2021 Volume 44 Issue 6 Pages 780-788
2021 Volume 44 Issue 6 Pages 780-788
Gastric cancer is one of the most common malignancies with a high mortality rate world. This study intends to make clear the role and mechanism of the Scutellarin (Scu), a flavonoid isolated from Erigeron breviscapus (Vant.) Hand.-Mazz, in regulating the evolvement of gastric cancer. We selected different doses of Scu to treat gastric cancer cells (MGC-803 and AGS). Then, cell counting kit-8 (CCK8) assay was conducted to verify the proliferation of tumor cells, while flow cytometry was adopted to test the apoptosis rate. Meanwhile, Western blot was conducted to examine epithelial–mesenchymal transition (EMT) markers and the expression of phosphatase and tensin homolog (PTEN)/phosphatidylinositol 3-kinase (PI3K) and apoptosis-related proteins (Bax, Bcl2 and Caspase3). Moreover, xenograft tumor experiment in nude mice was established to verify the effect of Scu on tumor growth. Furthermore, the knockdown model of PTEN was constructed, and the influence of PTEN on the anti-tumor effect of Scu was investigated. As a result, Scu inhibited cell proliferation, EMT and promoted the apoptosis in gastric cancer dose-dependently. Additionally, Scu attenuated tumor cell growth in vivo. Besides, Scu enhanced the expression of PTEN while reduced the phosphorylated level of PI3K. Moreover, the mechanistic study proved that Scu inactivated PI3K by up-regulating PTEN, thus dampening tumor progression. In conclusion, Scu dampened the growth and EMT of gastric cancer by regulating the PTEN/PI3K pathway.
Gastric cancer is one of the most frequent and fatal malignancies in human beings, which seriously affect the patients’ lives.1) The occurrence of gastric cancer is related to smoking, alcohol abuse and Helicobacter pylori infection.2) Clinically, about 20% of gastric cancer patients die within five years after diagnosis, mainly due to high lymphatic metastasis, recurrence and drug resistance.3) Currently, surgical treatment, radiotherapy and chemotherapy are the main therapeutic regimens for gastric cancer, while the prognosis and clinical outcome are still unsatisfactory.4) Therefore, it is crucial to explore alternative therapies for gastric cancer and improve the current situation.
Scutellarin (Scu) (Formula: C21H18O12) is a flavonoid isolated from Erigeron breviscapus (Vant.) Hand.-Mazz, which is also known as Scutellarin baicalensis in Chinese traditional medicine.5) Studies have discovered that Scu has antioxidative stress, anti-ischemic and anti-inflammatory. For example, Scu relieves the development of nonalcoholic fatty liver disease (NAFLD) via reducing excessive accumulation of hepatic lipids and oxidative injury of hepatocytes, the underlying mechanism is activating peroxisome proliferator-activated receptor gamma (PPARγ)/PPARγ coactivator-1 (PGC-1)α-nuclear factor-E2-related factor 2 (Nrf2) pathway.6) Also, Scu protected hepatocytes from hypoxia/reoxygenation (ischemia–reperfusion (I/R))-induced oxidative injury through regulating the Keap1/Nrf2/antioxidant response element (ARE) signaling pathway.7) Additionally, Scu also exerts an anti-tumor role. For instance, the angiogenesis and metastasis of colorectal cancer are both significantly attenuated by Scu, which is through targeting ephrinb2 signaling.8) However, the effect and mechanism of Scu on gastric cancer cells have not been evaluated so far.
Phosphatase and tensin protein homolog (PTEN) is frequently mutated in most cancers and is a tumor suppressor. Studies have confirmed that PTEN antagonizes the phosphoinositol 3-kinase (PI3K)/AKT pathway through lipid phosphatase on the plasma membrane.9) In addition to its typical PI3K inhibitory dependent function, PTEN can also serve as a tumor suppressor in a PI3K-independent manner, playing a specific role in cell proliferation, migration, invasion and apoptosis.10–12) Besides, PTEN has been proven to exert an anti-tumor role in colorectal cancer (CRC), non-small cell lung cancer (NSCLC), glioma and other tumors.13–15) For instance, studies have revealed that down-regulation of NR2F2-AS1 reduces cell proliferation and apoptosis in nasopharyngeal carcinoma (NPC) by up-regulating PTEN.16) Also, existing studies have demonstrated that F-box protein 11 promotes the growth, metastasis and epithelial–mesenchymal transition (EMT) of gastric cancer via activating PI3K/AKT pathway through repressing PTEN level.17) Interestingly, previous study has found that Scu shows inhibitive effects on the progression of human renal cancer by upregulating PTEN.18) Nevertheless, whether Scu modulates gastric cancer through PTEN pathway remains unclear.
Here, we explore the functions of Scu in the progression of gastric cancer. The results showed that Scu dampened cell proliferation and promoted the apoptosis of gastric cancer cells. Moreover, Scu inactivated PI3K and up-regulated PTEN expression. Therefore, we supposed that Scu is a promising anti-tumor drug in gastric cancer.
Scu (CAS No.: 27740-01-8) was obtained from MexChemExpress (NJ, U.S.A.). For in vitro experiment, Scu was dissolved in dimethyl sulfoxide (DMSO) and diluted by phosphate-buffered saline (PBS), Then the cells were treated with different doses of Scu (0–80 µM). For in vivo experiment, Scu was dissolved and diluted in 0.5% carboxymethyl cellulose (CMC)-Na/saline wate. The nude mice was treated with Scu (10–20 mg/kg body weight).
Cell CultureThe gastric cancer cell lines including MGC-803 and AGS, and gastric epithelial cell line GES-1 were obtained from the Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). All of the cells were cultured in RPMI1640 containing 10% fetal bovine serum (FBS, Invitrogen, Carlsbad, CA, U.S.A.), accompanied with 100 U/mL penicillin and 100 U/mL streptomycin (Invitrogen). Then the cells were incubated in a constant temperature incubator at 37 °C with 5% CO2 and saturated humidity. Different doses of Scu (0–100 µM) were applied to the gastric cancer cell lines as well as gastric epithelial cell line for 24 h, then the cells were collected for further usages.
Cell TransfectionMGC-803 cells at the logarithmic growth stage were inoculated in 6-well plates at 5 × 106/well after trypsinization and passage. Cells were transfected after stable growth. Briefly, small inference RNA targeting PTEN (si-PTEN) and corresponding negative control (si-NC) (GenePharma, Shanghai, China), were transfected into the above cells, respectively according to the specification of the FuGENE®HD Transfection Reagent (Roche, Shanghai, China). Afterward, the cells were cultured in an incubator at 37 °C with 5% CO2 and saturated humidity. After transfection for 24 h, total RNA was extracted for RT-PCR to monitor the expression change of PTEN in the cells.
Cell Counting Kit-8 (CCK-8) AssayFirst of all, 2 × 103 GES-1, MGC-803 and AGS cells were taken each, and inoculated into 96-well plates. After adherent culture for 24 h, the cells were treated with Scu for 24, 48, and 72 h then each well was filled with 10 µL CCK-8 solution. Subsequently, the culture plates were incubated for 4 h, and the absorbance at 450 nm was measured with a microplate reader. The proliferation alterations of the above cells were determined.
Flow CytometryAfter being treated with different factors, gastric cancer cells were trypsinized, and collected through centrifugation (1500 r/min, 3 min). The obtained cells were treated according to the instructions of the apoptosis detection kit (Shanghai Aladdin Biochemical Technology Co., Ltd., China) as follows: After washing cells with PBS twice, 400 µL pre-cooled PBS was added, and 10 µL AnnexinV-fluorescein isothiocyanate (FITC) and 5 µL propidium iodide (PI) were supplemented, respectively. Subsequently, the cells were incubated at 4 °C in the dark for 30 min, and then determined by flow cytometry immediately. The percentage of apoptotic cells was calculated by computer software.
RT-PCRThe cells in each group were collected after Scu treatment, and the total RNA was extracted with the TRIzol reagent (Invitrogen). After the purity test, the total RNA was reverse-transcribed into cDNA using RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, U.S.A.). The synthetic reaction conditions of cDNA were: 37 °C for 40 min, 85 °C for 5 s. RT-PCR was performed with the SYBRGreen method using PTEN specific primers, and cDNA served as the template. PCR reaction conditions were as follows: 95 °C for 30 s, 95 °C for 5 s, 60 °C for 30 s, 73 °C for 10 s, and 40 cycles were conducted. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal contrast of PTEN, and the relative expression of PTEN was calculated by 2−ΔΔCT method. Primer sequences:
PTEN: forward, 5′-AGG CAG TAG AAG GGG AGA GA-3′, reverse, 5′-TCC TCG GCT TCT CCT GAA AG-3′.
GAPDH: forward, 5′-CTC CTC CTG TTC GAC AGT CAG C-3′, reverse 5′-CCC AAT ACG ACC AAA TCC GTT-3′.
Western BlotAfter the cells were treated at different doses of Scu, the original culture medium was discarded, and the radio immunoprecipitation assay (RIPA) (containing 1% phenylmethylsulfonyl fluoride (PMSF)) lysate was used to trypsinize the cells. The cells were collected through low-speed centrifugation, and the total protein was extracted. Afterward, the Bradford method was adopted to quantify proteins, which were boiled for 5 min and cooled on ice. After that, the protein was centrifuged for 30 s, and the supernatant was taken for polyacrylamide gel electrophoresis at 100 v and transferred to the membranes for 1 h. Subsequently, 5% skim milk was employed to block the polyvinylidene fluoride (PVDF) membranes at room temperature for 1 h at 4 °C. Then, the membranes were incubated with Anti-E-caderin antibody (1 : 1000, ab40772, Abcam, MA, U.S.A.), Anti-Vimentin antibody (1 : 1000, ab92547, Abcam), Anti-N-cadherin antibody (1 : 1000, ab18203, Abcam), Anti-PTEN antibody (1 : 1000, ab267787, Abcam), Anti-PI3K antibody (1 : 1000, ab32089, Abcam), Anti-p-PI3K antibody (1 : 1000, ab182651, Abcam), Anti-Bax antibody (1 : 1000, ab32503, Abcam), Anti-Bcl-2 antibody (1 : 1000, ab32124, Abcam) and Anti-Caspase-3 antibody (1 : 1000, ab13847, Abcam) overnight. Next, the membranes were rinsed with TBST twice, and incubated with fluorescein-labeled anti-rabbit antibody for 1 h at room temperature. After 3 times of washing, the membranes were exposed with ECL colorant and imaged with a scanner.
Tumor Formation Experiment in Nude MiceThirty nude mice (4–6 weeks old, well developed, without underlying diseases) were selected and bred under aseptic conditions and randomly divided into three groups. The gastric cancer cell line MGC-803 at the logarithmic phase were adjusted to reach a cell concentration of 2 × 108 mL−1. Then, 0.1 mL cell suspension was injected subcutaneously in the left forearm of each nude mouse. After injection, the mice were kept raised. After 24 h, 10 and 25 mg/kg body weight of Scu were subcutaneously injected into the mice in two of the group, respectively, and 10 mg/kg sterile saline was subcutaneously injected into the other group. The mice in all of the three groups were then raised under the same sterile conditions. The survival rate, body weight and survival status of the mice were monitored within four weeks after injection. Meanwhile, tumor tissues of the three groups of newly dead nude mice were completely stripped and weighed, and the long diameter (a) and short diameter (b) of the tumors were accurately measured with a vernier caliper for 3 times each, and the mean value was taken to calculate the tumor volume (V), V = 0.5 × a × b2.
Statistical AnalysisIn the experiment, three repetitive wells were set for each group of cells, and cells in different batches were repeated at least three times. SPSS17.0 statistical software (SPSS Inc., Chicago, IL, U.S.A.) was adopted to analyze the experimental data. The measurement data with normal distribution were expressed as mean ± standard deviation (X ± s).
To confirm the cytotoxic effect of Scu on normal healthy cells, the gastric epithelial cell line GES-1 were induced by different doses of Scu and then the viability of the cells was detected. The result indicated that Scu had no obvious cytotoxic effect on GES-1 cells until the concentration reached to 40 µM (Fig. 1A). Next, the gastric cancer cells MGC-803 and AGS were treated with Scu at different doses (5, 10, and 25 µM) to preliminarily probe into the function of Scu in the growth and apoptosis of gastric cancer cells. CCK8 experiment was conducted to test cell proliferation in gastric cancer. It was discovered that Scu dampened the proliferation of gastric cancer cells dose-dependently (p < 0.05, Fig. 1B). Flow cytometry was implemented to verify the apoptosis of gastric cancer cells, and it was found that Scu significantly enhanced the apoptotic level (p < 0.05, Fig. 1C). Western blot was employed to monitor the expression of apoptotic proteins (Bax, Bcl2 and Caspase3). As a result, Scu promoted the pro-apoptotic proteins Bax and Caspase3, but attenuated the anti-apoptotic proteins Bcl2 (Figs. 1D, E). Further, the expression of EMT-related proteins (E-cadherin, Vimentin and N-cadherin) was compared by Western blot. Interestingly, Scu promoted E-cadherin, while dampened Vimentin and N-cadherin (Figs. 1F, G). The above results illustrated that Scu suppressed the proliferation, EMT of gastric cancer cells, and enhanced the apoptosis.
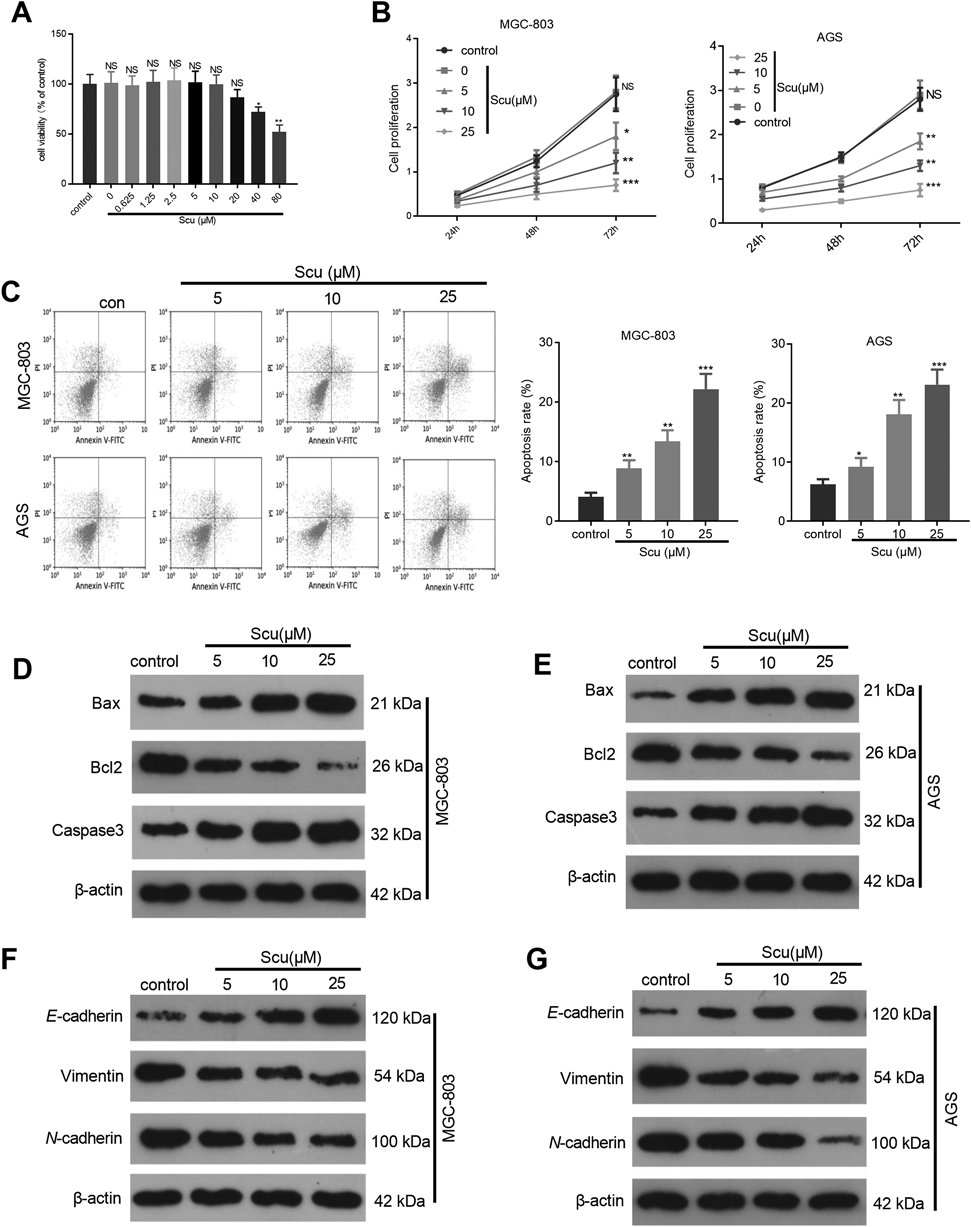
A.GES-1 cells were induced by different doses of Scu (0–80 µM) and their viability was detected by CCK8 assay. B. Gastric cancer cells (MGC-803 and AGS) were treated with different doses of Scu (0–25 µM) and their viability was detected by CCK8 assay. C. Flow cytometry was implemented to examine the apoptosis of gastric cancer cells. D, E. The level of apoptosis-related proteins (Bax, Bcl2 and Caspase3) was verified by Western blot. F, G. The expression of EMT markers (E-cadherin, Vimentin and N-cadherin) was monitored by Western blot. NS p > 0.05, * p < 0.05, ** p < 0.01, *** p < 0.0 1 (vs. control group). N = 3.
PTEN is a newly-discovered protein with anti-tumor effect. In this study, we were also curious about the expression characteristics of PTEN in gastric cancer cells. RT-PCR was carried out to determine the level of PTEN mRNA in gastric cancer cells, and the results testified that Scu distinctly up-regulated the expression of PTEN (p < 0.05, Figs. 2A, B). Further, the expression of the PTEN/PI3K signaling pathway was monitored by Western blot to explore the molecular mechanism of Scu affecting gastric cancer cells. As a result, the protein level of PTEN was up-regulated, while the phosphorylated PI3K was down-regulated with the increase of Scu doses (Figs. 2C, D). The above results illustrated that Scu inactivated PI3K by up-regulating PTEN.
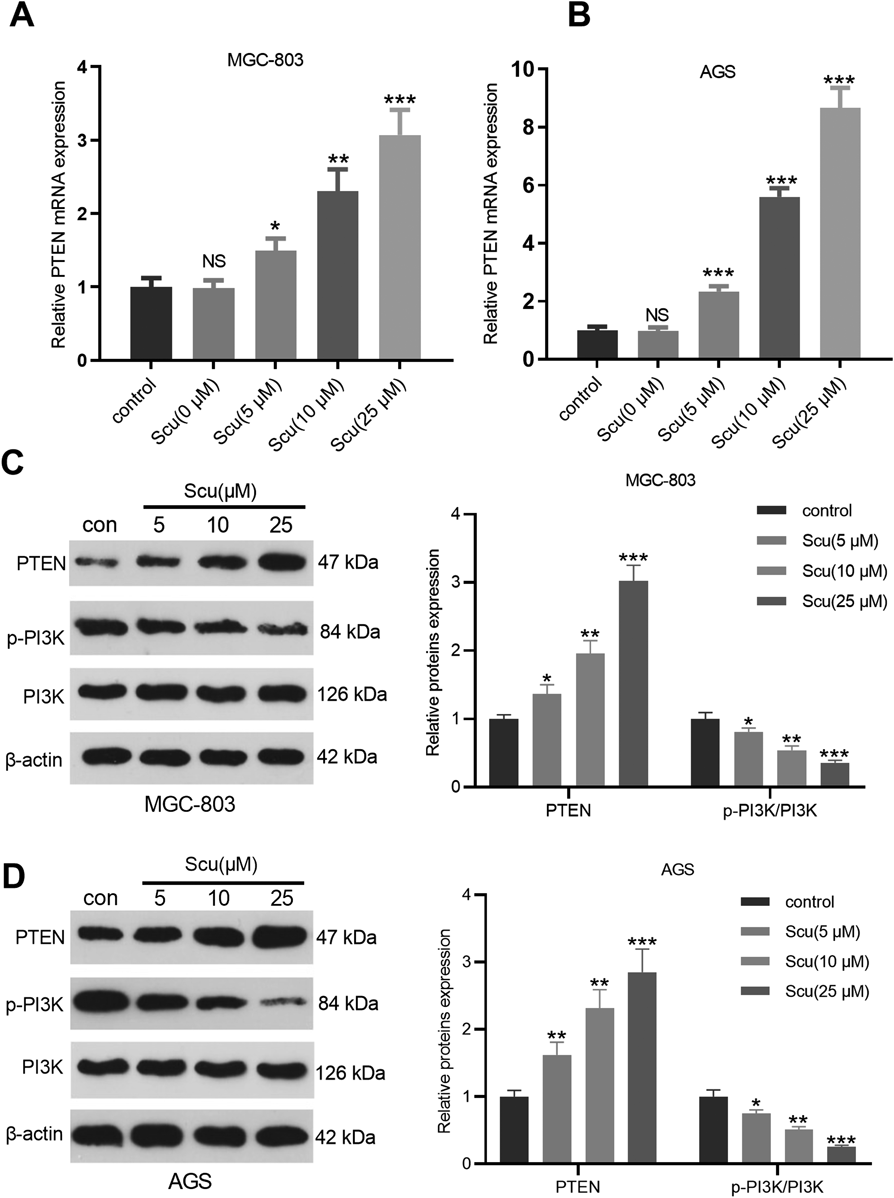
Different doses of Scu (0, 5, 10, and 25 µM) were applied to gastric cancer cell lines (MGC-803 and AGS) for 48 h. A, B. RT-PCR was performed to test the expression of PTEN mRNA in gastric cancer cells. C–D. The protein expression of PTEN/PI3K signaling pathway in gastric cancer cells was examined by Western blot. NS p > 0.05, * p < 0.05, ** p < 0.01, *** p < 0.01 (vs. control group). N = 3.
We conducted in vivo experiments to further explore the effect of Scu on gastric cancer cells. Briefly, nude mice aged 4–6 weeks were selected as experimental subjects to construct in vivo tumor models. The volume and weight of tumor tissues were measured, and the data indicated that they were markedly dampened by Scu (p < 0.05, Figs. 3A–C). Additionally, Western blot was implemented to verify the expression of apoptosis- and EMT-related proteins in nude mice. As a result, Scu enhanced Bax and caspase-3, and attenuated Bcl2 (Fig. 3D). Besides, the detection of EMT-related proteins manifested that Scu strengthened E-cadherin, while suppressed Vimentin and N-cadherin (Fig. 3E). Moreover, the detection of the PTEN/PI3K signaling pathway showed that Scu also up-regulated PTEN, while down-regulated the phosphorylated PI3K (Fig. 3F). These results illustrated that Scu dampened the growth and EMT of gastric cancer cells in vivo.

Nude mice aged 4–6 weeks were selected as experimental subjects to construct a nude mouse model in vivo, and then treated by Scu at different doses. A–C. The tumor volume and weight were measured. D–F. The expression of apoptosis-related proteins (Bax, Bcl2 and Caspase3), EMT markers (E-cadherin, Vimentin and N-cadherin) and the PTEN/PI3K signaling pathway in the tumor tussues was examined by Western blot. *** p < 0.001 (vs. control group). N = 5.
We constructed a PTEN knockdown model to clarify the anti-tumor mechanism of Scu via PTEN (p < 0.01, Fig. 4A). By detecting the mRNA expression of PTEN, we found that Scu enhanced PTEN level (Fig. 4B). Next, the proliferation and apoptosis of gastric cancer cells were detected. The results revealed that knockdown of PTEN promoted the growth and attenuated the apoptosis of gastric cancer cells (Figs. 4C, D). However, compared with si-NC + Scu group, the proliferation of gastric cancer cells in si-PTEN + Scu group was enhanced, while the apoptosis was mitigated (Figs. 4C, D), indicating that knockdown of PTEN reduced the anti-tumor effects of Scu. Furthermore, Western blot assay was implemented to test the level of apoptosis-related proteins. As a result, knocking down PTEN dampened the promotion of Scu on the pro-apoptotic proteins Bax and Caspase-3 and the inhibition of Scu on anti-apoptotic proteins Bcl2 (Fig. 4E). Meanwhile, knocking down PTEN abated the enhancement of Scu on E-cadherin, and the attenuation of Scu on Vimentin and N-cadherin expressions (Fig. 4F). What is more, the protein level of PTEN was promoted and phosphorylated PI3K was attenuated by Scu, and downregulating PTEN facilitated PI3K level (Fig. 4G). The above findings concluded that Scu modulated the proliferation, apoptosis and EMT of gastric cancer cells partly through the PTEN/PI3K signaling pathway.

A. Gastric cancer cells (MGC-803) were selected to construct the PTEN knockdown model. B. MGC-803 cells with lower level of PTEN were treated by Scu (10 µM), the PTEN mRNA level was detected by RT-PCR. C. CCK8 assay was adopted to verify the proliferation of gastric cancer cells. D. Flow cytometry was employed to examine the apoptosis of gastric cancer cells. E–G. The expression of apoptosis-related proteins (Bax, Bcl2 and Caspase3), EMT markers (E-cadherin, Vimentin and N-cadherin) and the PTEN/PI3K signaling pathway was examined by Western blot. * p < 0.05, ** p < 0.01, *** p < 0.01 (vs. control group).
Until now, gastric cancer remains one of the most fatal malignant tumors in the world. The 1-year relative survival rates (RSRs) of gastric cancer patients have been improved while the long-term survival rates still remained low, even the 10-year RSRs are less than 20%,3) so it is urgent to explore new treatment methods. In the current study, we investigated the anti-tumor effects of Scu on gastric cancer cells, and the results demonstrated that Scu attenuated the development of gastric cancer through modulating PTEN/PI3K signal pathway (Fig. 5).
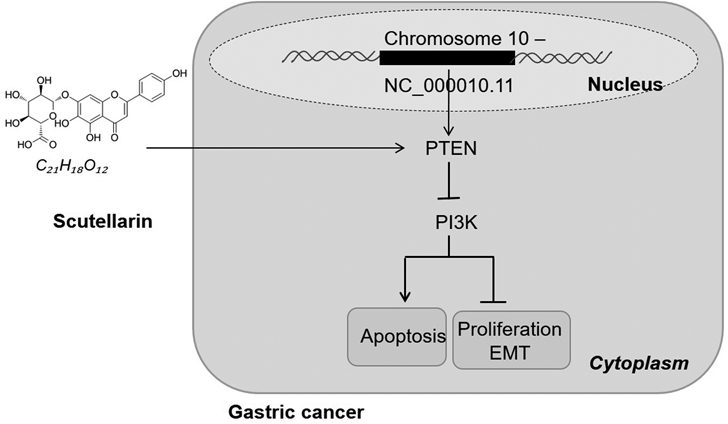
Scu inactivated PI3K to promote apoptosis and inhibited the proliferation and EMT of gastric cancer cells by up-regulating PTEN.
Scu, as an active flavone isolated from Scu baicalensis, has attracted increasing attention due to its inhibitory effect on tumors. Actually, Scu baicalensis is an important traditional Chinese medicine with the functions of clearing away heat and dampness, purging fire and detoxification.19) The active components of Scu baicalensis, such as baicalin, wogonoside and wogonin, all have been found to play a role in tumor progression. For example, baicalin inhibits the proliferation and aggravates the apoptosis of gastric cancer cells (BGC-823 and MGC-803) via downregulating Bcl-2.20) Wogonoside (Wg) mitigated cell viability and induced apoptosis in human GC cell lines AGS and SGC-7901 through induction of reaction oxygen species accumulation, mitochondrial dysfunction, and endoplasmic reticulum stress.21) What is more, Wogonin potentiates gastric cancer cells to 5-fluorouracil therapy through attenuating the activation of nuclear factor-kappa B (NF-kappaB) pathway.22) Interestingly, Scu has also proved to induce the development of multiple cancers, including bladder cancer,23) colorectal cancer24) and malignant melanoma.25) Here, we discovered that Scu abates the proliferation and invasion of gastric cancer cells and enhances the apoptosis in a dose-dependent manner both in vitro and in vivo. Therefore, Scu is believed to be another promising candidate in treating gastric cancer.
PTEN is one of the most frequent mutated tumor-suppressor genes in human cancer. Several studies have found that PTEN contributes to declining the growth of cancer cells. For example, PTEN exerts a critical role in modulating self-renewal, migration, and differentiation of hematopoietic stem cells (HSC) by neutralizing the PI3K/AKT/mammalian/mechanistic target of rapamycin (mTOR) pathway.26) Also, studies have shown that several drugs can suppress the proliferation and induces apoptosis of malignant tumors via modulating PTEN. For instance, Galangin has an anti-tumor role in cholangiocarcinoma via PTEN through inhibiting microRNA-21 expression.27) Shikonin suppresses endometrioid endometrial cancer development via modulating miR-106b/PTEN/AKT/mTOR signaling pathway.28) In this study, we found that PTEN level was significantly promoted by Scu, which was associated with the downregulation of PI3K pathway by Scu. To further confirm the role of PTEN in Scu induced effects, we established a PTEN knockdown model and found that the PTEN knockdown partly weakened the proliferation inhibitive and the apoptosis promotive functions of Scu on gastric cancer cells. Therefore, Scu could attenuate gastric cancer growth not only dependently via PTEN pathway.
The PI3K signaling pathway is a frequently altered pathway in human cancer and contributes to driving the occurrence and development of tumors.29) As a key link in the intracellular signaling pathway, PI3K has been found in diversified tumors and diseases, such as prostate cancer, breast cancer, pancreatic cancer, hematological malignancies, and bladder cancer.30–34) Therefore, the study of PI3K inhibitors has become a new clinical means to fight cancer, while the toxic and side effects of drugs limit their long-term clinical application. Studies have reported that PI3K or AKT inhibitors may make liver cells highly sensitive to apoptosis and liver injury induced by fatty acid synthase (FAS) or tumor necrosis factor-alpha (TNFα).35,36) Here, we discovered that Scu negatively regulated PI3K, and the knockdown of PTEN attenuated the inhibitory effect of Scu via upregulating PI3K. Hence, the PTEN/PI3K pathway is a potential pathway induced by Scu in gastric cancer (Fig. 5).
Overall, Scu facilitates the PTEN level and dampens the progression of gastric cancer cells. This study better explored the underlying mechanism of Scu in abating gastric cancer, providing new theoretical support for its treatment.
The authors declare no conflict of interest.