2022 年 45 巻 1 号 p. 51-62
2022 年 45 巻 1 号 p. 51-62
Methylglyoxal (MGO), which is produced as a byproduct of glucose metabolism, is the leading to diabetic cardiovascular complications. Salvia miltiorrhiza Bunge (Lamiaceae) has been reported as a potential plant to control diabetes and cardiovascular disease. However, no report exists on the effect of Salvia miltiorrhiza Bunge extract (SME) on MGO-induced glucotoxicity in human umbilical vein endothelial cells (HUVECs). We demonstrated the protective effects of SME (1, 5, and 10 µg/mL) and its components against MGO-induced endothelial dysfunction in HUVECs. Cytotoxicity was evaluated using the several in vitro experiments. Additionally, the protein expression of receptor of advanced glycation end-products (RAGE), mitogen-activated protein kinase (MAPK) pathway and glyoxalase system were measured. Then, the inhibitory effects of SME and its main components on MGO-induced oxidative stress, radical scavenging, formation of MGO-derived advanced glycation end products (AGEs), and MGO-AGEs crosslinking were evaluated. SME (10 µg/mL) strongly prevented expressed levels of RAGE, MGO-induced apoptosis and reduced reactive oxygen species (ROS) generation in HUVECs, comparing with 1 mM aminoguanidine. Additionally, SME (5 and 10 µg/mL) reduced the expression of proteins (e.g., p-extracellular signal-regulated kinase (ERK) and p-p38) in the MAPKs pathway and upregulated the glyoxalase system in HUVECs. SME (0.5–10 mg/mL), dihydrotanshinone (0.4 mM), and rosmarinic acid (0.4 mM) prevented MGO-AGEs formation and broke the MGO-AGE crosslinking. These results show that S. miltiorrhiza has protective effects against MGO-induced glucotoxicity by regulating the proteins involved in apoptosis, glyoxalase system and antioxidant activity. We expect that S. miltiorrhiza is a potential natural resource for the treatment of MGO-induced vascular endothelial dysfunction.
With the increasing prevalence of diabetes, diabetic complications are emerging as a huge social issue, especially diabetic cardiovascular complications. Diabetic cardiovascular complications are the main origin of morbidity and mortality among diabetic patients; they affect almost one-third of patients with diabetes, regardless of the type of diabetes.1)
Although the pathogenesis of diabetic cardiovascular complications is not yet fully understood, it is now established that endothelial dysfunction is a crucial factor for the advance of diabetic cardiovascular complications.2) Endothelial dysfunction refers to the malfunction of vascular endothelial cells. Vascular endothelial cells regulate hemodynamics through constriction or vasodilation, control vessel wall permeability, and seize circulating leukocytes, playing a vital role in inflammation.2,3) When these vascular endothelial cells are exposed to certain conditions, such as hyperglycemia, insulin resistance, or mechanical stretch, cell damage is induced by apoptosis, mitogen-activated protein kinase (MAPK) signaling activation, and reactive oxygen species (ROS) overproduction, which eventually leads to endothelial dysfunction.2–4) Recently, hyperglycemia-induced endothelial dysfunction has been shown to originate from dicarbonyl stress.5) In hyperglycemia and hyperfructosemia, sugar reacts with proteins and forms a Schiff base. Following this, the Amadori product undergoes a series of reactions to reactive α-dicarbonyls, and, finally, these precursors form advanced glycation end-products (AGEs).5,6) In a previous study, albumin, hemoglobin, fibrin, and collagen were modified to AGEs, which damaged the endothelial cells by changing the function of a protein or binding the receptor of AGEs (RAGE).7,8) The damaged endothelial extracellular matrix then promotes the expression of numerous cytokines, growth factors, procoagulators, and proinflammatory molecules that affect endothelial dysfunction.8–10)
Methylglyoxal (MGO) is one of the most powerful glycation agents. Most MGO is generated as a by-product of glycolysis by the fragmentation of glyceraldehyde-3-phosphate and dihydroxyacetone phosphate.4) Endothelial cells generate 85% of their ATP through glycolysis; thus, they are prone to have higher intracellular MGO concentrations when exposed to hyperglycemia.8) This high concentration of MGO exacerbates inflammation and apoptosis in vascular complications by affecting endothelial cells, such as inducing the activation of the MAPK signaling pathway, which induces cell death,11) and overproduction of ROS generation, which damages endothelial cells.4,12) Hanssen et al. reported the relationship between plasma MGO levels and cardiovascular disease in patients with type 1 and type 2 diabetes mellitus.10) Under normal conditions, MGO is formed but detoxified via a nuclear factor erythroid-2-related factor 2 (Nrf2) and glyoxalase 1 (GLO-1) interaction. However, in chronic hyperglycemia, MGO suppresses Nrf2 and leads to the downregulation of the glyoxalase defense system.8) Therefore, new solutions, such as Glo-1 inducers and anti-glycative agents, can be proposed to treat endothelial dysfunction and reduce the incidence of cardiovascular diseases.
Salvia miltiorrhiza Bunge (Lamiaceae) is widely distributed in Asia. The dried root of S. miltiorrhiza has been traditionally used to treat various cardiovascular diseases, such as cerebrovascular diseases and coronary heart disease, including Parkinson’s and Alzheimer’s diseases.13) More than 100 compounds from S. miltiorrhiza have been identified and non-polar diterpenoid and hydrophilic phenolic compounds have been studied for their anti-diabetic and anti-cardiovascular effects.14) Among them, tanshinone I, tanshinone IIA, salvianolic acid A, and salvianolic acid B has been reported to be effective in various in vitro and in vivo diabetic models.15,16) Particularly, salvianolic acid A have prevented early-stage diabetic nephropathy via the amelioration of glomerular endothelial hyperpermeability in type 2 diabetes mellitus rat model via AGE-RAGE associated signaling pathways.17) Additionally, tanshinone 2A has protected brain microvascular endothelial cells from MGO-induced apoptosis via the modulation of MAPK activity.18)
However, the potential effect of Salvia miltiorrhiza extract (SME) on MGO treated endothelial dysfunction in human umbilical vein endothelial cells (HUVECs) has not yet been reported. Therefore, in our study, we expected to determine the ameliorative effect of SME on MGO-induced endothelial dysfunction.
2′,7′-Dichlorodihydrofluorescein diacetate (DCF-DA; Cat. No. D6883), Thiazolyl blue tetrazolium bromide (MTT; Cat No. M5655), 2,2′-azino-di-(3-ethyl-benzothialozine-sulphonic acid) (ABTS), aminoguanidine hydrochloride (AG; Cat No. 396494 2,2-diphenyl-1-picrylhydrazyl (DPPH), MGO (Cat. No. M0252), rosmarinic acid (Cat. No. R4033), salvianolic acid B (Cat. No. SML0048), dihydrotanshinone I (Cat. No. D0947), tanshinone I (Cat. No. T5330), tanshinone IIA (Cat. No. T4952), and monoclonal anti-α-tubulin antibody (Cat. No. T5168) were obtained from Sigma-Aldrich (St. Louis, MO, U.S.A.). p38 (Cat. No. 9212), p-p38 (Cat. No. 9211), c-Jun N-terminal kinase (JNK) (p54/46 MAPK, Cat. No. 9252), p-JNK (p54/46 MAPK, Cat. No. 9251), extracellular signal-regulated kinase (ERK) (p44/42 MAPK, Cat. No. 9102), p-ERK (p44/42 MAPK, Cat. No. 9101), and poly(ADP-ribose)polymerase (PARP) (Cat. No. 9532) antibodies were obtained from Cell Signaling Technology (Danvers, MA, U.S.A.). Bax (Cat. No. sc493), Bcl-2 (Cat. No. sc492), GLO-I (Cat. No. sc67351), RAGE (Cat. No. sc365154), and Nrf2 (Cat. No. sc365949) antibodies were acquired from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.).
Preparation of AGEsAGEs were prepared via incubating bovine serum albumin (BSA) and sodium azide (pH 7.4) with MGO, which being diluted using phosphate-buffered saline (PBS), at 37 °C for 7 d. Mixtures were evaporated and filtered using Spin Desalting Columns (Thermo Scientific, Waltham, MA, U.S.A.; Cat. No. 89892), before being further dried in a freeze dryer.
ExtractionS. miltiorrhiza roots were purchased from Jeongdo Herbal Medicine (Gyeonggi Province, Korea) and voucher specimens were placed at the college of pharmacy (pharmacognosy lab) at Gachon University (KSY-010-2019-SM-01). Dried S. miltiorrhiza roots were extracted with 70% EtOH at 25 °C overnight. The extracts were filtered, evaporated, and frozen (yield: 16.9%).
Cell CultureHUVECs were obtained from PromoCell (Heidelberg, Germany). HUVECs were maintained in endothelial cell growth medium (EGM-2) with a supplement kit and 1% penicillin/streptomycin. HUVECs were maintained at 37 °C in a humidified incubator comprising 5% CO2. The cells used for all experiments were passages 4–8.
Cell Toxicity and Morphological InvestigationCell toxicity was evaluated using an MTT assay.9) Cells were seeded into 96-well plates (1.0 × 104 cells/well) for 24 h. After incubation, the cells were treated with several concentrations (1, 5, and 10 µg/mL) of SME for 1 h, followed via 500 µM MGO for 24 h. After incubation, the MTT solution was mixed to each well for 2 h. Then, the medium was suctioned and dimethyl sulfoxide (DMSO) added to each well. The cell viability was quantified by evaluating absorbance at 570 nm in the microplate reader (Molecular Devices, CA, U.S.A.). Then, the morphology and confluence percentage (viable cells) of the cells were measured using the IncuCyte Zoom analysis tool (Essen Bioscience, MI, U.S.A.).
Intracellular ROS ExposureHUVECs were seeded into 6-well plates (3.0 × 105 cells/well) and incubated at 37 °C in 5% CO2 for 24 h. After incubation, cells were treated with SME for 1 h, followed by 500 µM MGO treatment for 2 h. Cells were treated with 20 µM DCF-DA for 15 min in the supplement-free medium in the dark. The cells were observed using a JuLi live-cell imaging machine (NanoEnTek, Seoul, Korea).
Intracellular Superoxide Dismutase 1 (SOD1), SOD2, Catalase (CAT), and Glutathione Peroxidase (GSH-Px) LevelsHUVECs were seeded into 6-well plates (3.0 × 105 cells/well) and incubated at 37 °C in 5% CO2 for 24 h. After incubation, cells were treated with SME for 1 h, followed by 500 µM MGO treatment for 1 h. Cell lysates were prepared by using PRO-PREP™ Solution (iNtRON, Seoul, Korea; Cat. No. 17081), and then protein concentration were quantified using the Bradford assay protocol.5) Then levels of SOD1 (CuZn-superoxide dismutase, Abfrontier, Seoul, Korea; Cat. No. LFEK0101), SOD2 (Mn-superoxide dismutase, Abfrontier; Cat. No. LFEK0104), CAT (Biovision, U.S.A.; Cat. No. K773100), and GSH-Px (Abfrontier; Cat. No. LFEK0110) were analyzed by the enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s introductions.
Lactate Dehydrogenase (LDH) Generation AssayLDH generation was quantified using the CyQUANT™ LDH kit assay (Thermo Scientific). Briefly, HUVECs were seeded into 96-well plates (1.0 × 104 cells/well) for 24 h. After incubation, cells were pretreated with SME (1, 5, and 10 µg/mL) for 1 h, followed by 500 µM MGO for 24 h. After the conditioned medium (50 µL) was transferred and reaction mixture added to each well and incubated slow shaking for 30 min at 25 °C in the dark. After incubation, the color changed was determined by evaluating absorbance at 490 and 680 nm in the microplate reader (Molecular Devices, CA, U.S.A.).
AGEs Formation AssayThe AGEs formation and inhibition percentage were conducted to investigate in several steps of the glycation pathways, according to the methods of Kiho et al. and Lasker et al.19,20) As shown in Supplementary Fig. 1S, the formation and inhibition of 5 mM MGO-derived AGEs by SME were quantified by evaluating fluorescence at 355/460 nm in the VICTOR™ X3 multilabel plate reader (PerkinElmer, Inc., MA, U.S.A.).
AGEs Breaker AssayA 2,4,6-trinitrobenzene sulfonic acid (TNBSA) assay was conducted to measure the MGO-AGEs breaking ability of SME.5) According to previous our report, 1 mg/mL MGO-BSA-AGEs and MGO-Fibrinogen-AGEs breaking ability were quantified by measuring absorbance at 340 nm in the microplate reader (Molecular Devices, CA, U.S.A.).5,21)
ABTS Scavenging AssayThe ABTS free radical scavenging activities of SME were measured according to the method Jeong et al.22) Initially, 2.6 mM potassium phosphate was added to 7.4 mM ABTS and the solution was permitted to respond for 12–24 h at 25 °C in the dark to produce ABTS solution. After 24 h, the ABTS solution was diluted with PBS buffer to an absorbance of less than 0.70 ± 0.03 (mean ± standard deviation (S.D.)) at 732 nm. First, SME was diluted with distilled water (15.625, 31.25, 62.5, 125, and 250 µg/mL). Next, 50 µL of diluted SME was mixed with 950 µL of the ABTS solution. After incubation for 5 min at 25 °C in the dark, the ABTS scavenging activity was quantified by evaluating absorbance at 732 nm in the microplate reader (Molecular Devices, CA, U.S.A.). Then, ABTS free radical scavenging activity (%) = (1 − sample absorbance/control absorbance) × 100 was calculated by measuring absorbance at 340 nm in the microplate reader (Molecular Devices).
DPPH Scavenging AssayThe DPPH free radical scavenging activities were conducted according to the method Brand-Williams et al.23) Briefly, SME was diluted with distilled water (15.625, 31.25, 62.5, 125, and 250 µg/mL). Then, 100 µL of diluted SME was mixed with 100 µL of 0.1 mM DPPH in ethanol. After incubation for 30 min at 25 °C in the dark, the DPPH scavenging ability was quantified by evaluating absorbance at 517 nm in the microplate reader (Molecular Devices, CA, U.S.A.). Then, DPPH free radical scavenging activity (%) = (1 − sample absorbance/control absorbance) × 100.
Apoptosis AssayA fluorescein isothiocyanate (FITC) annexin V/propidium iodide (PI) double staining apoptosis kit (BD Biosciences Pharmingen, San Diego, CA, U.S.A.) was used to identify apoptosis and necrosis via fluorescence-activated cell sorting (FACS CaliburTM; Becton-Dickinson, San Jose, CA, U.S.A.). HUVECs were seeded into 60 mm dishes at a density of 8.0 × 105 cells/dish and incubated at 37 °C in 5% CO2 for 24 h. After incubation for 24 h, the cells were treated with SME at several concentrations (1, 5, and 10 µg/mL) for 1 h, followed by 500 µM MGO for 24 h. Then, the cells were washed, harvested, stained for 15 min at 25 °C in the dark with PI, and FITC in binding buffer, and analyzed via flow cytometry within 1 h (Becton-Dickinson).
Western Blot AnalysisThe cells were lysed in PRO-PREP™ Solution comprising phosphatase and protease inhibitors. After incubation for 24 h, cell lysates were centrifuged and quantified using the Bradford assay protocol.5) Then, equal amounts (20–30 µg) of each protein were separated in different percentage sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels. Then, the cells were transferred to an Immobilon-P polyvinylidene difluoride membrane using an ATTO Blotting System (ATTO Technology, Amherst, NY, U.S.A.). Membranes were blocked with 5% skimmed milk in TBST comprising 0.1% Tween-20 for 1–2 h at 25 °C. The membranes were then incubated with each primary antibody (1 : 1000) at 4 °C for 24 h. After, the membranes were detected with rabbit or mouse secondary antibody (1 : 2000) conjugated with horseradish peroxidase for 1–2 h at 25 °C. Protein band intensities were determined using a ChemiDoc XRS+ imaging machine (Bio-Rad, CA, U.S.A.).
HPLC AnalysisThe standardization of S. miltiorrhiza was performed using a Waters HPLC system (Waters Corp., Milford, MA, U.S.A.) comprising of separation modules (e2695) and a photodiode array (PDA) detector. 20 mg of dried S. miltiorrhiza powder was dissolved in methanol. Rosmarinic acid, salvianolic acid B, dihydrotanshinone I, tanshinone I, and tanshinone IIA were used as standard compounds and dissolved in water, methanol, and DMSO. The sample and standard were filtered using a 0.2-µm filter. After filtration, an aliquot containing 10 µL of the solution was injected into the HPLC system. For the analysis of each compound, a Kromasil C18 column (250 × 4.6 mm, 5 µm) was used. The mobile phase consisted of methanol-water (78 : 22 (v/v), containing 0.5% acetic acid).24) The calibration was linear and in the range of 0.1–10,000 µg/mL for these five compounds. The flow rate was 0.5 mL/min and the PDA detector was set at 340 nm to acquire chromatograms. The column temperature was set at 30 °C and 10 µL of the sample or standard was injected.
Statistical AnalysisAll data values are presented as the mean ± standard error of the mean (S.E.M.). The statistical analyses were conducted by a one-way ANOVA, followed by Bonferroni’s post-hoc test, using GraphPad Prism version 5.00 (GraphPad Software, Inc., CA, U.S.A.).
For optimization of MGO concentration, 100, 250, and 500 µM MGO were treated in HUVECs during 24 h. As shown in Fig. 1A, the cell viability of each concentration was 102.96 ± 1.64% for 100 µM MGO, 86.19 ± 1.98% for 250 µM MGO, and 61.38 ± 2.28% for 500 µM MGO, respectively. In the IncuCyte Zoom analysis, the treatment of 500 µM MGO was strongly decreased the area occupied by viable cells (Fig. 1B, Supplementary Fig. 2S). Thus, we selected the concentration of MGO as 500 µM for examining the prevent effects of SME. Meanwhile, 1, 5, and 10 µg/mL of SME was not shown any cytotoxicity (Supplementary Fig. 3S). Thus, we treated SME at the same concentration in HUVECs. The cell viability of HUVECs was significantly increased on treatment with 10 µg/mL of SME in MGO-induced glucotoxicity. (Figs. 1C, D).

(A) Cell viability of HUVECs treated with MGO (100, 250, and 500 µM) for 24 h. (B–D) Cell viability, confluence, and LDH production of HUVECs pretreated with SME (1, 5, and 10 µg/mL) for 1 h before stimulation with MGO (500 µM) for 24 h. (E) Representative flow cytometry with FITC and PI dyeing of MGO-induced HUVECs: (a) control; (b) 500 µM MGO; (c) 1 µg/mL SME; (d) 5 µg/mL SME; (e) 10 µg/mL SME; (f) 1 mM aminoguanidine. (F) Quantitative value of representative of flow cytometry with FITC/PI double staining. (G) Cells pre-treated with SME (1, 5, and 10 µg/mL) for 1 h followed via 500 µM MGO stimulation for 24 h. Representative Western blot band and intensities of apoptosis. (H) Relative band intensity of Bax/Bcl-2, and cleaved PARP/PARP. All data are indicated as mean ± S.E.M. n = 3 (## p < 0.01, ### p < 0.001 vs. control, * p < 0.05, ** p < 0.01, *** p < 0.001 vs. MGO 500 µM).
To investigate whether MGO-induced cell toxicity is related to necrosis and apoptosis, we used a flow cytometry technique based on FITC/PI staining. The MGO treatment group indicated a significant increase in the density of early and late apoptotic cells (Figs. 1E, F). However, SME pre-treatment decreased the density of apoptotic cells in HUVECs (Figs. 1E, F). In addition, the pre-treatment with 5 (* p < 0.05) and 10 µg/mL (*** p < 0.001) of SME decreased the ratio of Bax/Bcl-2 and cleaved PARP/PARP (Figs. 1G, 1H).
SME Inhibits MGO-Induced RAGE Expression in HUVECsIt was well reported that AGEs and RAGE interaction contributed to accelerate the inflammation, carcinogenesis and atherosclerosis.25) Thus, we investigated the effect of SME on the levels of RAGE expression in MGO-treated HUVECs (Figs. 2A, B). The MGO treatment group showed significantly increased the expressed levels of RAGE protein (### p < 0.001) in comparison with control group. Meanwhile, SME (*** p < 0.001) strongly suppressed the levels of RAGE protein expression at 10 µg/mL concentration, as similar that of positive control group (*** p < 0.001).

(A) Cells pretreated with SME (1, 5, and 10 µg/mL) for 1 h followed by 500 µM MGO stimulation for 24 h. (A, C) Representative Western blot of RAGE and glyoxalase system-related antibody. (B, D) Relative band intensity of Glo-1 and Nrf2. All data are indicated as mean ± S.E.M. n = 3 (# p < 0.05 vs. control, * p < 0.05, *** p < 0.001 vs. MGO 500 µM).
To evaluate whether the effects of MGO on the glyoxalase system are associated with Glo-1 and Nrf2, we performed Western blotting. The MGO treatment group (# p < 0.05) showed significantly decreased Glo-1 and Nrf2 protein expression in comparison with control group, but SME increased Glo-1 and Nrf2 expression in a concentration-dependent manner (Figs. 2C, D).
SME Decreases MGO-Induced ROS Production and MAPK Signaling in HUVECsWe evaluated whether the SME pre-treatment can decrease production of ROS in HUVECs or not. ROS production was quantified using DCF-DA staining and a JuLi live-cell imaging machine. MGO treatment significantly increased ROS generation, whereas SME pre-treatment decreased ROS generation in a concentration-dependent manner in HUVECs (Figs. 3A, B). Additionally, using Western blotting, we measured the effects of MGO treatment on proteins in the MAPKs (p-ERK/ERK, p-JNK/JNK, and p-p38/p38) signaling pathway in HUVECs. MGO treatment for 1 h significantly increased the phosphorylation of these MAPK signaling proteins. However, SME pre-treatment significantly decreased the ratios of p-ERK/ERK, p-JNK/JNK, and p-p38/p38, comparing with MGO treatment group (### p < 0.001) (Figs. 3C, D).
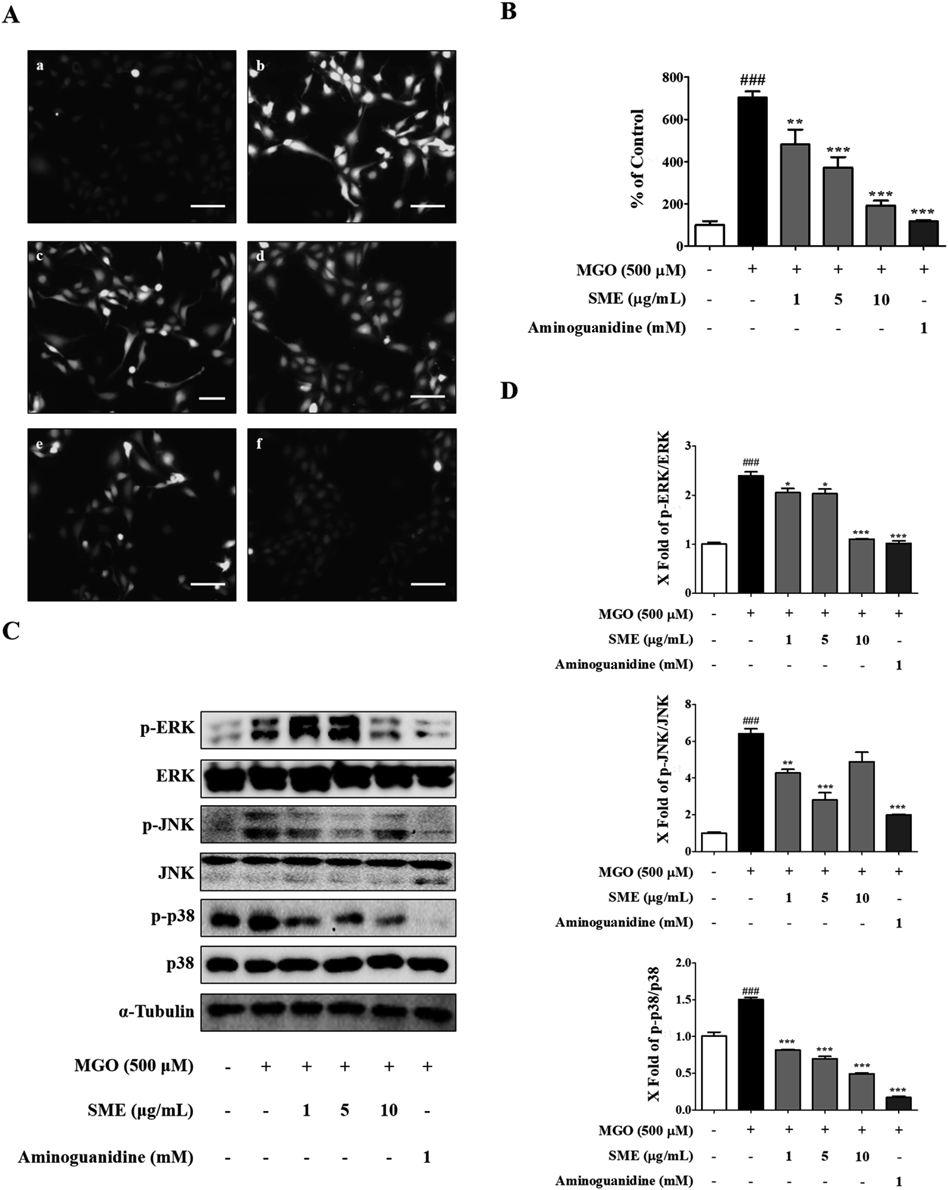
(A) HUVECs pre-treated with SME (1, 5, and 10 µg/mL) for 1 h followed by 500 µM MGO stimulation for 2 h. ROS production was evaluated by dyeing with DCF-DA dye using a JuLi live-cell imaging machine. Scale bar indicates 500 µm: (a) control; (b) 500 µM MGO; (c) 1 µg/mL SME, (d) 5 µg/mL SME; (e) 10 µg/mL SME; (f) 1 mM aminoguanidine. (B) Quantitative amounts of fluorescent intensity calculated using Image J software. (C) Cells pretreated with SME (1, 5, and 10 µg/mL) for 1 h followed by MGO stimulation for 1 h. Representative Western blot of MAPK (ERK, JNK, and p38) signaling pathway. (D) Relative band intensity of p-ERK/ERK, p-JNK/JNK, and p-p38/p38 ratio. All data are indicated as mean ± S.E.M. n = 3 (### p < 0.001 vs. control, * p < 0.05, ** p < 0.01, *** p < 0.001 vs. MGO 500 µM).
In our previous study, we found that ROS plays an important role in MGO-induced apoptosis.5) Thus, we performed to measure the antioxidant enzymes (e.g., SOD1, SOD2, CAT, and GPX-Px) in MGO-treated HUVECs. We found that each antioxidant enzymes significantly decreased by treating MGO in comparison with control, but they were restored with SME pre-treatment (Fig. 4). This result suggest that SME may prevent MGO-induced cell apoptosis mediated by ROS stress.

Cells were pre-treated with test compounds 1 h, and then treated with 500 µM MGO for 1 h. The activity of SOD1, SOD2, CAT, and GSH-Px was measured by ELISA kit. All data are indicated as mean ± S.E.M. n = 3 (## p < 0.01 and ### p < 0.001 vs. control, * p < 0.05, ** p < 0.01 and *** p < 0.001 vs. MGO 500 µM).
We conducted an MGO-AGEs formation assay by quantifying fluorescence. SME (1, 5, and 10 mg/mL) co-treatment significantly inhibited the formation of MGO-BSA or –fibrinogen in a concentration-dependent manner, comparing with control (Figs. 5A, B). Moreover, to investigate whether SME could break preformed MGO-BSA-AGEs or MGO-fibrinogen-AGEs crosslinks, a TNBSA assay was performed to evaluate the number of remaining free amines. SME significantly broke the MGO-BSA-AGEs or -fibrinogen-AGEs crosslink in a cocnentration-independent manner in comparison that of control (Figs. 5C, D).

(C, D) The several MGO-AGE-breaking abilities of SME were measured by breaking MGO-BSA-AGE and MGO-fibrinogen-AGE using the TNBSA assay. All data are indicated as mean ± S.E.M. n = 3 (*** p < 0.001 vs. MGO-BSA 5 mM, MGO-fibrinogen 5 mM, MGO-BSA-AGE 1 mg/mL, and MGO-fibrinogen-AGE 1 mg/mL).
We examined the inhibitory effects of the main components of SME on MGO-AGEs formation. Tanshinone IIA (*** p < 0.001), dihydrotanshinone I (* p < 0.05), and salvianolic acid B (*** p < 0.001) significantly inhibited the formation of MGO-BSA, comparing with control. However, tanshinone I and rosmarinic acid did not inhibit MGO-BSA formation (Fig. 6A). Additionally, rosmarinic acid (*** p < 0.001) and salvianolic acid B (*** p < 0.001) significantly inhibited the formation of MGO-fibrinogen in comparison with control, whereas tanshinone I, IIA, and dihydrotanshinone I did not show any effect (Fig. 6B). Moreover, the TNBSA assay was performed to explore whether SME-derived components could break the preformed MGO-AGEs crosslinks. Tanshinone IIA (** p < 0.01), dihydrotanshinone I (*** p < 0.001), and rosmarinic acid (*** p < 0.001) significantly broke the MGO-BSA-AGEs crosslink in comparison with control, but tanshinone I and salvianolic acid B were not effect. (Fig. 5C). Interestingly, five phytochemicals (*** p < 0.001) from SME significantly broke the MGO-fibrinogen-AGE crosslinks, comparing with control (Figs. 6C, 6D).

(A, B) Effects of SME components (0.4 mM) on the in vitro formation of MGO-BSA and MGO-fibrinogen quantified using the AGE formation assay. (D) The several MGO-AGE-breaking abilities of SME components (0.4 mM) was calculated by breaking MGO-BSA-AGEs and MGO-fibrinogen-AGEs using the TNBSA assay. All data are indicated as mean ± S.E.M. n = 3 (* p < 0.05, ** p < 0.01, *** p < 0.001 vs. MGO-BSA 5 mM, MGO-fibrinogen 5 mM, MGO-BSA-AGE 1 mg/mL, and MGO-fibrinogen-AGE 1 mg/mL).
In this study, five compounds, rosmarinic acid, salvianolic acid B, dihydrotanshinone I, tanshinone I, and tanshinone IIA, in SME were analyzed using an HPLC system. SME mainly contained salvianolic acid B (9.173 ± 0.042 mg/g), which was the main active component in SME against MGO-induced glucotoxicity. Rosmarinic acid (0.522 ± 0.006 mg/g), dihydrotanshinone I (0.575 ± 0.001 mg/g), tanshinone I (0.120 ± 0.001 mg/g), and tanshinone IIA (0.241 ± 0.006 mg/g) were also detected in SME (Table 1, Supplementary Fig. 4S).
| Samples | Content (mg/g) | ||||
|---|---|---|---|---|---|
| Rosmarinic acid | Salvianolic acid B | Tanshinone IIA | Tanshinone I | Dihydrotanshinone I | |
| SME | 0.522 ± 0.006 | 9.173 ± 0.042 | 0.241 ± 0.006 | 0.120 ± 0.001 | 0.575 ± 0.001 |
All data are indicated as mean ± S.E.M. n = 3.
We performed ABTS and DPPH radical assays using a microplate reader and ascorbic acid as the positive control. The ABTS and DPPH radical scavenging activities of SME and its components were measured, indicating the EC50 (effective concentration) value (Table 2). Interestingly, rosmarinic acid and salvianolic acid identified from SME showed radical scavenging ability than other compounds.
| Samples | EC50 | |
|---|---|---|
| Scavenging ability on ABTS radicals | Scavenging ability on DPPH radicals | |
| SME (µg/mL) | 14.35 ± 2.10 | 61.97 ± 4.10 |
| Rosmarinic acid (µM) | 14.69 ± 0.88 | 1.47 ± 0.01 |
| Salvianolic acid B (µM) | 38.83 ± 1.39 | 6.00 ± 0.05 |
| Dihydrotanshinone I (µM) | — | — |
| Tanshinone I (µM) | — | — |
| Tanshinone IIA (µM) | — | — |
| Ascorbic acid (µM) | 0.26 ± 0.01 | 0.03 ± 0.00 |
All data are indicated as mean ± S.E.M. n = 3.
The effect of MGO on endothelial dysfunction via the inducing of cell death has been widely reported.8) MGO can exacerbate diabetic vascular complications directly and by modifying the protein structure of MGO-AGEs.26,27) Furthermore, the MGO-AGEs/RAGE axis may induce the formation of ROS, which further increases cellular toxicity.5) In this study, we first determined the optimal MGO concentration that induces cytotoxicity in HUVECs and then examined the potential effect of SME on MGO-induced glucotoxicity using AG as a positive control (Fig. 1). Our in vitro cell viability assay results showed that SME has a concentration-dependent protective effect on MGO-induced cytotoxicity in HUVECs via the maintaining the integrity of cell membrane and mitochondrial function.
Schalkwijk et al. have demonstrated that MGO has induced endothelial dysfunction via the induction of oxidative stress, inflammation, and cell apoptosis.8) Also, MGO-induced cell apoptosis can be regulated by the Bcl-2 family.4) Bcl-2 is an anti-apoptotic protein that binds Bax. Bax is a pro-apoptotic effector that triggers apoptosis in healthy cell conditions. These two proteins are involved in intrinsic cell death pathways, which known as the mitochondrial pathway.4) Mitochondria may play key roles in activating apoptosis in mammalian cells. PARP is an enzyme that normally repairs DNA but, when apoptosis begins, it becomes a substrate for caspases. Cleaved PARP is a family of proteins involved in cellular process such as DNA repair and programmed cell death, forms as a product of the caspase reaction. Therefore, it is as a key indicator of cell apoptosis in HUVECs.4,11)
In the present study, MGO increased the ratio of Bax/Bcl-2 and cleaved PARP/PARP compared with the control, and SME was concentration-dependently decreased the ratio of Bax/Bcl-2 and cleaved PARP/PARP (Figs. 1G, H). Namely, SME significantly may reverse the MGO-induced cell apoptosis, comparing with MGO treatment. Based on an Annexin V-FITC/PI double staining apoptosis assay, our results suggested that SME ameliorates the mitochondrial pathway of apoptosis. We detected early apoptotic HUVEC cells by MGO pre-treatment and, under the same conditions, SME treatment significantly decreased the number of apoptotic cells compared with the control group (Figs. 1E, F).
MGO detoxification also plays a central role in the prevention of MGO accumulation. The lack of the MGO detoxification system can lead to excessive intracellular MGO accumulation and MGO-AGEs formation.8) The human body detoxifies MGO using the glyoxalase system,28) which consists of GLO-1, GLO-2, and a cofactor (glutathione). In this system, GLO-1 plays a vital role in MGO detoxification by acting in the rate-limiting step.8,28) Indeed, an in vivo experiment has reported that the overexpression of GLO-1 in a diabetic rat model reduces AGEs levels and oxidative stress.29) GLO-1 transcription is controlled by Nrf2 binding to the antioxidant response element.30) Therefore, we performed Western blotting to measure the change in GLO-1 expression by SME. In the negative control group (only MGO treatment), the expression of GLO-1 protein was significantly reduced in comparison with control group, whereas Nrf2 and the positive control group (aminoguanidine pre-treatment group) reversed this tendency. Interestingly, SME also restored the protein expression of GLO-1 and Nrf2 in a concentration-dependent manner. This result is the first to indicate that SME may protect human endothelial cells from MGO via the activation of Nrf2 and Glo-1 pathways (Figs. 2C, D).
ROS triggers cell apoptosis. MAPKs pathways may elicit several physiological responses including apoptosis in mammalian cells,2,4) and regulate apoptosis via the phosphorylation of downstream mediators of apoptosis.11) Indeed, it was already proven that MGO activated the MAPK pathways (i,e., ERK, JNK, p38) in HUVECs and associated with MGO-induced apoptosis.4) In our results, pre-treatment with SME reduced ROS production in a concentration-dependent manner (Figs. 3A, B). Additionally, SME pre-treatment reduced the phosphorylation of p38, JNK, and ERK (Figs. 3C, D). These experimental results suggest that SME may have a protective effect against MGO-induced apoptosis via the suppression of MAPKs signaling activation in HUVECs.
Our in vitro study showed that MGO induced significant damage to HUVECs through decreased cell viability, increased ROS production, and cell apoptosis, In addition, we found in this work that MAPK pathways were activated by MGO, whereas treatment with SME inhibited the activation of MAPKs. Moreover, we confirmed that pharmacological inhibition of MAPKs and apoptosis signaling using specific inhibitors involving ERK inhibitor (U120), JNK (SB203580), p38 (SP600125), and N-acetyl cysteine (ROS scavenger, NAC). As shown in Fig. 5S, All MAPK inhibitors and ROS scavenger showed that MAPK pathway is directly involved in MGO-induced apoptosis in HUVECs, and ROS play an important role in MGO-induced apoptosis.
Indeed, our present data displayed that oxidative stress significantly increased in HUVECs by treating MGO (Figs. 3A, B). It is several ways that MGO could increase the level of intracellular ROS. First, MGO-derived AGEs stimulate the generation of superoxide anion and hydrogen peroxide, and also the glutathione was used in MGO detoxification. Second, the MGO may inactivate the antioxidant enzymes that scavenge the ROS such as SOD1, SOD2, and GSH-Px.31) These results showed that SME significantly inhibited ROS generation, and increased the activities of antioxidant enzymes (e.g., SOD1, SOD2, CAT, and GSH-Px) in HUVECs (Fig. 4). Also, we examined the radical scavenging effects of SME and its bioactive compounds. As a result, SME and its bioactive compounds showed radical scavenging ability (i.e., ABTS and DPPH radicals) (Table 2). SME can contribute to the amelioration of oxidative stress by reducing the production of ROS or quenching the formed radicals. This antioxidant activity might be owing to its abundant compounds, such as rosmarinic acid and salvianolic acid B in SME (Figs. 3, 4, Table 2).
Meanwhile, RAGE, a receptor for AGEs, is expressed in cell surface of several types including endothelial cells and immune cells.32) The interactions of AGEs and RAGE initiates the inflammation and cell death responses.25,32) Thus, one of the major roles RAGE is involved in glycotoxins-induced inflammation and apoptosis signaling. In this work, we found that the expressed level of RAGE was increased by treating 500 µM MGO, whereas SME pre-treatment significantly reversed it in a concentration-dependent manner (see Figs. 2A and B). As a result, our data support that the protective effect of SME was due to down-regulate the RAGE pathway, and also it blocked the MGO induced apoptosis and inflammation in HUVECs (Figs. 1, 3).
Salvia is the largest genus in the Lamiaceae family and plants in this genus are widely used as therapies in worldwide because of the low incidence of side effects. Many Salvia species have been reported to have therapeutic effects on diabetes and diabetic complications.33,34) In Asia, S. miltiorrhiza is usually used as herbal medicine to treat problems related to circulation and diabetic complications. Additionally, recent studies have shown that S. miltiorrhiza decreases ROS production induced by a high glucose concentration in microvascular endothelial cells.35) In present study, we investigated the effects of SME in MGO-induced HUVECs apoptosis in vitro and the related possible mechanism involved. Our findings indicate that SME prevents MGO-induced HUVECs apoptosis via decreasing oxidative stress, inhibiting MAPK pathways and RAGE expression. According to our results and previous studies, the ability of SME to prevent MGO-induced oxidative stress and apoptosis might be protective against development of diabetic complications. We can also expect the potential activity of SME against age-related chronic diseases such as obesity, neurodegenerative disease, and cancer.8)
To identify the bioactive compounds in SME, we tested the anti-AGE activities of rosmarinic acid, tanshinone 1, tanshinone 2A, dihydrotanshinone, and salvianolic acid B, which are abundant in S. miltiorrhiza and potentially effective in endothelial dysfunction.14,35,36) To quantify the content of each component in SME, we performed HPLC analysis and observed that, among these compounds, salvianolic acid B was the most abundant in SME (Table. 1, Supplementary Fig. 4S). An AGE formation assay was conducted for each compound. Dihydrotanshinone, tanshinone IIA, and salvianolic acid B significantly reduced the formation of MGO-BSA in the listed order (Fig. 6A). Furthermore, rosmarinic acid and salvianolic acid significantly attenuated the formation of MGO-fibrinogen in comparison with control, but the other components did not show it (Fig. 6B). In the MGO-BSA-AGEs breaker assay, rosmarinic acid, dihydrotanshinone, and tanshinone IIA showed breaking ability in the listed order (Fig. 6C) and all five components in SME significantly broke the MGO-fibrinogen-AGE crosslinking, comparing with control (Fig. 6D). Interestingly, salvianolic acid B inhibited MGO-derived AGEs formation and dihydrotanshinone interfered with MGO-AGEs formation and breaking. However, tanshinone I had no significant effects on the in vitro anti-glycation assay. Although these three tanshinone type compounds are similar in structure, but they showed the quite different in anti-glucotoxicity activities. Therefore, further research on the structure–activity relationship (SAR) on anti-glucotoxicity of tanshinone derivatives are needed.
Taken together, our results strongly suggest that S. miltiorrhiza extract (SME) may protect endothelial cells from MGO toxicity via multiple pathways, including the reduction of oxidative stress, modulation of the apoptosis pathway, and induction of the glyoxalase system (see Fig. 7). These potential therapeutic effects could be owing to the synergic effect of bioactive compounds in SME. Our study provides insight into the application of S. miltiorrhiza and its compounds as therapeutic agents for the prevention and treatment of diabetic vascular dysfunction.
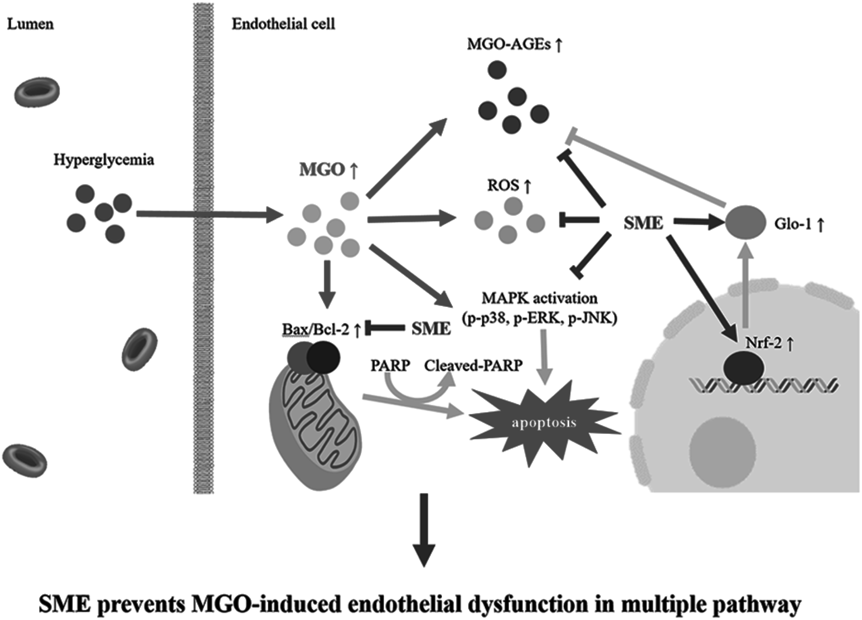
This schematic description of initiated in apoptosis by treating MGO (light gray arrow), whereas SME (gray arrow) prevented the MGO-endothelial dysfunction via several pathways.
In summary, our results demonstrated that the extract of S. miltiorrhiza (SME) prevented HUVECs against MGO-treated apoptosis via the modulation of MAPKs, Bax/Bcl-2 ratio, and PARP-related apoptosis pathways. We also showed that SME not only modulated the cell apoptosis pathway but also enhanced the glyoxalase system (Glo-1 and Nrf2). SME also decreased the production of ROS in a concentration-dependent manner and showed antioxidant activity. Furthermore, SME and its phytochemicals, dihydrotanshinone, tanshinone 2A, salvianolic acid, and rosmarinic acid, prevented the formation of MGO-AGEs and concentration-dependently broke preformed MGO-AGE crosslinks. Therefore, our results suggest that S. miltiorrhiza might have preventive effects against diabetes or diabetic complications-related vascular damage via the regulation of MGO-induced glucotoxicity and the reduction of oxidative stress. Further in vivo study on the therapeutic potentials of S. miltiorrhiza and its bioactive compounds on vascular endothelial dysfunction needs to be conducted.
This research was supported by the KIST Institutional Program (Project No. 2E31300-21-078) and a Grant from the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2018R1D1A1B07049500).
Conceptualization: S.Y.K. and J.H.L. Data curation: J.H.L. and J.S.K. Formal analysis: S.Y.K., J.H.L., and J.S.K. Funding acquisition: S.Y.K. Methodology: S.Y.K., J.H.L., J.S.K., S.M.H., and K.H.C. Supervision: S.Y.K. Writing, review, and editing: J.H.L., J.S.K., and S.Y.K.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.