2021 年 44 巻 5 号 p. 678-685
2021 年 44 巻 5 号 p. 678-685
To clarify the role of an amino acid residue in the pH-dependent efflux process in Chinese hamster ovary (CHO) cells expressing the human oligopeptide transporter hPEPT1 (CHO/hPEPT1), we determined the effect of extracellular pH on the hPEPT1-mediated efflux process. The efflux of glycylsarcosine (Gly-Sar), a typical substrate for hPEPT1, was determined using an infinite dilution method after cells were preloaded with [3H]-Gly-Sar. The efflux of [3H]-Gly-Sar was stimulated by 5 mM unlabeled hPEPT1 substrates in the medium. This trans-stimulation phenomenon showed that hPEPT1 mediated the efflux of [3H]-Gly-Sar from CHO/hPEPT1 and that hPEPT1 is a bi-directional transporter. We then determined the effect of extracellular pH (varying from 8.0 to 3.5) on the efflux activity. The efflux activity by hPEPT1 decreased with the decrease in extracellular pH. The Henderson–Hasselbälch-type equation, which fitted well to the pH-profile of efflux activity, indicated that a single amino acid residue with a pKa value of approximately 5.7 regulates the efflux activity. The pH-profile of the efflux activity remained almost unchanged irrespective of the proton gradient across the plasma membrane. In addition, the chemical modification of the histidine residue with diethylpyrocarbonate completely abolished the efflux activity from cells, which could be prevented by the presence of 10 mM Gly-Sar. These data indicate that the efflux process of hPEPT1 is also regulated in a pH-dependent manner by the protonation state of a histidine residue located at or near the substrate recognition site facing the extracellular space.
The human oligopeptide transporter 1 (hPEPT1/SLC15A1) is expressed predominantly on the apical membrane facing the lumen of the intestine, which plays an important physiological role in the absorption of small peptides derived from the digestion of dietary proteins.1–3) These absorbable peptides composed of two or three amino acids exhibit a variety of structures, charges, and hydrophilicities because the 20 constituent amino acids can undergo extensive combinations to form 400 dipeptides and 8000 tripeptides.4,5) Therefore, hPEPT1 exhibits a broad substrate specificity ranging from peptides to peptidomimetic therapeutic drugs such as beta-lactams,6–10) angiotensin converting enzyme inhibitor1,11) and antiviral prodrugs.12–14) hPEPT1 effectively absorbs the therapeutic prodrugs and facilitates enhancement of bioavailability.12–14) Currently, hPEPT1 has been considered extensively as an efficient absorption system for peptide-like prodrugs.15–17)
hPEPT1 actively transports substrates by coupling substrate transport with the movement of protons down an electrochemical gradient (Δμ̃H+), which is provided by the acid unstirred water layer (pH 5.5–6.0) in the intestinal lumen.18–20) It has been demonstrated clearly that the Δμ̃H+ activates the transport system by increasing maximal velocity with no significant effect on the affinity.18,19,21,22) On the other hand, hPEPT1 has an optimal pH at 5.5 to 6.0 and can function most efficiently in epithelial cells of the small intestine.18,19) Although Δμ̃H+ is an unequivocal energy source for hPEPT1, it is unclear as to why the transport activity decreases in a more acidic region where the driving force increases. To solve this question, we previously determined a relationship between the transport activity and extracellular pH under conditions where the only proton concentration difference across the membrane was dissipated and the membrane potential remained unchanged by the addition of monensin and nigericin and showed that the driving force is solely the membrane potential.23) We then demonstrated that the transport activity of hPEPT1 is regulated by the protonation/deprotonation of a single amino acid residue and that the bell-shape profile is due to the balance between the driving force and extent of regulation by a certain amino acid.23) The amino acid residue seemed to function as a pH sensor to regulate the available number of hPEPT1 involved in substrate transport. Chemical modification of the histidine residue in hPEPT1 with diethylpyrocarbonate (DEPC) further demonstrated that the histidine is located at or near the substrate-binding site and is involved in pH regulation of the transport activity. On the other hand, several questions remain unresolved: (1) Which stage of the transport cycle is regulated by the histidine residue; (2) whether the residue is involved in the regulation of the efflux activity; (3) whether this residue is involved in substrate recognition or binding and translocation of protons for energy coupling.
Using various techniques, hPEPT1 has been demonstrated to be a bi-directional transporter.8,9,24,25) Previously, we directly demonstrated that hPEPT1 transports neutral dipeptides bi-directionally in an electrogenic and proton-coupled co-transport mode using the patch-clamp technique.25) Notably, hPEPT1 mediates the efflux of oligopeptides from cells. One can determine the pH regulation of the hPEPT1-mediated transport activity by measuring the efflux activity with varying extracellular pH. In this way, the influence of protons on the substrate recognition site and the proton-binding site involved in energy coupling can be avoided. Under these experimental conditions, the sites of proton coupling and substrate binding are inside, whereas the regulation site by pH is outside.
Since Chinese hamster ovary (CHO)/hPEPT1 cells show a significant transport activity, we determined the effect of extracellular pH on the efflux activity. Our findings indicated that the protonation of the histidine residue at the extracellular site results in a decrease in the efflux activity, which is distinct from the sites of proton-coupled transport and substrate binding. Furthermore, we found that the decrease in extracellular pH reduced the turnover rate of transporters; in other words, the number of available transporters was reduced. The protonation/deprotonation of the putative histidine residue determines the states of the transporter; the active state participates in the transport cycle, whereas the other inactive state ceases the transport. These results provide an important insight into the operational mode of hPEPT1.
3H-Glycylsarcosine ([3H]-Gly-Sar) (148 GBq/µmol) was purchased from Moravek Biochemical, Inc. (Brea, CA, U.S.A.). Cephalexin, cephradine, dipeptides, Ham’s F-12 medium, and L-valine methyl ester (Val-OMe) were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Fetal bovine serum (FBS) was purchased from Gibco BRL Life Technologies (Grand Island, NY, U.S.A.). Blasticidin S was purchased from Kaken Pharmaceutical Co., Ltd. (Tokyo, Japan). All chemicals used were of analytical grade.
Cell CultureCHO/hPEPT1 cells overexpressing hPEPT1 were cultured as described previously.23) Cells were passaged in 75-cm2 culture flasks in Ham’s F-12 medium supplemented with 10% FBS and antibiotics (100 units/mL penicillin, 100 µg/mL streptomycin, and 10 µg/mL blasticidin S as a selection marker). At approximately 80% confluence, cells were harvested using 0.02% ethylenediaminetetraacetic acid and 0.05% trypsin and were seeded at a density of ten-fold dilutions. The culture medium was changed every third day. The transport experiments were performed on the fourth day after seeding the CHO cells.
Efflux Experiments in Monolayer CHO/hPEPT1 CellsCells were trypsinized when 80% confluence was noted in 75 cm2 flasks, and 2 × 105 cells were seeded per well in 6-well culture plates. After CHO cells reached confluency on the third day, efflux experiments were performed the next day. The composition of the buffer was 5 mM Good’s buffer containing 140 mM NaCl, 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2, and 10 mM glucose. N-2-Hydroxyethylpiperazine-N′-2-ethanesulfonic acid was used for pH 8.0–7.0 buffer; 2-(N-morpholino)ethanesulfonic acid for pH 7.0–5.0 buffer; and homopiperazine-N,N′-Bis-2-(ethanesulfonic acid) for pH 5.0–3.5 buffer. In the efflux experiments, we used non-metabolizable derivative, Gly-Sar as the efflux substrate via hPEPT1. For preloading Gly-Sar, cells were preincubated with [3H]-Gly-Sar (1 µCi/mL) and 10 µM Gly-Sar at pH 6.0 for 5 min and washed twice with ice-cold uptake buffer (pH 7.4). The cells were incubated again in 1 mL of the prewarmed buffer at various pH values to start [3H]-Gly-Sar efflux process from the cells. This dilution procedure was referred to as the infinite dilution method. In the trans-stimulation experiments, the cells were incubated in 1 mL of prewarmed buffer at pH 6.0 containing 5 mM compound. In the trans-stimulation experiments, the metabolism effects of cephradine, cephalexin and Val-OMe on the efflux activities were considered to exhibit minimal, because the excess amounts of these substrates were added to the medium. A 50-µL aliquot of the medium was removed at the designated time points (Fig. 1). At the end of the incubation period, the remaining medium was completely aspirated, and cells were washed twice with ice-cold buffer (pH 7.4). The cells were solubilized in 1 mL of 1 M NaOH and neutralized with 1 mL of 1 M HCl. The radioactivity remaining in cells was determined in an ACSII scintillation counting cocktail (Amersham/Pharmacia, Buckinghamshire, U.K.) using a liquid scintillation counter (LSC 2500, Packard, Meriden, CT, U.S.A.). The accumulated [3H]-Gly-Sar before initiating its efflux (total [3H]-Gly-Sar) was estimated by summing up the amounts of [3H]-Gly-Sar released into the medium and those remaining in the cells. The remaining [3H]-Gly-Sar in cells was normalized by the total [3H]-Gly-Sar (the amount at time zero). For preloading [3H]-Gly-Sar into the mock cells (CHO/pCIneo) transfected with pCIneo (empty vector),23) the cells were preincubated with [3H]-Gly-Sar (1 µCi/mL) and 10 µM Gly-Sar at pH 6.0 for 20 min. However, CHO/pCIneo exhibited a minimal accumulation, which could not be used for efflux assay. The protein content of the cell monolayers solubilized in 1.0 mL of 1 M NaOH was determined after the neutralization with 1 M HCl using a Bio-Rad protein assay kit (Bio-Rad Laboratories, Richmond, CA, U.S.A.) with bovine serum albumin as a standard.

Cells cultured on 6-well plates were preincubated with [3H]-Gly-Sar (1 µCi/mL) and 10 µM Gly-Sar at pH 6.0 for 5 min and washed twice with ice-cold uptake buffer (pH 7.4). The cells preloaded with [3H]-Gly-Sar were incubated again in 1 mL of prewarmed buffer at pH 6.0. Fifty microliters of the medium was removed at the designated time points. Panel (A) Time course of [3H]-Gly-Sar remaining in the cells after their infinite dilution. The amount of [3H]-Gly-Sar remaining in the cells is expressed as the fraction of that at time zero. Each point represents mean ± S.E. (control, n = 5; +5 mM Gly-Sar, cephradine, cephalexin, and Val-OMe, n = 6; +5 mM Val and Gly, n = 3). Panel (B) Trans-stimulation effect of hPEPT1 substrates on the efflux of [3H]-Gly-Sar. The preloaded cells were incubated again in 1 mL of prewarmed buffer at pH 6.0 containing 5 mM compound. * p < 0.05, ** p < 0.01 vs. control.
The efflux rates from CHO/hPEPT1 cells were assumed to be governed by the protonation/deprotonation of an amino acid residue in hPEPT1 protein. The mass spectrometric method was developed to determine the pKa values of histidine residues in a protein.26,27) An imidazole at the C2 position in histidine residue in a protein undergoes the exchanging between the hydrogen and deuterium or the hydrogen and tritium in a pH-dependent manner. The isotopic exchanging rate could be described based on Henderson–Hasselbälch equation.26,27) Thus, the following Henderson–Hasselbälch-type equation was fitted to the efflux rates in the absence and presence of 10 µM nigericin/monensin or 40 µM carbonyl cyanide 3-chlorophenylhydrazone (CCCP):
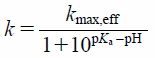 | (1) |
where k represents the initial rate of Gly-Sar transport, kmax,eff is the maximum transport rate value, pH is the medium pH, and pKa is the association of the proton. The pKa values were determined by an iterative nonlinear least-squares method using SigmaPlot (ver14.0; Systat Software Inc., San Jose, CA, U.S.A.).
Statistical AnalysesAll experiments were conducted at least three times, and the results are expressed as the mean ± standard error (S.E.). Statistical differences between two groups were assessed using one-way ANOVA followed by Dunnett’s multiple comparisons test with the SigmaPlot. Vales with p < 0.05 were considered statistically significant.
The efflux of Gly-Sar from CHO/hPEPT1 cells was determined with an infinite dilution method after cells were preloaded with 10 µM [3H]-Gly-Sar. The time course of [3H]-Gly-Sar remaining in cells is depicted in Fig. 1. The decay of [3H]-Gly-Sar remaining in cells was exponential (Fig. 1A). The efflux rate was determined from the slope of the logarithmic phase. The efflux rate of [3H]-Gly-Sar was significantly enhanced by unlabeled Gly-Sar, Val-OMe, cephradine, and cephalexin in the medium, but not by the amino acids Gly and Val (Fig. 1B), indicating that the efflux of [3H]-Gly-Sar from CHO/hPEPT1 was stimulated in the presence of inwardly directed gradients of substrates for hPEPT1. The extent of the trans-stimulation of efflux process is governed by the substrate uptake rate by an excess amount of substrate in the medium, which enhances the available transporter number inside the cells.7,8,28,29) As shown in Fig. 1, the different extents of trans-stimulation effects among Gly-Sar, cephradine, cephalexin and Val-OMe might be involved in the difference in the uptake activities attributed to their affinities against hPEPT1, because the rank order of the affinity for hPEPT1 was Gly-Sar > Val-OMe > cephradine, cephalexin.3,10,30) This phenomenon indicates that this efflux process is mediated via hPEPT1 and that hPEPT1 is a bi-directional transporter.8,23,24)
Effect of Extracellular pH on the Efflux Activity by hPEPT1We further examined the effect of extracellular pH on the efflux of [3H]-Gly-Sar from CHO/hPEPT1 cells. The decay slope of [3H]-Gly-Sar from cells decreased as extracellular pH decreased (Fig. 2A). The efflux was completely abolished at pH <4.0. The efflux ceased at pH 3.5, followed by the recovery of efflux activity after extracellular pH was increased to 6.0 (Fig. 2B). Even though the efflux activity was abolished at pH 3.5, the return of extracellular pH to 6.0 recovered the efflux activity. The relationship between the efflux rate and extracellular pH showed the profile of the Henderson–Hasselbälch-type equation, which fitted well to Eq. 1, where the pKa and kmax,eff values was calculated to be 5.74 ± 0.04 (± calculated standard deviation (S.D.)) and 0.144 ± 0.003 min−1 (± calculated S.D.), respectively. Notably, the efflux activity was also dependent on extracellular pH and was regulated by the protonation/deprotonation of a single amino acid residue. In this study, we used a zwitter-ionic peptide, Gly-Sar, as the model substrate. The pKa values of the amino and carboxylic groups in Gly-Sar are 8.5 and 2.8, respectively,3) and the ionic state of Gly-Sar remained almost unchanged in the pH range of 4.0 to 7.5, at which the pH-dependence of Gly-Sar efflux activity was determined (Figs. 2, 3).
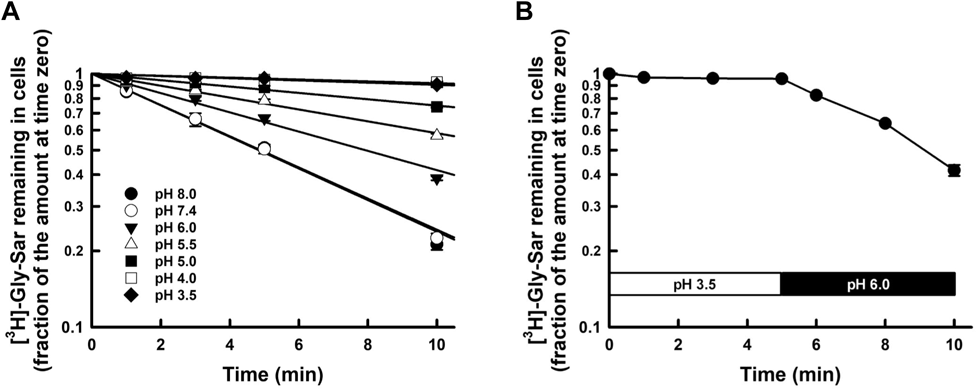
The cells preloaded with [3H]-Gly-Sar were incubated again in 1 mL of the prewarmed buffers ranging from pH 3.5 to 8.0 after washing with ice-cold buffer at the corresponding pH. Panel (A) The time course of [3H]-Gly-Sar remaining in the cells after infinite dilution. The amount of [3H]-Gly-Sar remaining in the cells is expressed as the fraction of that at time zero. Each point represents mean ± S.E. (pH 7.4, n = 6; other pH, n = 3). Panel (B) Reversibility of the inhibited efflux activity at pH 3.5 by returning extracellular pH to 6.0. The preloaded cells were incubated in the prewarmed buffer at pH 3.5 for 5 min and then replaced in the buffer at pH 6.0.

The efflux rates of [3H]-Gly-Sar from CHO/hPEPT1 cells were determined from the efflux profiles at various pH values, as described in the legend to Fig. 2. The line in the figure represents the fitted line, the Henderson–Hasselbälch-type equation (Eq. 1). The pKa and kmax,eff values were calculated to be 5.74 ± 0.04 (± calculated S.D.) and 0.144 ± 0.003 min−1 (± calculated S.D.), respectively.
The good fit of the Henderson–Hasselbälch-type equation with a single pKa (approximately 6.0) to the pH profile of the efflux activity suggested that the histidine residue might be involved in the pH regulation of the efflux process. In addition, histidine residues have been identified as key amino acid residues involved in the substrate transport of PEPT1, in both substrate recognition as well as transport activity. Therefore, we chemically modified histidine with DEPC7,19,21,23) to determine whether these crucial histidine residues are also involved in the pH profile of efflux activity. One millimolar DEPC treatment completely abolished the transport activity of CHO/hPEPT1 (Fig. 4), indicating that a histidine residue was also involved in the efflux process. On the other hand, this abolishment was almost prevented by the incorporation of 10 mM Gly-Sar, which was observed only in the presence of the substrate of PEPT1, indicating that the histidine residue is located at or near the substrate recognition site. These data indicate that the histidine residue located at or near the substrate recognition site is involved in the pH regulation of transport activity for the efflux process.
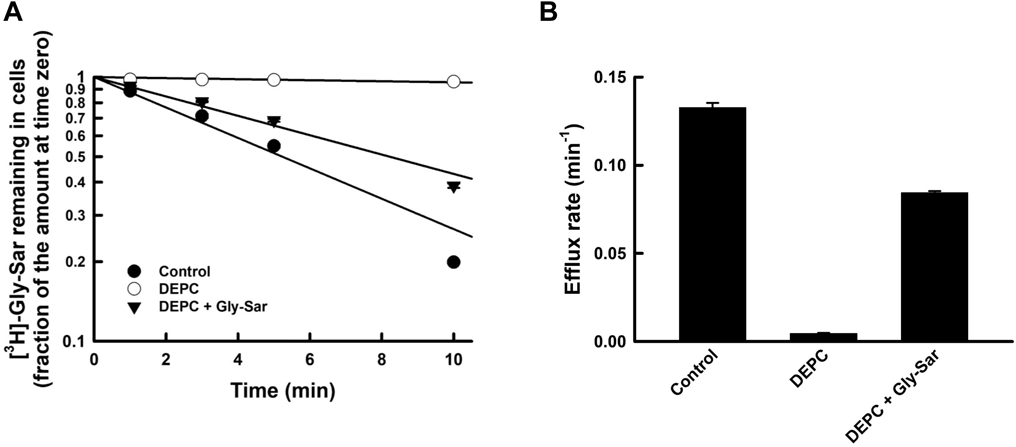
After cells were preloaded with [3H]-Gly-Sar (10 µM), the cells were incubated at 4 °C for 10 min with 1 mM DEPC (pH 6.0) in the absence (○) or presence (▼) of 10 mM Gly-Sar. The efflux rates of [3H]-Gly-Sar from CHO/hPEPT1 cells were measured at pH 7.4, as described in the legend to Fig. 1. Each point represents the mean ± S.E. (n = 6).
hPEPT1 is an electrogenic H+/dipeptide co-transporter and a bi-directional transporter. The inward driving force increases with a decrease in pH. In other words, the decrease in the outward driving force might explain the decrease in efflux activity. To exclude this possibility, the efflux activity was determined under conditions in which only the proton gradient across the membrane was dissipated by 10 µM nigericin/monensin31,32) (Fig. 5). The pH dependency of the efflux activities exhibited almost similar profiles irrespective of the inward proton gradient across the membrane. The protonophore CCCP slightly affected the pH profile of the efflux activity by hPEPT1. The efflux process was scarcely affected by the proton gradient across the membrane, which is consistent with the evidence obtained previously in the whole-cell patch (WCP)-clamp experiment.25)

The efflux rates of [3H]-Gly-Sar from CHO/hPEPT1 cells were measured in the presence of 10 µM nigericin/monensin or 40 µM CCCP at various pH values, as described in the legend to Fig. 1. The pH profiles were fitted to Eq. 1 by an iterative nonlinear least-squares method. The pKa values were estimated to be 5.41 ± 0.16 (± calculated S.D.) and 5.60 ± 0.13 (± calculated S.D.) in the presence of 10 µM nigericin/monensin and 40 µM CCCP, respectively. The kmax,eff values was calculated to be 0.144 ± 0.010 min−1 (± calculated S.D.) and 0.144 ± 0.009 min−1 (± calculated S.D.) in the presence of 10 µM nigericin/monensin and 40 µM CCCP, respectively. Each point in nigericin/monensin treatment represents mean ± S.E. (n = 3–6: pH 6.5, n = 6; pH 6, n = 6; pH 5.5, n = 5; other pH, n = 3). Each point in CCCP treatment represents mean ± S.E. (n = 3). The dotted line represents the control from the fitted line in Fig. 3. The dashed and dash-dot-dashed lines represent the fitted lines in the presence of nigericin/monensin and CCCP, respectively.
In the present study, employing hPEPT1 efflux assay, we aimed to investigate the role of extracellular pH in the hPEPT1 efflux activity. Previously, we determined the transport currents caused due to the uptake and efflux processes of substrates in the hPEPT1 expression system. Furthermore, we demonstrated that hPEPT1 could transport dipeptides bi-directionally in an electrogenic and proton-coupled co-transport mode.25) The present results are in accordance with our previous reports23,25) (Fig. 1). The efflux activity of hPEPT1 was determined using the infinite dilution method after its typical substrate, [3H]-Gly-Sar was preloaded. The preloaded [3H]-Gly-Sar was promptly released from CHO/hPEPT1 cells and its efflux rate was enhanced by the extracellular repletion of its substrates. The trans-stimulation phenomenon has been used extensively as the definitive criteria for transporter-mediated flux process28); the transportable substrate preloaded on the trans side (opposite side) across the plasma membrane accelerates the transport of substrate applied on the cis side into the trans side.7,8,29) The trans-stimulation of the flux increases the number of available transporters at the cis side due to the faster trans-to-cis translocation (return) of the transporters loaded with substrate than that of the unloaded transporters. Thus, the efflux activity via hPEPT1 might reflect the number of available transporters. Alternatively, the trans-stimulation phenomenon in the efflux process indicates that hPEPT1 mediates the efflux of Gly-Sar and that hPEPT1 can transport dipeptides bi-directionally.
DEPC reaction with proteins has considered to be specific for histidine within the pH range from 5.5 to 7.5,33) although DEPC can react in part with sulfhydryl and hydroxyl residues. The involvement of key histidine residues in pH-dependent transport activity via PEPT1 also has been demonstrated in Caco-2 cells21) and PEPT1-expressed mammalian cells23,34) and PEPT1 expressed Xenopus laevis oocytes35) under conditions of DEPC pretreatment in the presence and absence of PEPT1 substrate. Alternatively, it has been demonstrated that the chemical modification of hydroxyl group with phenylmethyl sulfate exhibited minimal effect on the pH-dependent transport activity by CHO/hPEPT1 cells.23) Miyamoto et al. also demonstrated using renal brush-border membrane vesicles that a complete inhibition of the pH-dependent Gly-Sar uptake by DEPC treatment was not reversed with 1,4-dithiothreitol, 2,3-dimercaptopropanol, or mercaptoethanol, revealing that reaction of DEPC with sulfhydryl groups in the transporter was not involved in the inhibition of the pH-dependent Gly-Sar uptake.19) Therefore, it seems very plausible that DEPC inhibition of [3H]-Gly-Sar efflux from CHO/hPEPT1 cells is attributed to the modification of essential histidyl residues in hPEPT1.
The results clearly showed the pH dependency in the efflux activity; the hPEPT1-mediated efflux activity decreased as extracellular pH decreased. The efflux was completely abolished at pH <4.0. The efflux ceased at pH 3.5, followed by the recovery of efflux activity after extracellular pH was increased to 6.0 (Fig. 2B), indicating that the abolished efflux at lower pH is not attributed to the damage of protein and/or cell membrane by acidification but rather the protonation of certain amino acid residues in hPEPT1. To further understand which amino acid residue is involved in the pH profile of the efflux activity, the efflux activity was plotted against extracellular pH (Fig. 3). The Henderson–Hasselbälch-type equation with a single pKa (Eq. 1) described the pH dependency of efflux activity. The pKa value was calculated to be 5.74 ± 0.04 (± calculated S.D.). Notably, the pH profile of the efflux activity remained unchanged, irrespective of the inward proton gradient; the only proton gradient across the membrane was dissipated with 10 µM nigericin/monensin31,32) (Fig. 5). Consistently, CCCP did not affect the pH profile of the efflux activity either. These results strongly suggest that the efflux process was scarcely affected by the proton gradient across the membrane.17,36–41)
Furthermore, the pH titration curve reflects the protonation state of the amino acid residue with a pKa of approximately 6. The imidazole ring in histidine exhibits a pKa value within the pH range from 5.5 to 7.442,43); at pH 5.0, the imidazole ring is protonated and positively charged, whereas, at pH 7.0, it is electrically neutral. The protonation profile in the ring seems to be consistent with the pH-dependent efflux activity. To determine whether a histidine residue is involved in efflux activity, we determined the effect of chemical modification of histidine residues by DEPC treatment on the pH profile of efflux activity after preloading cells with [3H]-Gly-Sar. The results showed that the chemical modification abolished the efflux activity (Fig. 4). On the other hand, we found that the addition of 10 mM Gly-Sar to the extracellular medium almost protected hPEPT1 from DEPC modification, corroborating that the crucial DEPC-sensitive residue involved in the efflux transport faced the outside surface and was located near the substrate recognition site. It is plausible that the number of available transporters was governed by the protonation state of the DEPC-sensitive histidine residue.19,21,23)
In general, the transporter is a tulip-shaped molecule that spans across the plasma membrane. As shown in Fig. 6, according to kinetic analysis, the transporter is hypothesized to exist in two conformational states in the flux cycle: one with the substrate recognition site exposed on the outer surface of the plasma membrane and the other with the recognition site exposed on the inner surface. The overall flux of the substrate involves four sequential processes: (1) the binding of the substrate to the recognition site exposed on one surface, (2) the translocation of the recognition site with the substrate loaded from one face of the membrane to the other, (3) release of the substrate from the loaded site, and (4) the translocation of the empty recognition site to the starting site, where the transporter is ready to transport another substrate molecule. The directions of influx and efflux transport depend on the side where the substrate is initially loaded. It has been hypothesized empirically that the turnover rate of the transporter is determined by the return of the empty recognition site to the initial surface because the empty transporter migrates more slowly than the loaded transporter. The empirical hypothesis is supported by overwhelming studies regarding the trans-stimulation phenomenon7,8,28,29): an acceleration of the transporter-mediated flux of substrate was observed when membrane vesicles and cells were loaded with another substrate on the trans (opposite) face. The efflux rate was also increased by the excess amount of Gly-Sar (Fig. 1), which could be feasibly explained by the empirical hypothesis: the excess amount of Gly-Sar loaded on the outer face accelerated the reorientation of the substrate recognition site from the outer to the inner face and thereby induced an increase in the availability of substrate recognition site on the inner surface. It should be noted that the reorientation rate of the transporter also reflects the number of available transporters, i.e., the entire transporter activity.
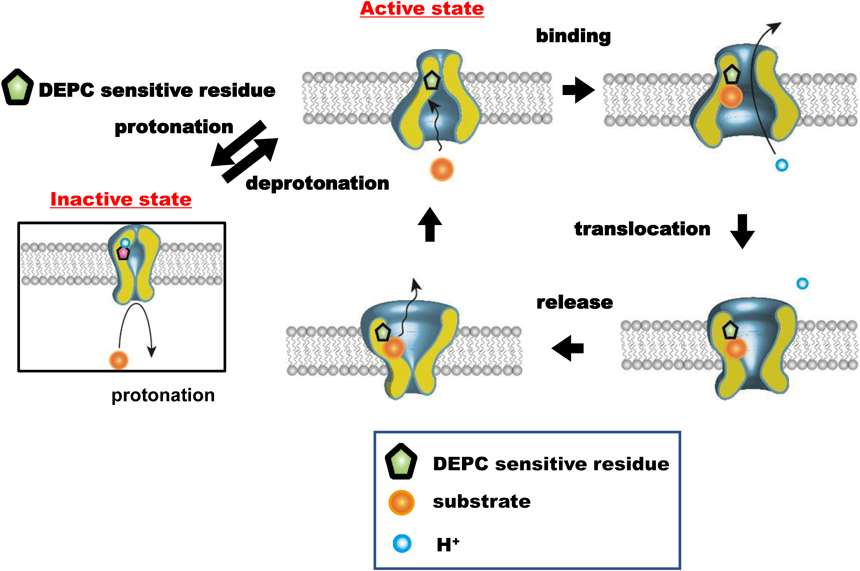
The preloaded substrate binds to the recognition site facing the intracellular space and then further translocates to the extracellular face, concomitant with a proton. The substrate is released from the recognition site, followed by translocation of the empty recognition site to the intracellular face. Now, hPEPT1 is ready to transport another substrate molecule. The translocation of the empty recognition site in hPEPT1 from the outer to the inner surface constitutes the rate-limiting state of the efflux process since the empty site migrates more slowly than the loaded site. Importantly, the number of available transporters reflects the reorientation rate of transporter. The protonated DEPC-sensitive amino acid residue, a histidine residue, inhibits the PEPT1 transport cycle and decreases the number of available transporters involved in the efflux process. Therefore, the histidine residue, exposed to the extracellular space, functions as a pH sensor to regulate the number of available recognition sites. In other words, once the histidine is protonated, it is impossible to initiate the translocation of the recognition site, culminating in the arrest of the transport cycle. (Color figure can be accessed in the online version.)
Based on kinetic data, the reduction of the efflux activity in the range of low extracellular pH can be explained feasibly by the decline of the reorientation rate of the empty recognition site to the inner face. The efflux activity was reduced as extracellular pH decreased, and the pH profile was superimposed on the Henderson–Hasselbälch-type equation with pKa 6.0. Therefore, we speculated that the protonation of the crucial histidine in hPEPT1 inhibited the reorientation step in the transport cycle, resulting in a decrease in the number of available recognition sites. As shown in Fig. 6, the protonation/deprotonation of the putative histidine residue determines the states of transporter availability; the activated state can participate in the transport cycle, whereas the inactivated state ceases the transport. Therefore, a crucial histidine residue functions as a pH sensor exposed to the outer space to regulate the number of available sites. In other words, once the histidine is protonated, it is impossible to initiate the translocation of the recognition site, culminating in the arrest of the transport cycle. The histidine regulates the ratio of the active and inactive forms to the protonation state. This reorientation process does not require proton translocation, but the histidine residue can extract the proton from somewhere. However, this reorientation does not require the concomitant translocation of protons.
To date, studies have focused on two crucial histidine residues. Site-directed mutagenesis studies revealed that histidine (His)-57 in the second transmembrane domain (TMD2) is the most plausible residue involved in H+ binding/dissociation.21,22,35,44,45) Alternatively, site-directed mutagenesis of His-121 in TMD4 showed that it is a substrate-binding site.21,35,45) Both His-57 and His-121 were located near the substrate recognition site. In our study, it was difficult to consider that the histidine residue formed the substrate-binding pocket because the substrate recognition site facing the inner space was involved in the efflux. Therefore, the most probable histidine residue seems to be the proton-coupling site, His-57. However, it is unclear how His-57 regulates the transport activity in a pH-dependent manner. As shown in Figs. 3 and 5, the efflux activity increased with increasing pH and reached a maximum at a pH >7.5. It is highly feasible that His-57 may extract H+ molecules from somewhere in the first state. This step is indispensable for initiating the transport cycles. At lower pH, His-57 is protonated and positively charged before the first state of transport cycle, which culminates in the arrest of transport. It is difficult to elucidate the exact role of His-57 in the pH regulation of transport activity because the mutagenesis of His-57 with other amino acid residues resulted in the complete loss of transport function.
Alternatively, hPEPT1 belongs to the evolutionarily conserved proton-dependent oligopeptide transporter (POT) family.17,36–41) The POT family transporters utilize the inwardly directed Δμ̃H+ to drive the uptake of nutrients into the cell. Recent studies on the structural and biochemical features of the transporters have focused on resolving two questions: (1) how POT family transporters can recognize structure-unrelated substrates within a single binding site; (2) how the substrate transport is driven by the proton movement and the molecular mechanism of substrate transport via hPEPT1. In particular, the oligopeptide transporter from Shewenella oneidensis (PEPTso) exhibited high functional and structural similarity with hPEPT136–39); the pH profile of the transport activity of PEPTso also showed a bell-shaped curve, which was governed by a key residue His-61, corresponding to His-57 in hPEPT1.37,39) In the His61Asp variant of PEPTso, the pH optimum of the transport activity was shifted to a more acidic region,39) indicating that His61 might function as the site of proton binding, release residue, and regulate pH of the transporter activity. However, further investigation is required for determining how His-57 is involved in the pH regulation of the transport activity because the mutagenesis of His-57 with other amino acid residues completely abolished the transport function.
In summary, we used CHO/hPEPT1 cells showing a huge transport activity to determine the effect of extracellular pH on efflux activity. Our findings indicated that the protonation of the histidine residue at the extracellular site results in a decrease in the efflux activity, which is distinct from the site of proton coupling for transport operation and the site of substrate binding site. Furthermore, we found that the decrease in extracellular pH reduced the turnover rate of transporters; in other words, the number of available transporters in the cycle was reduced. The protonation/deprotonation state of histidine determines the transport activity; the deprotonated histidine residue can participate in the transport cycle, whereas the protonated histidine residue can cease the transport. These results provide an important clue regarding the operational mode of hPEPT1-mediated efflux activity.
This work was supported by Grant-in-Aid for Scientific Research (C) from the Japanese Ministry of Education, Culture, Sports, Science, and Technology [18K06608].
The authors declare no conflict of interest.