2021 Volume 44 Issue 6 Pages 789-797
2021 Volume 44 Issue 6 Pages 789-797
Sleep curtailment negatively affects cardiac activities and thus should be ameliorated by pharmacological methods. One of the therapeutic targets is melatonin receptors, which tune circadian rhythms. Ramelteon, a melatonin MT1/MT2 receptor agonist, has recently been developed to modulate sleep-wake rhythms. To date, the sleep-promoting effect of ramelteon has been widely delineated, but whether ramelteon treatment physiologically influences cardiac function is not well understood. To address this question, we recorded electrocardiograms, electromyograms, and electrocorticograms in the frontal cortex and the olfactory bulb of unrestrained rats treated with either ramelteon or vehicle. We detected vigilance states based on physiological measurements and analyzed cardiac and muscular activities. We found that during non-rapid eye movement (non-REM) sleep, heartrate variability was maintained by ramelteon treatment. Analysis of the electromyograms confirmed that neither microarousal during non-REM sleep nor the occupancy of phasic periods during REM sleep was altered by ramelteon. Our results indicate that ramelteon has a remedial effect on cardiac activity by keeping the heartrate variability and may reduce cardiac dysfunction during sleep.
Functional alterations in the autonomic and somatic nervous system induce various changes in organ systems during sleep.1) When sleep is divided into rapid eye movement (REM) and non-REM sleep states based on physiological measurements,2,3) for instance, sympathetic nervous activity is shown to increase and decrease during REM and non-REM sleep, respectively, compared to parasympathetic activity.4) Moreover, some metabolic and physiological functions are modulated and maintained during sleep.1,5,6) In particular, non-REM sleep is considered the most restorative state.1,7) Conversely, once sleep is chronically curtailed or insufficient, several peripheral (e.g., cardiovascular, respiratory, pulmonary, gastrointestinal, and renal) functions are impaired in humans.8–10) Indeed, inadequate sleep duration in humans may confer a risk for cardiovascular dysfunctions.11,12) Thus, pharmacologic strategies have been undertaken to improve such sleep disorders.
One of the therapeutic targets for sleep disorders is the melatonin receptor family composed of MT1 and MT2. Melatonin endogenously acts through MT1/MT2 receptors in the brain and fine-tunes circadian rhythms.13–17) This physiological evidence has led to the development of a tricyclic synthetic analog of melatonin called ramelteon, a selective MT1/MT2 receptor agonist.18–25) To date, the sleep-promoting effects of ramelteon for managing chronic insomnia have been widely documented. In addition to the effects on the central system, the cardioprotective effects of ramelteon have been investigated at the molecular and cellular levels.26–30) However, it remains uncertain whether and how ramelteon physiologically modulates the cardiac system during waking and sleep periods.
To address this question, we simultaneously recorded electrocardiograms (ECGs), electromyograms (EMGs), and electrocorticograms (ECoGs) in the frontal cortex (Fr) and the olfactory bulb (OB) of unrestrained rats treated with ramelteon and vehicle.31–33) We classified murine behavior into three vigilance states (i.e., awake, REM sleep, and non-REM sleep) based on neural oscillatory activity in the OB and the Fr and physiologically investigated the modulation of cardiac signals by ramelteon administration.
Animal experiments were performed with the approval of the Animal Experiment Ethics Committee at the University of Tokyo (Approval No. P29-7) and according to the University of Tokyo guidelines for the care and use of laboratory animals. These experimental protocols were carried out in accordance with the Fundamental Guidelines for the Proper Conduct of Animal Experiments and Related Activities of the Academic Research Institutions (Ministry of Education, Culture, Sports, Science and Technology, Notice No. 71 of 2006), the Standards for Breeding and Housing of and Pain Alleviation for Experimental Animals (Ministry of the Environment, Notice No. 88 of 2006) and the Guidelines on the Method of Animal Disposal (Prime Minister’s Office, Notice No. 40 of 1995). While our experimental protocols have a mandate to humanely euthanize animals if they exhibit any signs of pain, prominent lethargy, and discomfort, we did not observe such symptoms in any of the rats tested in this study. All efforts were made to minimize the animals’ suffering.
AnimalsA total of six male 8- to- 10-week-old Wistar rats (Japan SLC, Shizuoka, Japan) with preoperative weights of 180–300 g were housed individually under conditions of controlled temperature and humidity (22 ± 1 °C, 55 ± 5%) and maintained on a 12 : 12-h light–dark cycle (lights off from 7:00 to 19:00) with ad libitum access to food and water unless otherwise specified. Rats were habituated to an experimenter by daily handling.
PreparationA recording interface assembly was prepared as previously described.31–33) In short, the assembly was composed of an electrical interface board (EIB) (EIB-36-PTB, Neuralynx, Inc., Bozeman, MT, U.S.A.) and shell and core bodies custom-made by a three-dimensional printer. The EIB had a sequence of metal holes for connections with wire electrodes. A given individual hole was conductively connected with one end of the insulated wire (approx. 5 cm) using attachment pins, whereas the opposite end was soldered to a corresponding individual electrode during surgery.
SurgeryWe performed the following stereotaxic surgery in accordance with our previous literature,34) and basically conformed to almost the same procedure for reliable data.
We induced and maintained general anesthesia for rats using 2–3 and 1–2% isoflurane gas, respectively, followed by the application of veterinary ointment to the rats’ eyes to prevent drying. We sterilized the skin with povidone iodine and 70% ethanol whenever we were about to make an incision.
After we confirmed that depth of anesthesia was adequate, we implanted electrodes for ECGs, EMGs, and ECoGs as previously described.33,35) Briefly, we placed a rat onto a stereotaxic apparatus (SR-6R-HT, Narishige, Tokyo, Japan).36) We attached one wire electrode to the left pectoralis muscle to record cardiac signals (ECGs), whereas we implanted another electrode into the trapezius to record myogenic potentials (EMGs). Following the careful removal of the scalp with a surgical knife, we performed a small circular craniotomy. To record ECoGs, we stereotaxically implanted screw electrodes bilaterally into the Fr (3.2 mm anterior and 3.0 mm lateral to bregma) and the OB (10.0 mm anterior and 1.0 mm lateral to bregma). In addition, two other stainless-steel screws were implanted in the bone above the cerebellum (9.6 mm anterior and 1.0 mm bilateral to bregma) as ground and reference electrodes.
After the surgery, we allowed each animal to recover from anesthesia. The animal was housed individually in a cage with free access to water and food. For the first 2 d after surgery, we carefully monitored the health of all animals. After full recovery from surgery and sufficient habituation to experimenters, we moved on to electrophysiological recordings.
DrugRamelteon (184-03371, FUJIFILM Wako, Osaka, Japan) was dissolved in dimethyl sulfoxide (DMSO) at a concentration of 200 mg/mL. This solution was diluted 100 times with saline solution at a final concentration of 2 mg/mL immediately before use. Saline with 1% DMSO was used as a vehicle control solution.
In Vivo ElectrophysiologyAfter full recovery from surgery and familiarization with the home chamber,35) we recorded electrophysiological signals for two days. We started the experiments at 9 : 30 a.m. for either day. On the first day, each rat was gently placed in the open field, and the recording electrode assembly was connected to a digitally programmable low-noise amplifier (C3324, Intan Technologies, CA, U.S.A.). The output of the head stage was conducted through an SPI cable (C3216, Intan Technologies) and a commutator to the acquisition board (Open Ephys, MA, U.S.A.).37) We recorded ECoGs, EMGs and ECGs for 3 h. Following the recording, either saline or ramelteon (5 mL/kg for either) was intraperitoneally administered to each rat at 12 : 30 p.m. (i.e., half past noon during the dark (nocturnal) period for rats). Immediately (approx. 10 min) after the administration, we placed each rat in the open arena and recorded its biosignals for 3 h. On the second day, we performed the same experiment with the other drug of saline or ramelteon in a randomized crossover design. For all recordings, the electrophysiological signals were amplified and digitized at 2 kHz.
HistologyAfter the recording, rats were anesthetized with an overdose of isoflurane gas and transcardially perfused with 4% paraformaldehyde (PFA) in 0.01 M phosphate-buffered saline (pH 7.4) followed by decapitation. Brains were soaked in 4% PFA for post-fixation overnight and coronally sectioned at a thickness of 100 µm using a vibratome. Acute serial slices were mounted on glass slides and processed for cresyl violet staining to localize the recording electrode. For cresyl violet staining, the slices were rinsed in water, ethanol and xylene, counterstained with cresyl violet, and coverslipped with a mounting agent. The positions of all screw electrodes were confirmed by identifying dents on the superficial layer in histological tissue. Data were excluded from subsequent analysis if the electrode position was out of the target brain region. Cresyl violet-stained images were acquired using a phase-contrast microscope (BZ-X710, Keyence, Osaka, Japan) (Fig. 1).

Representative post hoc histology of the recording site shown in the Nissl-stained section and the simplified brain atlas. The screw electrode placement was indicated by the red dot. Fr, frontal cortex. (Color figure can be accessed in the online version.)
All data analyses were performed using custom-made MATLAB routines (MathWorks, MA, U.S.A.). The summarized data are reported as the mean ± the standard error of the mean (S.E.M.). The null hypothesis was statistically rejected when p < 0.05 unless otherwise specified.
Here we analyzed EMGs and ECGs along with ECoGs from the OB and the Fr, whereas we previously analyzed EMGs simultaneously with ECoGs from the OB, the primary motor cortex, and the primary somatosensory cortex.34)
The recording periods dominated by apparent electrical noise caused by physical impact when a rat hit its head on the walls were manually excluded from analysis. To define awake and sleep states, we first divided the recording periods into 5-s segments. We band-pass filtered OB ECoGs into the gamma (50–70 Hz) frequency band using fast Fourier transform (FFT). We calculated the gamma power for each segment to obtain the consecutive gamma power signal.38) The two vigilance states were then detected by applying the gamma signal to a Schmitt trigger circuit with two thresholds, followed by manual post hoc adjustments based on visual inspection of EMGs.39) We further employed the difference in the value before and after drug injection, which is hereafter signified as Δ.
We next calculated the ratio of the power in the delta band (0.3–4 Hz) to theta band (4–8 Hz) of Fr ECoGs for each 5-s segment to obtain the consecutive delta-theta ratio signal. We further classified sleep states into REM and non-REM sleep states based on the delta-theta ratio, followed by manual cross-validation.39)
To elucidate the effect of ramelteon on cardiac functions, we calculated heartrate and its variability (i.e., HRV). As an index of HRV, we calculated the standard deviation of R-R (interbeat) intervals (i.e., SDRR), the time intervals between two successive R-waves of the QRS complex on the ECGs. In general, SDNN, the standard deviation of normal-to-normal intervals, is also used as an index of HRV. To calculate SDNN, abnormal (e.g., ectopic) beats are first removed (because they may mirror cardiac artifacts and/or dysfunctions that masquerade as normal beats), and the normal R-waves must be detected.40) In this study, we did not observe abnormal beats in the ECGs of rats tested. Thus, we speculated that SDNN and SDRR were similar in the current study.40) Moreover, strictly speaking, sleep/waking time varies between ramelteon- and vehicle-treated rats. However, the absolute values of total sleep time were not significantly different between ramelteon- and vehicle-treated conditions for pre-administration (p = 0.34, t4 = 1.07, n = 5 rats, paired t-test). This is also the case for post-administration (p = 0.25, t4 = 1.36, n = 5 rats, paired t-test).
Because the EMGs that did not meet our criteria were discarded, the data of five rats were used in subsequent analysis. To identify microarousal events during non-REM sleep,41–43) we downsampled the EMGs to 100 Hz and set three times the standard deviation of the downsampled EMGs as the threshold. We defined a microarousal event as transiently fast motor burst activity, where suprathreshold EMGs lasted for at least 0.3 s. In contrast, REM sleep comprises tonic and phasic muscular activity.44,45) To quantify the phasic periods of REM sleep, we downsampled raw EMGs from 2 kHz to 100 Hz and determined the 99th percentile of the downsampled EMG signals during the first 5 s of each REM bout. We determined muscle twitches as instantaneous motor events that exceeded the 99th percentile threshold. A given 5-s REM bout was classified as phasic REM sleep if muscle twitches were detected.44)
We further analyzed time–course changes in HRV (indicated by SDRR) using the FFT and represented in the frequency-based domain. Using the Fourier spectra, we calculated the low-frequency (LF; 0.04–1 Hz) and high-frequency (HF; 1–3 Hz) powers and the ratio of the low-frequency power to the high-frequency power.46) We regarded the HF power as an index of parasympathetic nerve activity, whereas sympathetic nervous function was evaluated based on the power ratio between LF and HF powers.
We simultaneously recorded the ECoGs of Fr and OB and ECGs of freely behaving rats before and after treatment with either saline or ramelteon in a randomized crossover manner (Figs. 1, 2). Using the gamma power of the OB ECoGs,38) we divided the recording periods into sleep and awake states. We then band-passed the Fr ECoGs in the delta and theta frequency ranges (Fig. 2). With the delta-to-theta power ratio during the sleep states (see Materials and methods), we further discriminated between REM and non-REM periods (Fig. 3).
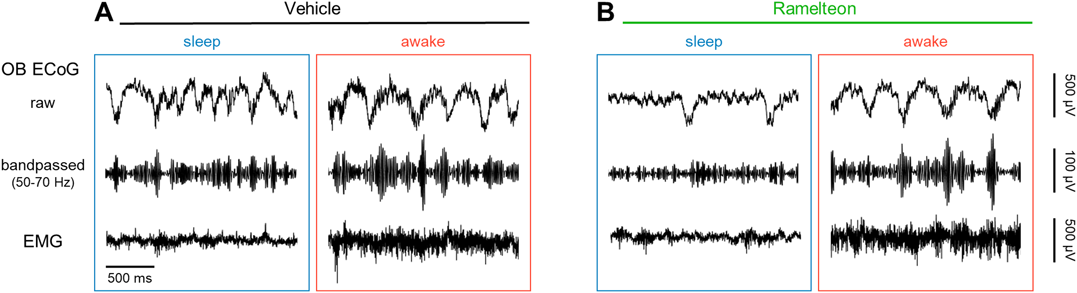
A, Left: Representative raw (first row) and band-pass filtered (50–70 Hz (second)) traces of ECoGs in the OB during sleep in vehicle-treated rats. Right: The same as left, but during wakefulness. B, The same as A, but for ramelteon-treated rats. ECoG, electrocorticogram; EMG, electromyogram; OB, olfactory bulb. (Color figure can be accessed in the online version.)
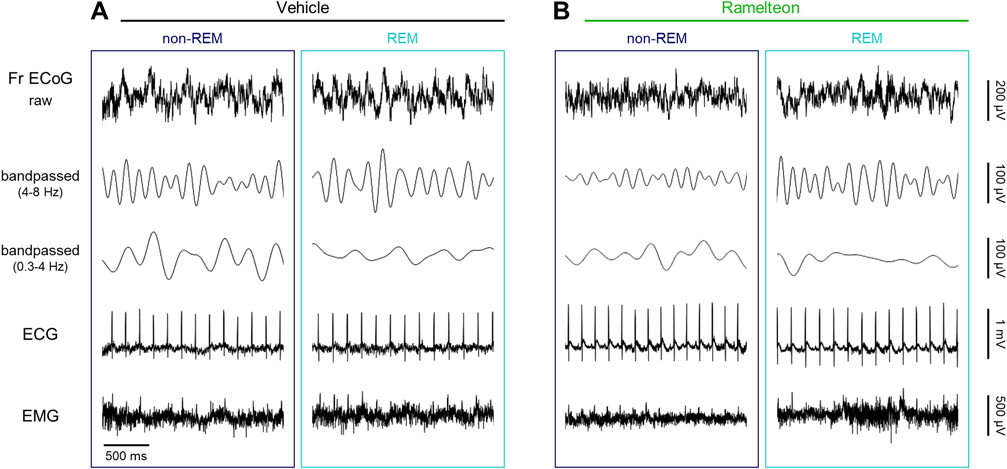
A, Left: Representative traces of raw (first row) and band-pass filtered (4–8 Hz and 0.3–4 Hz (second and third, respectively)) Fr ECoGs, ECGs (fourth), and EMGs (fifth) during non-REM sleep in vehicle-treated rats. Right: The same as left, but during REM sleep. B, The same as A, but for ramelteon-treated rats. ECoG, electrocorticogram; ECG, electrocardiogram; EMG, electromyogram; Fr, frontal cortex; REM, rapid eye movement. (Color figure can be accessed in the online version.)
To investigate whether and how ramelteon modulated cardiac activity during the awake, REM and non-REM states (Fig. 3), we calculated the heartrate in four conditions (i.e., before vehicle administration, after vehicle administration, before ramelteon administration, after ramelteon administration) (Supplementary Table 1). We then subtracted the heartrate after drug (i.e., vehicle or ramelteon) administration from that before drug administration, which was indicated by ‘ΔHeartrate’ (Fig. 4). However, we did not find any significant differences in ΔHeartrate between the two conditions during any of the states (non-REM, −0.12 ± 0.29 Hz (vehicle) vs. 0.04 ± 0.13 Hz (ramelteon), p = 0.61, t4 = 0.54, n = 5 rats, paired t-test (Fig. 4A); REM, −0.16 ± 0.27 Hz (vehicle) vs. 0.03 ± 0.12 Hz (ramelteon), p = 0.56, t4 = 0.63, n = 5 rats, paired t-test (Fig. 4B); awake, −0.18 ± 0.10 Hz (vehicle) vs. −0.38 ± 0.23 Hz (ramelteon), p = 0.45, t3 = 0.86, n = 4 rats, paired t-test (Fig. 4C)).
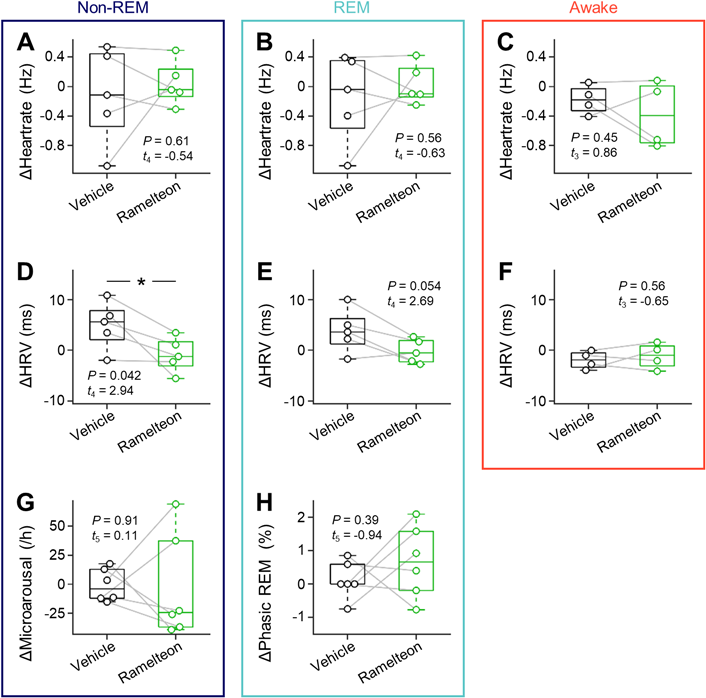
A, Differences in heartrate during non-REM sleep before and after drug (vehicle vs. ramelteon) injection (n = 5 rats). B, The same as A, but during REM sleep (n = 5 rats). C, The same as A, but during wakefulness (n = 4 rats). D, The same as A, but for heartrate variability (n = 5 rats). E, The same as D, but during REM sleep (n = 5 rats). F, The same as D, but during wakefulness (n = 4 rats). G, Increased event rate of microarousals during non-REM sleep (n = 6 rats). H, The occupancy of the phasic periods in the total REM sleep time (n = 6 rats). Statistics was compiled by paired t-test for A–H. HRV, heartrate variability; REM, rapid eye movement. (Color figure can be accessed in the online version.)
To examine whether ramelteon affected the regularity of heartbeats, we calculated the HRV during the three vigilance states under the four conditions (Supplementary Table 1) and estimated ‘ΔHRV’ in a similar way to ΔHeartrate as described above. We found a significant decrease in ΔHRV when ramelteon was administered compared to vehicle during non-REM sleep (5.0 ± 2.1 ms (vehicle) vs. −0.9 ± 1.5 ms (ramelteon), p = 0.042, t4 = 2.94, n = 5 rats, paired t-test (Fig. 4D)). Compared to vehicle, acute ramelteon administration was likely to decrease ΔHRV during REM sleep (3.8 ± 1.9 ms (vehicle) vs. −0.2 ± 1.1 ms (ramelteon), p = 0.054, t4 = 2.69, n = 5 rats, paired t-test (Fig. 4E)), whereas we did not find any significant differences in ΔHRV during the awake period between the two conditions (–2.0 ± 0.9 ms (vehicle) vs. −1.1 ± 1.3 ms (ramelteon), p = 0.56, t3 = −0.65, n = 4 rats, paired t-test (Fig. 4F)).
We further evaluated motor activity-related events during non-REM and REM sleep independently. We first quantified microarousals during non-REM sleep to investigate whether sleep was disturbed.47) We estimated ‘ΔMicroarousal’ in a similar way as described above, but there was no significant difference in ΔMicroarousal (during non-REM sleep) between the two conditions (−0.8 ± 5.7 events/h (vehicle) vs. −3.0 ± 18.4 events/h (ramelteon), p = 0.91, t5 = 0.11, n = 6 rats, paired t-test (Fig. 4G)). We next analyzed REM sleep comprised of both tonic and phasic periods. Tonic REM sleep is characterized by stereotypical periods of muscle atonia, whereas phasic REM sleep is identified by periodic muscle twitches.44) To better elucidate the microstructure of REM sleep, we further classified REM sleep into phasic and tonic episodes based on nuchal muscle twitches.44) We estimated ‘ΔPhasic REM’ in a similar way as described above, but did not observe significant differences in phasic REM bouts between vehicle- and ramelteon-treated conditions (0.1 ± 0.2% (vehicle) vs. 0.7 ± 0.4% (ramelteon), p = 0.39, t5 = −0.94, n = 6 rats, paired t-test (Fig. 4H)).
We additionally calculated the LF and HF power of the R-R intervals using the fast Fourier transform and estimated the ratio of the LF power to the HF power (LF-to-HF ratio)46) (Fig. 5). We estimated ‘ΔLF-to-HF ratio’ and ‘ΔHF power’ to evaluate the sympathetic and parasympathetic activity during three vigilance states under vehicle- and ramelteon-treated conditions, respectively. We found significant differences in ΔLF-to-HF ratio during REM-sleep between the two conditions (−0.82 ± 0.08 (vehicle) vs. 0.66 ± 0.49 (ramelteon), p = 0.04, t4 = −2.96, n = 5 rats, paired t-test (Fig. 5)), whereas there were no significant differences in ΔLF-to-HF ratio during non-REM-sleep or wakefulness (Fig. 5, Supplementary Table 2). On the other hand, we did not find significant differences in ΔHF power between the two conditions for awake, REM sleep, and non-REM sleep (Fig. 5, Supplementary Table 2).
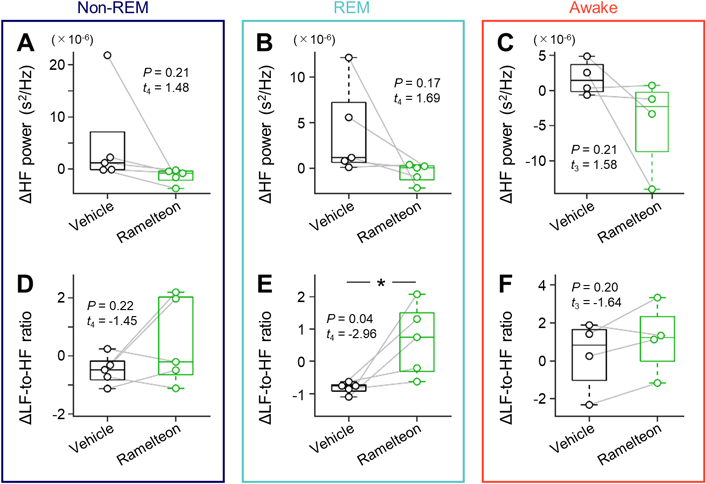
For pre- and post-drug (i.e., vehicle (black) and ramelteon (green)) administration cases, time–course changes in heartrate variability (indicated by the standard deviation of R-R intervals in ECGs) was analyzed using the fast Fourier transform and represented in the frequency-based domain. Using the Fourier spectra, the low-frequency (0.04–1 Hz) and high-frequency (1–3 Hz) powers and the ratio of the low-frequency power to the high-frequency power were calculated. We subtracted the values for post-administration from those for pre-administration for each drug. A, Differences in the low-frequency power during non-REM sleep before and after drug (vehicle vs. ramelteon) injection (n = 5 rats). B, The same as A, but during REM sleep (n = 5 rats). C, The same as A, but during wakefulness (n = 4 rats). D, The same as A, but for ratio of the low-frequency power to the high-frequency power (n = 5 rats). E, The same as D, but during REM sleep (n = 5 rats). F, The same as D, but during wakefulness (n = 4 rats). Statistics was compiled by paired t-test for A–F. LF, low-frequency; HF, high-frequency; ECG, electrocardiogram; REM, rapid eye movement. (Color figure can be accessed in the online version.)
In this study, we found that ramelteon treatment stabilized the heartrate variability in rats, especially during non-REM sleep bouts. Moreover, our analyses of peripheral muscle activity demonstrated that ramelteon administration did not affect microstates during either REM or non-REM sleep compared to vehicle treatment.
As for the mechanism of reduced ΔHRV by ramelteon treatment, we consider three possibilities: (I) autonomic balance, (II) blood pressure and (III) gas exchange.40) Based on the spectral analysis on HRV, we did not find any significant differences in the HF power (as an index of parasympathetic activity) or the LF-to-HF ratio (as an index of sympathetic activity) during non-REM sleep (Fig. 5), suggesting that ramelteon-induced stabilization of HRV is not associated with autonomic balance. Nevertheless, HF and LF components in the frequency domain sometimes merge into a single component (called entrainment) especially when the frequency of breathing decreases.48) Respiratory rates of rats are lower during REM and non-REM sleep than during wakefulness.49) Thus, we are not completely able to exclude the possibility that our spectral analysis misestimated LF and HF powers during sleep.
We next consider the other possibilities for the reduction in ΔHRV. As for blood pressure, previous literatures demonstrated anti-hypertensive effects of ramelteon in both humans and rats.50–52) In addition, high values of blood pressure are associated with lower HRV in both humans and rats.53–56) Thus, we excluded this possibility for our findings. The remaining possibility is pulmonary gas exchange. Improved efficiency of pulmonary gas change is associated with HRV in humans.57,58) This respiratory component of HRV is called respiratory sinus arrhythmia, a phenomenon observed in humans57,58) and rats.59) Although the mechanisms for systemic O2 (oxygen) transport system underlying gas exchange are similar in rats and humans,60) there are some quantitative differences in parameters for the O2 transport system such as p50 (representing hemoglobin-oxygen affinity61)) and PaO2 (standing for the partial pressure of arterial oxygen60)). While ramelteon is known not to worsen gas exchange in humans,62) acute effects of ramelteon treatment on gas exchange in rats remain elusive, to the best of our knowledge. We speculate that ramelteon may affect the pneumonic functions of rats in a different way from those of humans, which leads to the decrease in ΔHRV after ramelteon treatment in rats.
As ramelteon acts through melatonin MT1 and MT2 receptors expressed in the suprachiasmatic nucleus, a responsible region for controlling circadian rhythms,16) the cardiac effect of ramelteon treatment could be associated with the administration timing. We intraperitoneally injected drugs into rats during the dark period and found that HRV was likely stable even after ramelteon administration, whereas vehicle altered HRV (Fig. 4). This phenomenon could result from the difference in the basal (i.e., pre-administration) values of HRV (and its standard deviation (S.D.)) during each vigilance state. The other factors may be that HRV (represented by SDRR) was higher in the dark period than the light period63) and pulmonary gas exchange was more enhanced in the dark period.64) One possible mechanism for HRV-increasing effects of vehicle observed in this study might be that susceptibility of cardiac and pulmonary function to intraperitoneal administration of drugs, although this possibility has not been empirically tested.
In this study, we used naïve (healthy) rats to investigate the cardiac effect of acute ramelteon administration in focus of ‘natural’ HRV. In contrast, unnatural HRV characterizes cardiac arrhythmia. One of the mechanisms underlying cardiac arrhythmia is abnormal impulse formation.65) The abnormal impulse is generally classified into (i) aberrant automaticity and (ii) triggered activity. (i) First, as for automaticity, pacemaker cells in the sinoatrial node in the right atrium are capable of firing spontaneously called normal automaticity. While we assume that cardiac automaticity is normal in the current study, abnormal automaticity occurs when cardiac cells lying outside the sinus node spontaneously fire, producing premature heartbeats. Although every cardiac cell potentially fires spontaneously, such ‘abnormal’ discharge emerges at a lower rate and thus is masked by the normal heartbeat. Under pathological conditions, cardiac cells other than pacemakers may take over. Nevertheless, in the current study, we do not have any direct evidence for involvement of ramelteon in cardiac automaticity. (ii) Next, cardiac muscles sometimes contract twice within a restricted time, called triggered activity. The triggered activity is induced by early and/or delayed afterdepolarizations and leads several types of fatal irregularity of the heartrate, which sometimes deteriorates into sudden cardiac death. The lethal irregular heartbeat is provoked and exacerbated by several factors.66–68) For example, blockers of voltage-gated potassium channels (e.g., amiodarone, nifekalant, sotalol, and quinidine) sometimes induce early afterdepolarizations and eventually cause drug-induced long QT syndrome that increases a risk of torsades de pointes, premature ventricular contraction, and polymorphic ventricular tachycardia.69–73) Another possible factor of the triggered activity is an overloaded Ca2+ store in the sarcoplasmic reticulum. Then, enhanced Ca2+ leak from the sarcoplasmic reticulum via hyperphosphorylated ryanodine receptor 2 leads to delayed afterdepolarizations, which sometimes depolarize the cardiac membrane towards the action potential threshold. This intracellular Ca2+ overload is aggravated by sympathetic nervous hyperactivity.74–79) While the sympathetic nerves innervate the heart and modulate cardiac β adrenergic receptor activity, the parasympathetic nerves, which are derived from vagal nerves, also effectively regulate cardiac activity via M2 muscarinic acetylcholine receptors. These β adrenergic and M2 muscarinic receptors are coupled with Gs and Gi proteins, respectively and have opposite effects on intracellular signaling. MT1 receptors, which are highly expressed in the heart, are coupled with Gi proteins, as well as M2 receptors.80–83) This possible parasympathetic-like function of MT1 receptors may contribute to improvement of cardiac arrhythmia.
For representative clinical use, ramelteon has been proven safe and well tolerated for ameliorating obstructive sleep apnea syndrome.84,85) This syndrome is accompanied by cardiovascular comorbidities, including arrhythmias, hypertension, stroke, and coronary heart disease, in human patients.86) As in humans, a rat model of obstructive sleep apnea syndrome exhibited fast and irregular QRS waves associated with reduced P waves and increased amplitudes of R waves during hypopnea events.87) Moreover, a systematic review study concluded that obstructive sleep apnea is potentially related to sudden cardiac death.88) Herein, we demonstrated that cardiac rhythm variations during sleep are stable in response to ramelteon treatment. Thus, this cardio-friendly effect of ramelteon might be beneficial for reducing the risk of cardiac mortality during sleep, which is in line with the putative antiarrhythmic effect of melatonin, by elevating the repetitive extrasystole threshold,30,89) an index of vulnerability of the ventricular myocardium.90,91)
This work was supported by JST ERATO (JPMJER1801), Institute for AI and Beyond of the University of Tokyo, and JSPS Grants-in-Aid for Scientific Research (18H05525, 20K15926).
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.