2022 年 45 巻 10 号 p. 1404-1411
2022 年 45 巻 10 号 p. 1404-1411
Proton pumping ATPases, both F-type and V/A-type ATPases, generate ATP using electrochemical energy or pump protons/sodium ions by hydrolyzing ATP. The enzymatic reaction and proton transport are coupled through subunit rotation, and this unique rotational mechanism (rotational catalysis) has been intensively studied. Single-molecule and thermodynamic analyses have revealed the detailed rotational mechanism, including the catalytically inhibited state and the roles of subunit interactions. In mammals, F- and V-ATPases are involved in ATP synthesis and organelle acidification, respectively. Most bacteria, including anaerobes, have F- and/or A-ATPases in the inner membrane. However, these ATPases are not believed to be essential in anaerobic bacteria since anaerobes generate sufficient ATP without oxidative phosphorylation. Recent studies suggest that F- and A-ATPases perform indispensable functions beyond ATP synthesis in oral pathogenic anaerobes; F-ATPase is involved in acid tolerance in Streptococcus mutans, and A-ATPase mediates nutrient import in Porphyromonas gingivalis. Consistently, inhibitors of oral bacterial F- and A-ATPases, such as phytopolyphenols and bedaquiline, strongly diminish growth and survival. Herein, we discuss rotational catalysis of bacterial F- and A-ATPases, and discuss their physiological roles, focusing on oral bacteria. We also review the effects of ATPase inhibitors on the growth and survival of oral pathogenic bacteria. The features of the catalytic mechanism and unique physiological roles in oral bacteria highlight the potential for proton pumping ATPases to serve as targets for oral antimicrobial agents.
The oral ecosystem comprises the oral flora and the oral microbiome.1) Some oral pathogens are responsible for dental caries and periodontal disease, resulting in loss of teeth.2) In addition, chronic oral infection with these microorganisms is associated with systemic diseases such as cancers, type 2 diabetes, and Alzheimer’s disease.3–5) However, antimicrobial agents have rarely been used for therapy or prevention of dental caries and periodontal disease, since continuous use of these drugs induces drug-resistant strains and disrupts the oral microbiome. Therefore, there is a need to identify novel targets in pathogens to develop oral antimicrobial agents.
Proton pumping ATPases are membrane-embedded, multisubunit enzymes coupling proton or sodium ion transport and ATP synthesis or hydrolysis through subunit rotation.6) They are classified into F-type and V/A-type ATPases.6) F-type ATPase (F-ATPase, also known as ATP synthase) is found in chloroplasts, mitochondria, and bacterial membranes.7) Vacuolar-type ATPase (V-ATPase) is present in eukaryote cells, localized in endomembrane organelles such as vacuoles, lysosomes, endosomes, and Golgi apparatus, as well as the plasma membrane.8) Archaeal type ATPase (A-ATPase) in bacterial membrane appears to be the ancestors of V-ATPase.9) Therefore, A-ATPase is also called V/A-ATPase.
The unique structures and rotational mechanism of proton pumping ATPases have been intensively studied.10–13) Specifically, single-molecule studies on rotational catalysis by proton pumping ATPases have revealed their dynamics, torque generation, and intrinsic features such as stochastic rotation rate and catalytically inhibited state, none of which could be determined by bulk phase analysis.14–17) Studies involving single-molecule assays, crystal structures, and inhibitors of proton pumping ATPases have revealed the importance of subunit interactions for smooth rotation.18–21)
Eukaryotic F- and V-ATPases are involved in ATP synthesis and organelle acidification, respectively.6) F- and V-ATPases are essential in mammals since their deletion is embryonic lethal.22,23) Most bacteria, including anaerobes, possess F-ATPase and/or A-ATPase in the inner membrane. However, these ATPases are believed to be unessential for energy metabolism in anaerobic bacteria because these organisms generate sufficient ATP through anaerobic glycolysis without depending on ATP synthesis via oxidative phosphorylation.24,25) Recently, it was reported that F- and A-ATPases in oral pathogenic anaerobes play indispensable roles in acid tolerance by secreting protons from the cytoplasm, and in amino acid import by generating a proton gradient.26–28) These findings suggest that F- and A-ATPases could serve as potential targets for antimicrobial agents against oral pathogens.
Consistently, proton pumping ATPase inhibitors diminish the growth and survival of oral pathogenic bacteria including Streptococcus mutans and Porphyromonas gingivalis.27,28) Most inhibitors are toxic to both bacteria and mammals, probably due to their structural similarity, whereas phytopolyphenols and the antituberculosis drug bedaquiline are less toxic to mammals.20,29–32) This is possibly because bedaquiline selectively affects bacterial F-ATPase, and phytopolyphenols are rapidly metabolized and/or less readily absorbed in mammals.33,34) Therefore, we predict that these less toxic inhibitors could serve as seeds for novel antidental caries and antiperiodontitis agents.
Herein, we focus on bacterial F- and A-type proton pumping ATPases. We review the mechanism underlying rotational catalysis of F- and A-ATPases, discuss the physiological roles of these ATPases in oral pathogenic bacteria, and describe the effects of ATPase inhibitors on the growth and survival of pathogenic bacteria. Understanding the chemomechanical coupling mechanism of proton pumping ATPases will provide pharmacophores for these ATPases, and accelerate the discovery of seed compounds for oral pathogen-specific antimicrobial agents.
F-ATPase (Fig. 1A) consists of a catalytic F1 sector (α3β3γδε) and a transmembrane proton pumping FO sector (ab2c8–14).35–38) The rotor complex composed of γεc8–14 subunits rotates against the stator complex built from α3β3δab2 subunits, which couples catalysis and proton translocation.7) Rotational catalysis of F-ATPase has been studied by single-molecule observation and crystal structure analysis.7) The γ subunit in Escherichia coli F1 rotates relative to the α3β3 hexamer in distinct 120° steps during ATP hydrolysis39) (Fig. 1B). During 360° rotation of the γ subunit, the β subunit sequentially passes through three different conformations: βE (no nucleotide), βTP (bound ATP), and βDP (bound ADP; Figs. 1B, C).40,41) In each 120° step, ATP binds to βE, βTP hydrolyzes ATP to ADP + Pi (inorganic phosphate), and Pi and ADP are released from βDP (Fig. 1C; β subunit highlighted by an orange line).40,41)

(A) Schematic model of bacterial F-ATPase (FOF1). F1 is the membrane extrinsic catalytic sector (α3β3γδε), and FO is the membrane-embedded proton pathway (ab2c10). (B) Crystal structure of the F-ATPase F1 sector (PDB ID: 2JDI45)). Structure of α3β3γ viewed from the bottom (black arrow in A) shown with bound nucleotide (green space-filling representation). The β subunits with ATP (βTP) and ADP (βDP), and without a nucleotide (βE) bound to the catalytic site are colored yellow. A red arrow represents the direction of the γ subunit rotation under ATP hydrolysis conditions. (C) Proposed scheme of rotational catalysis in E. coli F1. The β subunits are colored yellow with binding site occupancy labeled, and the γ subunit is colored cyan. The α subunits are omitted for simplicity. (D) Interface between β and γ subunits. The sites of the interfaces between βDP and the N-terminal region of the γ subunit (β−γN interface), βTP and the C-terminal region of the γ subunit (β−γC interface), and α and β subunits (α−β interface) are indicated by orange circles. γM23 and βR398 (E. coli numbering) are shown in gray space-filling representation.
To analyze the rotational mechanism of F1, 40 or 60 nm gold beads were attached to the γ subunit of the F1 sector immobilized on a glass surface through a histidine (His)-tag attached to the α subunit.19–21,39,41–44) Rotation of the attached gold beads relative to the α3β3 sector was observed under laser light illumination.43) The viscous drag of the probe was low enough to observe high-speed rotation of the γ subunit (approx. 500 rotations per second under Vmax conditions).43) The rotation rate was stochastic, and, interestingly, the γ subunit underwent continuous rotation (active state) and pausing (catalytically inhibited state) every approx. 1 s.43,44) The ε subunit, an intrinsic inhibitor of F-ATPase, lowers the rotation rate in the active state and extends the duration of the inhibited state.19,44)
We performed thermodynamic analysis of single-molecule rotation by observing temperature-dependent rotation of the γ subunit.20,41,44) We found that the activation energy of catalytic steps was similar, suggesting there is no obvious rate-limiting step throughout the rotation.41) In other words, γ subunit rotation mediates the highly cooperative reaction mechanism and lowers energy barriers for ATP hydrolysis/synthesis by F-ATPase.41)
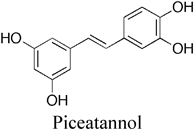
2.2. Subunit Interactions of F-ATPaseSingle-molecule assays together with thermodynamic and crystal structure analyses revealed the importance of subunit interactions.20,41,44) N- and C-terminal regions of the γ subunit interact with βDP and βTP, respectively45) (Fig. 1D). The γM23K mutation (E. coli F1 numbering) results in an additional hydrogen bond between βDP and the N-terminal region of the γ subunit.46) Piceatannol, an F-ATPase inhibitor, binds to the interface between βTP and the C-terminal region of the γ subunit.47) Thermodynamic analysis indicated that the γM23K mutation and piceatannol synergistically increased the activation energy in the rate-limiting step by preventing the β subunit from triggering γ rotation, suggesting that interactions between β and γ subunits at both interfaces influence the rate-limiting transition state.20,41)
The region including βArg398 of βDP interacts with that including αGlu402 of αDP45) (Fig. 1D). Mutational and molecular docking studies suggest that the F-ATPase inhibitors curcumin and citreoviridin bind to the region around βArg398 and disrupt the interaction between α and β subunits.21) These inhibitors prevent conformational transmission of the β subunit,21) suggesting that the α–β interaction plays a role in the β subunit conformational changes required for triggering γ subunit rotation. These studies revealed that interactions at the α–β and β–γ interfaces of F-ATPase are crucial for smooth progression through the reaction cycle, and suggest that compounds binding to these interfaces may inhibit rotational catalysis.20,41) Therefore, these interfaces could serve as pharmacophores for in silico screening of F-ATPase inhibitors.7,21)
2.3. Rotational Catalysis of A-ATPaseA-ATPase consists of a catalytic A1 (A3B3DF) sector, a transmembrane proton pumping AO sector (acxd), and a stalk sector (E2G2), but the number of c subunits varies between bacterial species48,49) (Fig. 2A). The rotor complex of the DFcxd subunits rotates against the stator complex of the A3B3E2G2a subunits.48,49) The structure, subunit composition, and rotational catalysis of the enzyme resemble those of F-ATPase.12,50) The D subunit in A1 rotates relative to the A3B3 hexamer in distinct 120° steps51,52) (Fig. 2B). Similar to F-ATPase, A-ATPase is characterized by a stochastic rotation rate and a catalytically inhibited state,51,53) but A-ATPase of Enterococcus hirae does not adopt the inhibited state.51) Amino acid residues in the catalytic sites of A and B subunits are similar to those in β and α subunits of F-ATPase.54,55) During rotation, pairs of A and B subunits pass through three different conformations, empty, bound, and tightly bound, corresponding to αEβE, αTPβTP, and αDPβDP of F-ATPase, respectively50) (Figs. 1B, 2B). According to the crystal structure of the enzyme, the N- and C-terminal helices of the D subunit interact with the A subunit to trigger rotation, suggesting that subunit interactions mediate rotational catalysis.50) Further studies including thermodynamic analysis will likely reveal the detailed rotational mechanism of A-ATPase and the essential interfaces that may serve as pharmacophores for specific inhibitors.

(A) Schematic model of bacterial A-ATPase. A1 is the membrane extrinsic catalytic sector (A3B3DE2FG2), and AO is the membrane-embedded proton pathway (acxd). (B) Crystal structure of the A-ATPase A1 sector (PDB ID: 3VR650)). The structure of A3B3D is viewed from the bottom (black arrow in A). The three pairs of A and B subunits, tightly bound to nucleotide (ACRBCR), bound to nucleotide (ACBO), and empty (AOBC),50) are shown with nucleotide (green space-filling representation). A red arrow represents the direction of the D subunit rotation under ATP hydrolysis conditions.
F-ATPase in the bacterial inner membrane is essential for the production of ATP in aerobic bacteria such as Mycobacterium tuberculosis, the etiological agent of tuberculosis.56)M. tuberculosis lowers its metabolism when entering the nongrowing (dormant) state under hypoxic conditions, and becomes recalcitrant to most antituberculous drugs.56) Importantly, F-ATPase inhibitors decrease bacterial growth in the optimal replicating state, and diminish survival in the hypoxic dormant state.57) These studies suggest that F-ATPase plays an important role in maintaining low ATP levels in the dormant state in M. tuberculosis, hence inhibitors could decrease bacterial survival in the dormant state.
On the other hand, F-ATPase is believed to be unessential in anaerobic bacteria since they can bypass the requirement for oxidative phosphorylation when grown on carbohydrates. Consistently, knockout of F-ATPase genes does not significantly decrease the growth of anaerobic bacteria such as E. coli and Staphylococcus aureus under nutrient-rich conditions.24,25) However, recent studies indicate that F-ATPase is essential in pathogenic oral streptococci such as S. mutans and Streptococcus anginosus, even though they lack a respiratory chain for oxidative phosphorylation.58)
S. mutans is considered an etiological agent of dental caries; this bacterium produces organic acids through metabolism of dietary carbohydrates, which cause demineralization of the tooth enamel surface.59) Highly evolved acid tolerance mechanisms support bacterial growth and survival in the acidic microenvironment generated by the bacterium.60) F-ATPase likely contributes to an acid tolerance mechanism since expression of F-ATPase and membrane ATPase activity of S. mutans are elevated under acidic conditions.61) Additionally, we showed that F-ATPase inhibitors significantly decreased the growth rate and colony-forming ability of S. mutans under acidic conditions, but they had no effect under neutral conditions.27) These findings suggest that F-ATPase is involved in acid tolerance of S. mutans, probably by secreting protons from the cytoplasm.
S. anginosus is a pathogenic streptococcus found in the human oral cavity, and gastrointestinal and genital tracts.62) This bacterium causes serious purulent abscesses in various tissues, and subacute infective endocarditis,63,64) and it has been linked to gastrointestinal and esophageal cancers.65–67)S. anginosus has high acid tolerance and membrane ATPase activity, similar to S. mutans.26) Recent studies and our unpublished data showed that knockout of the F-ATPase β subunit and F-ATPase inhibitors significantly decreased acid tolerance of S. anginosus, suggesting that the enzyme also plays a role in acid tolerance.26)
By contrast, low pathogenic oral streptococci, such as S. sanguis, have lower acid tolerance and lower membrane ATPase activity than S. mutans and S. anginosus.26,68) This correlation between expression of F-ATPase, acid tolerance, and pathogenicity in oral streptococci implies that F-ATPase is involved in virulence by secreting protons from cytoplasm. Therefore, the enzyme could be a target for prevention of dental caries and diseases related to oral pathogenic streptococci.
3.2. Physiological Roles of A-ATPaseA-ATPase is found in the membrane of archaea such as Methanosarcina mazei Gö1 and Thermus thermophilus, where it functions in ATP synthesis.48) Some nonarchaeal anaerobes also possess this enzyme. Recent studies suggest that A-ATPase is essential for functions other than ATP synthesis in nonarchaeal oral pathogenic bacteria including P. gingivalis and Enterococcus faecalis.
P. gingivalis is a major causative agent of periodontitis.69) This bacterium is asaccharolytic, and utilizes amino acids and oligopeptides as energy sources.70) P. gingivalis lacks F-ATPase but expresses A-ATPase in the bacterial membrane.28) Proton pumping ATPase inhibitors significantly reduced ATPase activity in the bacterial membrane, where A-ATPase is located, and decreased bacterial growth.28) These findings suggest that the enzyme is essential for growth of P. gingivalis.28) Since the bacterium imports amino acids and oligopeptides through proton-dependent oligopeptide transporters (POTs), A-ATPase likely plays a role in generating the proton gradient for nutrient transport.71)
E. faecalis, found in a variety of environments including the root canal, causes infectious diseases such as endocarditis and endodontic failure.72,73) Interestingly, this bacterium possesses both F- and A-ATPases. E. faecalis F-ATPase synthesizes ATP, and A-ATPase functions in sodium extrusion from cytoplasm under high salt or alkaline conditions.74)
As described above, A-ATPase in nonarchaeal anaerobes mediates nutrient transport and salt tolerance, whereas it functions in central metabolism in archaea. It is likely that nonarchaeal bacteria adopted A-ATPase for various purposes during evolution. Since mammals do not have A-ATPase, it is a potential therapeutic target for oral infectious diseases such as periodontitis.
There are various types of F-ATPase inhibitors, including dicyclohexylcarbodiimide (DCCD), azide, chloro-4-nitrobenzo-2-oxa-1,3-diazo1e (NBD-Cl), and aurovertin. Most inhibit both bacterial and mitochondrial F-ATPase since the structures of these enzymes are similar.31,75–77) Therefore, F-ATPase inhibitors are presumed to be toxic to mammals and unsuitable as antimicrobial agents. However, recent studies revealed that bedaquiline selectively affects bacterial F-ATPase, hence it is less toxic to mammals than previously expected.29)
On the other hand, only a few compounds including diethylstilbestrol and phytopolyphenols have been reported to inhibit A-ATPase.28,78) As described above, mammals do not possess this enzyme, and the structure of mammalian F- and V-ATPases is different from that of bacterial A-ATPase.28) Thus, A-ATPase inhibitors would presumably selectively kill bacteria.
4.1. BedaquilineBedaquiline, a diarylquinoline derivative, selectively binds to the membrane-spanning part of the c subunit of mycobacterial F-ATPase79) and functions as a H+/K+ ionophore80) (Table 1). It is likely that bedaquiline is accumulated in the mycobacterial membrane by binding to F-ATPase, which disrupts transmembrane proton and potassium gradients, resulting in inhibition of ATP synthesis.80) Amino acid residues essential for binding to bedaquiline are not conserved in mycobacterial and mitochondrial enzymes,29,79) explaining the selective binding of bedaquiline to mycobacterial F-ATPase. As described above, M. tuberculosis enters a nongrowing dormant state that is not very susceptible to known antituberculous drugs.56) Unlike these drugs, bedaquiline potently kills the bacterium in both the optimal replicating state and the dormant state57,81) (Table 1). Therefore, bedaquiline has recently been approved in 109 countries for treating multidrug-resistant tuberculosis.82)
| Compound | F-ATPase | A-ATPase | ||
|---|---|---|---|---|
| Effect on enzymatic activity | Effect on bacterial growth | Effect on enzymatic activity | Effect on bacterial growth | |
 | IC50 = 5.3 nM (ATP synthesis by M. bovis BCG inverted membrane vesicles)80) | MIC = 5.4 nM and 54 nM (M. bovis BCG and M. tuberculosis H37Rv, respectively)29) IC50 = 5 µM (S. mutans, acidic condition)27) | NT a) | NT a) |
 | IC50 = 48 µM (ATP hydrolysis by E. coli F1)20) IC50 = 39 µM (ATP hydrolysis by S. mutans F1)27) | IC50 = 68 µM (S. mutans, acidic condition)27) | NT a) | IC50 = 143 µM (P. gingivalis)28) |
 | IC50 = 4 µM (ATP hydrolysis by E. coli F1)32) IC50 = 6 µM (ATP hydrolysis by S. mutans F1)27) | IC50 = 18 µM (E. coli, succinate medium)32) IC50 < 30 µM (S. mutans, acidic condition)27) | IC50 = 3 µM (ATP hydrolysis by P. gingivalis membrane)28) | IC50 = 3 µM (P. gingivalis)28) |
 | IC50 = 3 µM (ATP hydrolysis by E. coli F1)32) IC50 = 4 µM (ATP hydrolysis by S. mutans F1)27) | IC50 = 5 µM (E. coli, succinate medium)32) IC50 = 6 µM (S. mutans, acidic condition)27) | NT a) | NT a) |
a) Not tested.
Bedaquiline also decreases the growth and survival of S. mutans under acidic conditions, although the IC50 value for S. mutans is approx. 100 times higher than that for M. tuberculosis27,29) (Table 1). More recently, it was reported that bedaquiline analogs and other compounds binding to the bedaquiline binding site display more potent antituberculosis activity and/or less toxicity to mammals than bedaquiline.83,84) These inhibitors could be potential candidates for antidental caries agents.
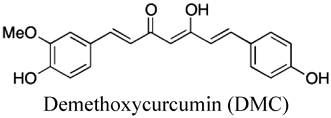
4.2. PhytopolyphenolsPhytopolyphenols are a group of natural products found in plants including fruits and vegetables.85) Our group and others have shown that E. coli F-ATPase is inhibited by phytopolyphenols, such as piceatannol, resveratrol, quercetin, curcumin, thymoquinone, safranal, and olive phenolics20,32,86–88) (Table 1). As described above, the interface between the β subunit and the C-terminal regions of the γ subunit is important for triggering rotation of the γ subunit,23) and piceatannol, resveratrol, and quercetin bind to this interface.47) Mutagenesis and molecular docking studies suggest that curcumin and a 3′-demethoxy analog (demethoxycurcumin; DMC) interact with residues in interface between the α and β subunits of F1.21,89) Piceatannol, curcumin, and DMC decrease E. coli growth by inhibiting oxidative phosphorylation, consistent with their inhibitory effects on F1 ATPase activity20,30,32) (Table 1). These phytopolyphenols also inhibit S. mutans F-ATPase, and reduce bacterial growth and survival only under acidic conditions27) (Table 1), suggesting that they lower acid tolerance of pathogenic streptococci.
Curcumin lowers ATPase activity in the P. gingivalis membrane, where A-ATPase is located, and thereby inhibits bacterial growth28) (Table 1). Piceatannol and its analogs have similar chemical structures to diethylstilbestrol, an A-ATPase inhibitor, and they strongly decrease bacterial growth (Table 1). These findings suggest that phytopolyphenols exhibit antimicrobial activity against P. gingivalis by inhibiting A-ATPase.
Phytopolyphenols such as piceatannol and curcumin also inhibit ATPase activity in the mitochondrial membrane, where F-ATPase is located,90) and these compounds are not toxic to mammals, unlike other F-ATPase inhibitors. This is probably because piceatannol and its analogs are rapidly metabolized and eliminated in mammals.34) In addition, curcumin and its analogs are poorly soluble and unstable in water.33) It is unlikely that these phytopolyphenols inhibit F-ATPase in mammalian tissues due to their low bioavailability. On the other hand, these compounds are expected to inhibit F-ATPase in oral pathogenic bacteria since they can be used at high concentrations through oral administration without absorption, distribution, metabolism, and excretion (ADME) restrictions. Therefore, these phytopolyphenols could be potential seeds for novel antidental caries or periodontal agents.
Proton pumping ATPases in most organisms are involved in ATP synthesis, but these enzymes are presumed to be unessential in anaerobes that can grow without oxidative phosphorylation. Recent studies indicated that these enzymes in oral pathogenic anaerobic bacteria play indispensable roles in acid tolerance and nutrient transport, rather than ATP synthesis. Bedaquiline and phytopolyphenols affect proton pumping ATPases in these bacteria and inhibit the bacterial growth rate, and they are less toxic to mammals.27–29,32) Therefore, these compounds could be seeds for antimicrobial agents against oral pathogenic bacteria.
Studies on bacterial proton pumping ATPases based on single-molecule observations have revealed the essential mechanism of rotational catalysis, including the roles of the β−γ and α−β interfaces.7,20,21,41) Piceatannol and curcumin bind to these interfaces and inhibit proton pumping ATPases,20,21,47) suggesting that these interfaces may be applicable as pharmacophores for in silico screening of pathogen selective inhibitors.
Proton pumping ATPases are composed of many subunits, and various subunit interfaces essential for rotational catalysis have been identified.7) This implies that inhibitors binding to different interfaces of the enzymes could be developed. Antimicrobial resistance has become a worldwide problem. Cocktails of such inhibitors could more effectively kill bacteria than single administration and suppress the emergence of drug-resistant microbes.
Dr. M. Nakanishi-Matsui is thanked for helpful advice and critical discussion. This work was supported in part by Japan Society for the Promotion of Science (JSPS) KAKENHI Grants (JP18K06629, JP21K06546, and 21H02627), and by the Keiryokai Research Foundation (Grant No. 142).
The author declares no conflict of interest.