC-PK1 cells expressing human P-gp (LLC-PK1/P-gp) and mock cells2,14) were obtained from Riken Cell Bank (Tsukuba, Japan). Caco-2 cells were obtained from the American Type Culture Collection (Manassas, VA, U.S.A.). All other chemicals were of reagent grade.
Preparation of HCF Cell Culture InsertsHCF made of polycarbonate (PC) was prepared by a simple solution casting process in FUJIFILM Corp. (Odawara, Japan). A polymer solution was cast onto a glass substrate under humidified conditions. Water droplets were generated by condensation at the interface of the solution with humidified air. During subsequent drying of the polymer solution, a honeycomb-patterned water droplet array was induced by surface tension. Once the drying procedure was complete, pores were formed in the polymer film by the process of water droplet drying. Amphiphilic polymer (CAP) was synthesized by copolymerizing N-dodecylacrylamide and 6-acylamidehexanoic acid. PC and CAP were subsequently dissolved in chloroform (mixing weight ratio of PC/CAP: 10/1) to a final concentration of 1–5 mg/mL. HCF (pore size, 3.0 µm; porosity, 50%) were then fabricated using experimental apparatus controlling casting and drying conditions.12) Microscopic images of HCF and Transwell® membranes were obtained by confocal microscopy (VK-8710, Keyence, Osaka, Japan), as shown in Fig. 1. Finally, the HCF was attached to a cell culture insert (surface area, 0.33 cm2) for use in a 24-well plate.
Cell CultureLLC-PK1/P-gp and mock cells were cultured in Medium 199 containing 10% fetal bovine serum (FBS), 100 units/mL penicillin, 100 µg/mL streptomycin, and 500 µg/mL G418 at 37 °C in an atmosphere of 5% CO2. For transcellular transport studies, HCF inserts were coated with type I-C collagen (Cellmatrix Type I-C, Nitta Gelatin, Osaka, Japan). LLC-PK1/P-gp and mock cells were seeded at 0.9 or 1.8 × 105 cells/cm2 on HCF inserts in a 24-well plate. These cells were then cultured for 5, 6, 7, and 8 d. For the assay using the TEM inserts, LLC-PK1/P-gp and mock cells were seeded at 3.6 × 105 cells/cm2 on Transwell® inserts (pore size, 3.0 µm; surface area, 0.9 cm2) in a 12-well plate and cultured for 6 d. Culture medium was replaced with fresh medium at 3 and 5 d after seeding.
Caco-2 cells were cultured in Dulbecco’s Modified Eagle’s Medium containing 10% FBS, 1% non-essential amino acids, 2 mM glutamine, 100 units/mL penicillin, 100 µg/mL streptomycin, and 500 µg/mL G418 at 37 °C in an atmosphere of 5% CO2. For transcellular transport assays, Caco-2 cells were seeded at 2.6 × 105 cells/cm2 on collagen I-C-coated HCF inserts in a 24-well plate or on Transwell® in a 12-well plate. In both cases, cells were cultured for 21 d. Culture medium was replaced with fresh medium every other day after seeding. Transepithelial electrical resistance (TEER) was measured by MILLICELL-ERS (Merck Millipore, Billerica, MA, U.S.A.) as an indicator of tight-junction formation.
Transcellular Transport ExperimentsThe transcellular transport assay procedure was performed as described previously.18) Briefly, cells were pre-incubated in Hanks’ balanced salt solution (HBSS, 137 mM NaCl, 5.4 mM KCl, 0.95 mM CaCl2, 0.81 mM MgSO4, 0.44 mM KH2PO4, 0.39 mM Na2HPO4, 25 mM D-glucose, and 10 mM N-(2-hydroxyethyl)piperazine-N′-2-(ethanesulfonic acid) (HEPES), adjusted to pH 7.4) at 37 °C for 15 min. For the assay assessing the dependence of permeability on lipophilicity in LLC-PK-1/mock and Caco-2 cells, the transport reaction was initiated by adding HBSS containing a mixture of drugs (5 µM acebutolol, 50 µM atenolol, 5 µM alprenolol, 5 µM corticosterone, 5 µM metoprolol, 5 µM probenecid, 5 µM propranolol, 5 µM sotalol, and 5 µM testosterone) to apical (AP) chambers. Cells were continuously incubated at 37 °C. A 200 µL aliquot of the medium (the sample) was removed from the basolateral (BL) chamber at 5, 10, 15, 20, 30, 45, and 60 min, and then replaced with the same volume of fresh HBSS. For LLC-PK1/P-gp and mock cells, the transport reaction was initiated by adding HBSS containing rhodamine 123 (10 µM) or a paracellular marker lucifer yellow (125 µM) to AP or BL chambers to measure AP to BL or BL to AP transport, respectively. Rhodamine 123 and lucifer yellow concentrations were measured using a fluorescent plate reader (1420 ARVO MX/Light; PerkinElmer, Inc., Waltham, MA, U.S.A.) at excitation and emission wavelengths of 405 and 535 nm, respectively. The concentrations of all other drugs were measured by LC-tandem mass spectrometry (LC-MS/MS) as described below. Transport activity was estimated in terms of a permeation coefficient (in units of centimeters per minute) according to the following equation:
where Q, A, and C0 are the amount of substrate transported over time, the area of the membrane surface, and the initial concentration of substrate in the donor medium, respectively.
Measurement of Drugs by LC-MS/MSIndividual compounds concentrations were measured by LC-MS/MS (LCMS-8050; Shimadzu, Kyoto, Japan) coupled to an LC-30A system (Shimadzu). The analytical column was a Luna 5 µm C18(2) LC Column (50 × 2 mm; Phenomenex, CA, U.S.A.) maintained at 40 °C. The mobile phase was composed of a 0.1% formic acid aqueous solution (solvent A) and acetonitrile containing 0.1% formic acid (solvent B). The flow rate was 0.4 mL/min and the injection volume was 5 µL. The gradient profile was as follows: 5% solvent B for 1.0 min; linear ramp to 95% solvent B in 3.0 min; then return to initial conditions in 0.5 min. The detection conditions of each compound by LC-MS/MS are summarized in Table 1. The lower limit of detection for each compound was 1 nM.
Table 1. LC-MS/MS Detection Conditions for Tested Drugs
| Compound | Log D | RT (min) | Ion mode | m/z monitored | CE (V) |
|---|
| Atenolol | −2.14 | 0.96 | Positive | 267.20 > 145.20 | −26.0 |
| Sotalol | −1.56 | 0.85 | Positive | 273.00 > 255.00 | −12.0 |
| Probenecid | −1.0 | 3.71 | Positive | 286.10 > 121.00 | −25.0 |
| Acebutolol | −0.29 | 2.52 | Positive | 337.00 > 116.00 | −21.0 |
| Metoprolol | 0.07 | 2.50 | Positive | 268.30 > 116.10 | −19.0 |
| Alprenolol | 1.0 | 2.74 | Positive | 250.20 > 117.00 | −35.0 |
| Propranolol | 1.54 | 2.68 | Positive | 260.20 > 155.00 | −35.0 |
| Corticosterone | 1.89 | 3.35 | Positive | 347.20 > 329.30 | −16.0 |
| Testosterone | 3.31 | 3.60 | Positive | 289.20 > 97.00 | −35.0 |
| Carvedilol (IS) | — | 2.82 | Positive | 407.40 > 210.25 | −26.0 |
RT, retention time; IS, internal standard; CE, Collision Energy.
The statistical significance of differences in mean values was determined using a Student’s t-test. Differences with a p-value less than 0.05 were considered statistically significant.
RESULTS
Optimization of LLC-PK1 and Caco-2 Cell Culture Conditions in the HCF InsertsTo optimize transcellular assays using the HCF inserts, the effects of culture time and cell seeding density on TEER and transcellular transport of lucifer yellow as a paracellular marker were evaluated. The results are shown in Fig. 2. The TEER value obtained with LLC-PK1/mock cells seeded on the HCF inserts increased until day 7, since the value at day 7 was comparable to that at day 8. Furthermore, while TEER was higher until day 6 after seeding at a cell density of 1.8 × 105 cells/cm2 compared with seeding at a cell density of 0.9 × 105 cells/cm2 (Fig. 2A), TEER values were comparable between days 7 and 8 (238 ± 4 and 227 ± 3 Ω cm2 at a density of 1.8 × 105 cells/cm2 and 208 ± 14 and 236 ± 2 Ω cm2 at a density of 0.9 × 105, respectively). In addition, while the Papp of lucifer yellow decreased until day 7 (Fig. 2B), the values at day 7 (2.57 ± 0.34 × 10−6 cm/s at a density of 1.8 × 105 cells/cm2 and 4.64 ± 0.32 × 10−6 cm/s at a density of 0.9 × 105 cells/cm2) were comparable to those at day 8 (3.03 ± 0.09 × 10−6 cm/s at a density of 1.8 × 105 cells/cm2 and 2.85 ± 0.10 × 10−6 cm/s at a density of 0.9 × 105 cells/cm2). The TEER and Papp value obtained using HCF inserts were comparable to those obtained using TEM inserts (Fig. S1). Following these preliminary experiments, all assays using LLC-PK1/mock cells on HCF inserts were conducted under optimal conditions: cell density of 1.8 × 105 cells/cm2; day 7. The TEER of Caco-2 cells on HCF inserts at day 21 (mean TEER, 510 Ω cm2; n = 2) was comparable to the TEER of Caco-2 cells on TEM inserts (586 ± 37 Ω cm2) (Fig. 2C). However, until day 14, the TEER of Caco-2 cells on HCF inserts was lower than the TEER on TEM inserts. Thus, Caco-2 cells were cultured on HCF inserts for 21 d prior to assay.
Transcellular Transport Study Using Drugs of Diverse LipophilicityA transcellular transport of nine compounds (Table 1) was performed using LLC-PK1/mock and Caco-2 cells (Fig. 3). In LLC-PK1/mock cells and Caco-2 cells, the Papp value of the tested drugs increased with an increase in lipophilicity of each drug. In addition, the Papp values of the tested drugs tended to be higher when using the HCF inserts than the TEM inserts.
P-gp-Mediated Transcellular Transport of Rhodamine 123 in LLC-PK1/P-gp CellsTo evaluate P-gp activity, directional transport of rhodamine 123 in LLC-PK1/P-gp and mock cells on HCF and TEM inserts was investigated. The BL to AP permeability Papp(BL→AP) of rhodamine 123 in LLC-PK1/P-gp cells on HCF and TEM inserts was 2.33 and 2.39 times higher (respectively) than the AP to BL permeability Papp(AP→BL) (Fig. 4). While the efflux ratio (Papp(B-A)/Papp(A-B)) of rhodamine 123 in LLC-PK1/P-gp cells cultured on HCF inserts (2.23 ± 0.13) was comparable to the efflux ratio in LLC-PK1/P-gp cells cultured on TEM inserts (2.39 ± 0.27), the amount of rhodamine 123 transported in both directions was significantly higher using HCF inserts than when using TEM inserts.
DISCUSSION
Assessments of drug membrane permeability are widely used during evaluation of the pharmacokinetic properties of drugs, including intestinal absorption, renal clearance, and distribution across the blood–brain barrier. Moreover, this methodology is routinely applied during drug discovery stages in the pharmaceutical industry. However, application of the technique to lipophilic drugs is limited. For example, the intracellular accumulation and adsorption on equipment of lipophilic drugs affect the evaluation of the Papp value.19) These problems could be improved by using buffers containing binding proteins. On the other hand, permeation across filter membrane and unstirred water layer could still be a problematic. Thus, the Papp values of highly lipophilic drugs in Caco-2 cells tend to plateau as apparent permeability is restricted by permeation across the unstirred water layer,1,9) and this affects evaluations of the intrinsic permeability of each drug. This issue may be overcome by using HCF membranes with a uniform diameter and a higher porosity than conventional TEM membranes, such as Transwell® membranes (Fig. 1). Accordingly, the present study investigated the usefulness of HCF in the transcellular transport assay. First, the culture conditions for LLC-PK1 and Caco-2 cells on HCF inserts were optimized (Fig. 2). The TEER value of LLC-PK1/mock cells cultured on HCF inserts reached over 200 Ω cm2, which is comparable to values obtained using TEM inserts in our previous study.20) Moreover, the Papp value for transport of the paracellular permeability marker lucifer yellow across a cell monolayer seeded on HCF inserts at day 7 (2.5 × 10−6 cm/s), was slightly higher to the Papp value obtained using TEM inserts (1.0 × 10−6 cm/s).18) For optimization of culture conditions with Caco-2 cells, the TEER values on the HCF inserts at day 21 were comparable to those on the TEM inserts. Therefore, while LLC-PK1 were used at a cell density of 1.8 × 105 cells/cm2 and cultured for 7 d prior to use, Caco-2 cells were cultured for 21 d.
The results of a transcellular transport study of nine different drugs of varying lipophilicities are presented in Figs. 3A and B. The Papp values obtained using Caco-2 and LLC-PK1/mock cells on HCF inserts both attained apparently maximum at a Log D value of 2. This was higher than previously reported in experiments using Caco-2 cells cultured on TEM inserts,1) where a maximum Papp was obtained at Log D value of 0. Accordingly, it was suggested that the use of the HCF inserts enhance the increase in permeability observed with increasing Log D value. Furthermore, HCF inserts even enhanced the apparent transcellular transport of hydrophilic compounds. HCF inserts have a higher aperture ratio than commercially available TEM inserts. Thus, the high aperture ratio of HCF inserts might enhance the apparent transcellular transport of hydrophilic as well as lipophilic compounds by increasing the permeation across the filter membrane. On the other hand, the Papp of acebutolol was lower than expected from the Log D value. Although the reason is uncertain, since acebutolol is acetylated by non-CYP enzymes to diacetolol,21) the metabolism might reduce the Papp of acebutolol. In addition, the Papp in Caco-2 cells could be affected by P-gp, since acebutolol was transported by P-gp.22) Collectively, these results are consistent with the proposal that apparent permeability was improved through using the highly porous HCF inserts, although an effect on the unstirred water layer cannot be completely dismissed.
Membrane transporters expressed in the intestine play an important role in oral absorption of drugs.23,24) P-gp, which is localized on the apical membrane of enterocytes, decreases intestinal absorption of a diverse range of drugs.25) For example, the plasma concentration of orally administered digoxin is significantly affected by P-gp in the intestine in humans.26) To clarify the usefulness of the HCF inserts for carrier-mediated permeation studies, transcellular transport of the P-gp substrate rhodamine 123, Log D 1.5,27) in LLC-PK1/P-gp cells was examined (Fig. 4). The values of Papp(AP→BL) and Papp(BL→AP) obtained using HCF inserts were higher than those of TEM inserts. These results suggest that passage through the filter membrane is a rate-determining step in the transcellular transport of rhodamine 123, and that this effect was reduced using HCF inserts. On the other hand, the efflux ratio obtained using the HCF inserts was 2.33, which was equivalent to the efflux ratio 2.39 of the TEM inserts. This is considered to be due to the high aperture ratio of the HCF inserts. This porosity of HCF inserts may lead to an increase of the P-gp-mediated transport of rhodamine 123 as well as the simple diffusion. In addition, transcellular transports of rhodamine 123 across the both LLC-PK1/P-gp and mock cells were much lower than expected from the Log D value of 1.5. While rhodamine 123 is widely used as a probe for P-gp, rhodamine 123 has various characteristics such as high accumulation in the mitochondrial matrix and inhibition of its function,28) and metabolism by intracellular esterase.29) These characteristics of rhodamine 123 might make it difficult to simply predict membrane permeation from the Log D value. However, the efflux ratio obtained using the HCF inserts provide evidence that carrier-mediated transport can be evaluated using the HCF inserts.
As an additional application of cell culture using HCF inserts, cell–cell interactions may be assessed following co-culture of different types of cells on either side of the membrane. Because HCF is characterized by high porosity, the synergetic effect of co-culture of different types of cells is enhanced compared with conventional TEM inserts. Although only a few studies on cell–cell communication in pharmacokinetic studies have been reported,30–33) cell–cell communication is potentially important for precise analysis of cell function. For example, the functions of cerebral microvessels, which constitute the blood–brain barrier, are regulated by astrocytes, which interact with brain capillary endothelial cells.34) Moreover, the barrier function of Caco-2 cells was decreased by co-culture with immune THP-1 cells activated by lipopolysaccharide (LPS) and interferon-γ (IFN-γ).35) Using high-porosity HCF inserts, secreted factors released from activated cells on one side of the membrane may more easily access cells on the opposite side of the membrane, thus improving cell–cell interaction studies.
In conclusion, use of HCF inserts in transcellular transport studies can alleviate the rate-determining effect of the filter membrane on transcellular transport of highly permeable lipophilic compounds as well as hydrophilic compounds compared with conventional insert film. Accordingly, it is expected that HCF inserts provide a better prediction of transcellular transport of compounds and evaluation of substrate recognition when transporters are involved in the transport process.
Acknowledgments
This work was supported by JST A-STEP Grant No. JPMJTM19F9, Japan.
Conflict of Interest
This work was financially supported by FUJIFILM Corporation.
Supplementary Materials
The online version of this article contains supplementary materials.
REFERENCES
- 1) Artursson P, Karlsson J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem. Biophys. Res. Commun., 175, 880–885 (1991).
- 2) Kunze A, Huwyler J, Poller B, Gutmann H, Camenisch G. In vitro–in vivo extrapolation method to predict human renal clearance of drugs. J. Pharm. Sci., 103, 994–1001 (2014).
- 3) Kurosawa T, Tega Y, Higuchi K, Yamaguchi T, Nakakura T, Mochizuki T, Kusuhara H, Kawabata K, Deguchi Y. Expression and functional characterization of drug transporters in brain microvascular endothelial cells derived from human induced pluripotent stem cells. Mol. Pharm., 15, 5546–5555 (2018).
- 4) Ueda K, Okamura N, Hirai M, Tanigawara Y, Saeki T, Kioka N, Komano T, Hori R. Human P-glycoprotein transports cortisol, aldosterone, and dexamethasone, but not progesterone. J. Biol. Chem., 267, 24248–24252 (1992).
- 5) Nakanishi T, Ohya K, Shimada S, Anzai N, Tamai I. Functional cooperation of URAT1 (SLC22A12) and URATv1 (SLC2A9) in renal reabsorption of urate. Nephrol. Dial. Transplant., 28, 603–611 (2013).
- 6) Matsushima S, Maeda K, Kondo C, Hirano M, Sasaki M, Suzuki H, Sugiyama Y. Identification of the hepatic efflux transporters of organic anions using double-transfected Madin–Darby canine kidney II cells expressing human organic anion-transporting polypeptide 1B1 (OATP1B1)/multidrug resistance-associated protein 2, OATP1B1/multidrug resistance 1, and OATP1B1/breast cancer resistance protein. J. Pharmacol. Exp. Ther., 314, 1059–1067 (2005).
- 7) Arakawa H, Amezawa N, Kawakatsu Y, Tamai I. Renal reabsorptive transport of uric acid precursor xanthine by URAT1 and GLUT9. Biol. Pharm. Bull., 43, 1792–1798 (2020).
- 8) Chen Y, Jin JY, Mukadam S, Malhi V, Kenny JR. Application of IVIVE and PBPK modeling in prospective prediction of clinical pharmacokinetics: strategy and approach during the drug discovery phase with four case studies. Biopharm. Drug Dispos., 33, 85–98 (2012).
- 9) Naruhashi K, Tamai I, Li Q, Sai Y, Tsuji A. Experimental demonstration of the unstirred water layer effect on drug transport in Caco-2 cells. J. Pharm. Sci., 92, 1502–1508 (2003).
- 10) Zhang X, Zheng N, Zou P, Zhu H, Hinestroza JP, Rosania GR. Cells on pores: a simulation-driven analysis of transcellular small molecule transport. Mol. Pharm., 7, 456–467 (2010).
- 11) Shimomura M, Koito T, Maruyama N, Arai K, Nishida J, Grasjo L, Karthaus O, Ijiro K. Photonic and electronic applications of mesoscopic polymer assemblies. Mol. Cryst. Liq. Cryst. Sci. Technol. Mol. Cryst. Liq. Cryst., 322, 305–312 (1998).
- 12) Yamazaki H, Ito K, Yabu H, Shimomura M. Formation and control of line defects caused by tectonics of water droplet arrays during self-organized honeycomb-patterned polymer film formation. Soft Matter, 10, 2741–2747 (2014).
- 13) Yabu H. Fabrication of honeycomb films by the breath figure technique and their applications. Sci. Technol. Adv. Mater., 19, 802–822 (2018).
- 14) Kawano T, Nakamichi Y, Fujinami S, Nakajima K, Yabu H, Shimomura M. Mechanical regulation of cellular adhesion onto honeycomb-patterned porous scaffolds by altering the elasticity of material surfaces. Biomacromolecules, 14, 1208–1213 (2013).
- 15) Tanaka M, Takayama A, Ito E, Sunami H, Yamamoto S, Shimomura M. Effect of pore size of self-organized honeycomb-patterned polymer films on spreading, focal adhesion, proliferation, and function of endothelial cells. J. Nanosci. Nanotechnol., 7, 763–772 (2007).
- 16) Zhu Y, Sheng R, Luo T, Li H, Sun J, Chen S, Sun W, Cao A. Honeycomb-structured films by multifunctional amphiphilic biodegradable copolymers: surface morphology control and biomedical application as scaffolds for cell growth. ACS Appl. Mater. Interfaces, 3, 2487–2495 (2011).
- 17) Tanaka M, Nishikawa K, Okubo H, Kamachi H, Kawai T, Matsushita M, Todo S, Shimomura M. Control of hepatocyte adhesion and function on self-organized honeycomb-patterned polymer film. Colloids Surf. A Physicochem. Eng. Asp., 284–285, 464–469 (2006).
- 18) Arakawa H, Kubo H, Washio I, Staub AY, Nedachi S, Ishiguro N, Nakanishi T, Tamai I. Rat kidney slices for evaluation of apical membrane transporters in proximal tubular cells. J. Pharm. Sci., 108, 2798–2804 (2019).
- 19) Krishna G, Chen K, Lin C, Nomeir AA. Permeability of lipophilic compounds in drug discovery using in-vitro human absorption model, Caco-2. Int. J. Pharm., 222, 77–89 (2001).
- 20) Shirasaka Y, Kuraoka E, Spahn-Langguth H, Nakanishi T, Langguth P, Tamai I. Species difference in the effect of grapefruit juice on intestinal absorption of talinolol between human and rat. J. Pharmacol. Exp. Ther., 332, 181–189 (2010).
- 21) Lilja JJ, Raaska K, Neuvonen PJ. Effects of grapefruit juice on the pharmacokinetics of acebutolol. Br. J. Clin. Pharmacol., 60, 659–663 (2005).
- 22) Terao T, Hisanaga E, Sai Y, Tamai I, Tsuji A. Active secretion of drugs from the small intestinal epithelium in rats by P-glycoprotein functioning as an absorption barrier. J. Pharm. Pharmacol., 48, 1083–1089 (1996).
- 23) Tamai I, Nakanishi T. OATP transporter-mediated drug absorption and interaction. Curr. Opin. Pharmacol., 13, 859–863 (2013).
- 24) Nakanishi T, Tamai I. Interaction of drug or food with drug transporters in intestine and liver. Curr. Drug Metab., 16, 753–764 (2015).
- 25) Szakács G, Váradi A, Ozvegy-Laczka C, Sarkadi B. The role of ABC transporters in drug absorption, distribution, metabolism, excretion and toxicity (ADME-Tox). Drug Discov. Today, 13, 379–393 (2008).
- 26) Greiner B, Eichelbaum M, Fritz P, Kreichgauer HP, von Richter O, Zundler J, Kroemer HK. The role of intestinal P-glycoprotein in the interaction of digoxin and rifampin. J. Clin. Invest., 104, 147–153 (1999).
- 27) Forster S, Thumser AE, Hood SR, Plant N. Characterization of rhodamine-123 as a tracer dye for use in in vitro drug transport assays. PLOS ONE, 7, e33253 (2012).
- 28) Huang M, Camara AK, Stowe DF, Qi F, Beard DA. Mitochondrial inner membrane electrophysiology assessed by rhodamine-123 transport and fluorescence. Ann. Biomed. Eng., 35, 1276–1285 (2007).
- 29) Jancis EM, Carbone R, Loechner KJ, Dannies PS. Estradiol induction of rhodamine 123 efflux and the multidrug resistance pump in rat pituitary tumor cells. Mol. Pharmacol., 43, 51–56 (1993).
- 30) Jeon TI, Seo YK, Osborne TF. Gut bitter taste receptor signalling induces ABCB1 through a mechanism involving CCK. Biochem. J., 438, 33–37 (2011).
- 31) Arakawa H, Ohmachi T, Ichiba K, Kamioka H, Tomono T, Kanagawa M, Idota Y, Hatano Y, Yano K, Morimoto K, Ogihara T. Interaction of peptide transporter 1 with D-glucose and L-glutamic acid; possible involvement of taste receptors. J. Pharm. Sci., 105, 339–342 (2016).
- 32) Arakawa H, Sugiura S, Kawanishi T, Shin K, Toyoda H, Satoh T, Sakai Y, Kanamori T, Kato Y. Kinetic analysis of sequential metabolism of triazolam and its extrapolation to humans using an entero-hepatic two-organ microphysiological system. Lab. Chip., 20, 537–547 (2020).
- 33) Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci., 7, 41–53 (2006).
- 34) Gaillard PJ, van der Sandt IC, Voorwinden LH, Vu D, Nielsen JL, de Boer AG, Breimer DD. Astrocytes increase the functional expression of P-glycoprotein in an in vitro model of the blood–brain barrier. Pharm. Res., 17, 1198–1205 (2000).
- 35) Kämpfer AAM, Urbán P, Gioria S, Kanase N, Stone V, Kinsner-Ovaskainen A. Development of an in vitro co-culture model to mimic the human intestine in healthy and diseased state. Toxicol. In Vitro, 45, 31–43 (2017).