2022 年 45 巻 11 号 p. 1678-1683
2022 年 45 巻 11 号 p. 1678-1683
The skin is an important barrier that protects against invasion by foreign substances, including irritants and harmful microorganisms, and holds water in the body. Washing the skin with cleansers and shampoos containing anionic surfactants, for example sodium dodecyl sulfate (SDS), is important for maintaining skin homeostasis. However, surfactants can cause dermatitis, cutaneous hypersensitivity (e.g., alloknesis), and pruritus in humans. Our previous studies revealed an alloknesis response in the skin with SDS-induced dermatitis in C57BL/6 mice. In addition, we found that alloknesis responses and afterdischarge responses following stimulation with light touch are related because they are observed contemporaneously. In this study, we used Hos:HR-1 hairless mice to establish a mouse model to evaluate long-term drug application for alloknesis responses. Alloknesis was observed in HR-1 mice with SDS-induced dermatitis. The mean number of c-Fos (a marker of neural activity) immunopositive neurons was increased in the lamina 1-2 (L1-2) spinal dorsal horn, but not in L3-4, of SDS-treated HR-1 mice compared to vehicle-treated mice. We also discovered that afterdischarge responses were observed in neurons in L1-2. There was also a correlation between the intensity of the afterdischarge responses and depth of the recording site. Thus, the following were suggested: 1) neurons that mediate these afterdischarge responses are located on the superficial layer of the spinal cord; 2) afterdischarge responses can be an index of alloknesis responses, and 3) the mouse model of SDS-induced dermatitis is an appropriate alloknesis model.
The skin is an important barrier that protects against invasion by foreign substances (such as viruses and harmful microorganisms).1,2) Body/hand soaps and shampoos play an important role in washing these agents from the skin. However, they can cause various adverse reactions in the skin, such as dermatitis, skin dryness, cutaneous hypersensitivity (e.g., alloknesis), and pruritus.3,4) Such adverse reactions are likely to occur with anionic surfactants, which cause skin irritation. A typical example is sodium dodecyl sulfate (SDS), an agent often used experimentally to induce irritant contact dermatitis (ICD).5,6) We previously reported that repeated SDS application of the skin promotes the nerve elongation into the epidermis and ICD with chronic itching in C57BL/6 mice.7,8) Interestingly, C57BL/6 mice with SDS dermatitis exhibit itching-related scratching behavior in response to non-noxious stimuli such as light touch from a filament.9) Based on these reports, increased nerve elongation in the epidermis might be involved in filament-induced scratching. In C57BL/6 mice, skin inflammation disrupts the hair cycle and accelerates hair growth. Under such circumstances, it is difficult to evaluate the effects of long-term application of a substance. Therefore, to establish a mouse model that can evaluate these effects, we experimented with hairless mice instead of C57BL/6 mice.
In the spinal dorsal horn (SDH), especially lamina 2 (L2) is one of the principal areas for synaptic processing of noxious information (e.g., pain or itch).10) Itch information is conveyed via primary afferents from the skin and processed in the superficial SDH. In the SDH of C57BL/6 mice with SDS-induced ICD, neurons expressing c-Fos, a marker for neural activity, were increased in L1 and L2 compared to that in the control mice group.9) Considering the increase in c-Fos-immunoreactive neurons observed in these layers as reported by this study, it is presumed that the neurons in L1-2 are involved in alloknesis responses. We hypothesized that there may be regions where neurons associated with alloknesis responses exist and that these alloknesis-related neurons may have different firing frequencies at the individual level when compared to other neurons. Thus, in this study, we first investigated whether SDS dermatitis–derived alloknesis responses observed in hairless HR-1 mice were similar to those observed in C57BL/6 mice. Next, we assessed the presence of spinal cord neurons with a high frequency of filament-induced afterdischarge firing. Finally, we evaluated the areas where these neurons were found.
All animal experiments were conducted in accordance with the guidelines of the Physiological Society of Japan, and were approved by the Animal Experiment Committees of the University of Toyama. All efforts were made to minimize animal suffering and animal count in these studies.
AnimalAdult (7 weeks old) male Hos:HR-1 mice were used for this study (Japan SLC, Shizuoka, Japan). The animals were caged in a room temperature kept at 21–24 °C with a relative humidity of 45–60%. Food and water were provided ad libitum.
SDS TreatmentThe hair on the back of the right caudal dorsal part was shaved at least 3 d prior to the start of the study. SDS (Nacalai Tesque, Inc., Kyoto, Japan) was dissolved in 70% ethanol. Ten percent SDS was topically application of the shaved skin at a volume of 50 µL for three consecutive days.
Assessment of Cutaneous Barrier Disruption, Skin Dryness and Dermatitis SeverityCutaneous barrier disruption was investigated by measuring transepidermal water loss (TEWL, g/m2 per hour) using a VapoMeter (model SWL4002; Keystone Scientific, K.K., Tokyo, Japan). Skin dryness was assessed by measuring stratum corneum (SC) hydration (%) with a moisture checker MY-808S (Scalar Co., Tokyo, Japan). The severity of dermatitis was scored as follows: 0, no redness; 1, subtle-to-mild redness (< 25% of the hair removal region); 2, moderate redness (≥ 25% but <50%); and 3, severe redness (≥ 50%) and/or hemorrhage.
Behavioral ExperimentsScratching behavior was observed as described in previous studies.7,11,12) To acclimate the animals to the experimental environment, they were placed individually in an acrylic observation cage for at least 30 min. After the acclimation period, their behavior was video-recorded by personnel kept out of the observation room. The video files were played back to count the hind-paw scratching of the rostral back. A series of these movements were counted as a single bout of scratching. Alloknesis was assessed as follows: at 10 s intervals, an innocuous light touch with a 0.07 g bending force by a von Frey filament (vFF) was applied to the treated rostral back skin five times per set for three sets. The force was sufficiently low to be insensitive under normal conditions. The presence or absence of a hindlimb scratch bout following each stimulus was noted (maximum alloknesis score = 15).13)
Immunohistochemical Staining of Spinal CordThe spinal cord samples were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS). The lumbar 4-5 segment was embedded in paraffin, and sectioned at 5 µm thickness. After dewaxing in xylene and antigen retrieval using a commercial DAKO target retrieval solution (pH 6.1; DAKO Corporation, Carpinteria, CA, U.S.A.) overnight at 60 °C, the sections were blocked in PBS containing 0.1% Tween 20 and 1.5% fetal bovine serum at room temperature for 30 min and incubated overnight at 4 °C with the following primary antibodies: rabbit polyclonal anti-c-Fos H-125 antibody (1 : 100, Cat# sc-7202, Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.) and biotinylated Griffonia simplicifolia lectin I isolectin B4 (IB4) (1 : 100; Cat# B-1205, Vector Laboratories, CA, U.S.A.) antibody. After washing in PBS, the sections were treated with the following secondary antibodies for 2 h at room temperature: fluorophore-labeled anti-rabbit immunoglobulin G (IgG) (1 : 500; Invitrogen Corp., Waltham, MA, U.S.A.) and streptavidin fluorophore-conjugated (1 : 500; Invitrogen Corp.) IgG. The nuclei were counterstained with 4′,6-diamidino-2-phenylindole. Immunofluorescence was observed using a fluorescence microscope (model BZ-8000; Keyence, Osaka, Japan), and each image was captured under 20× magnification. Cells with immunoreactive signal throughout the cytoplasm were counted in superficial layer (L1-2) and deep layer (L3-6 separately using an image analyzer (BZ-II Analyzer, version 2.2; Keyence).
In vivo Extracellular Recording from Superficial SDH (L1-2) NeuronsThe in vivo preparation and recordings from L1-2 neurons were similar to described in a previous report.9) Briefly, adult male HR-1 mice were anesthetized with urethane (1.5 g/kg, intraperitoneal). Thoracolumbar laminectomy was carried out to remove the spinal column (lumbar 1-5 vertebrae), and the HR-1 mouse was then placed in a brain-spinal stereotaxic apparatus. The spinal cord surface was bath-applied with Krebs solution (15 mL/min) containing (in mmol/L) 117 NaCl, 3.6 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 11 glucose, and 25 NaHCO3, equilibrated with 95% O2–5% CO2 at 37 ± 1 °C. Extracellular single-unit activity of superficial SDH (L1-2) neurons were performed as previously described.9,14–16) Signals were recorded, amplified (EX1; Dagan corporation, Minneapolis, MN, U.S.A.), digitized (Digidata 1400 A; Molecular Devices, Union City, CA, U.S.A.) and displayed online using a Clampfit 10.2 (Molecular Devices). We detected for the receptive field on the skin where cotton wisp (innoxious stimulation) or forceps (noxious stimulation) produced a neural mechanical response. Mechanical stimulation with the vFF was applied to the receptive field on the skin for 5 s.13,17) The afterdischarges following termination of the vFF stimulus were also analyzed for 10 s.
Statistical AnalysesData were expressed as mean ± standard error of the mean (S.E.M.). Statistical analyses were performed using the Shapiro–Wilk test and F-test, and group means were compared using unpaired t-test or Mann–Whitney U test, or ANOVA followed by post hoc Holm–Šidák tests. Correlations between the frequency of afterdischarge and recording depth were analyzed using Spearman’s correlation coefficients. All statistical data analyses were carried out using Prism version 8 software (GraphPad Software Inc., San Diego, CA, U.S.A.), with p < 0.05 indicating significance.
At 24 h after the final topical application with 10% SDS, skin induced dermatitis (Figs. 1A, D), reduced SC hydration (Fig. 1B), and raised TEWL (Fig. 1C). These changes were significantly observed in SDS-treated mice, whereas not in VH-treated mice (Figs. 1B–D).
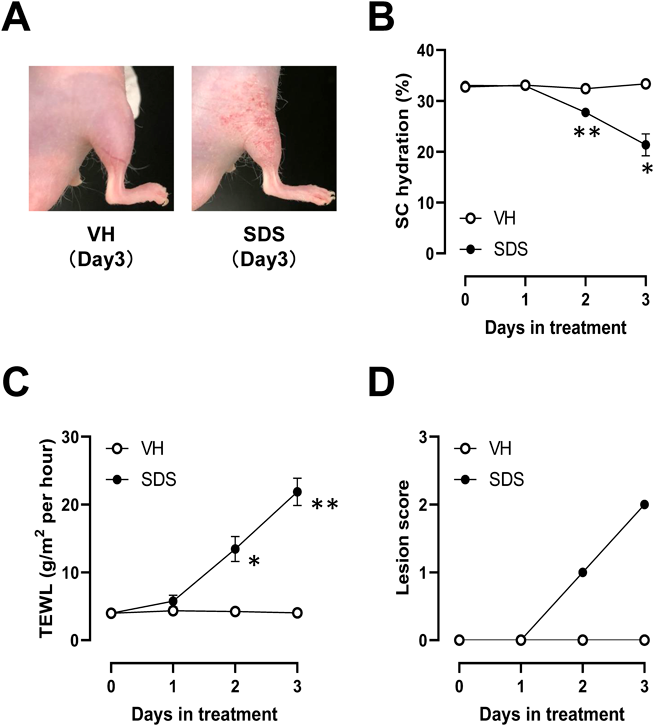
HR-1 mice receive repeated topical applied of 10% SDS (surfactant) or vehicle (VH, 70% ethanol) to the right caudal dorsal skin for 3 consecutive days. (A) Representative pictures of mouse skin at 24 h after the final VH or SDS treatment. (B–D) VH and SDS treated mice are evaluated by their SC hydration (B), TEWL (C), daily dermatitis scores (D) (n = 6 mice for B–D). * p < 0.05, ** p < 0.01 vs. VH (Holm–Šidák test).
Repeated application of 10% SDS to the skin provoked spontaneous scratching behavior and increased alloknesis (Fig. 2).

(A) Bar graph shows the mean number of scratch bouts per hour for VH or SDS treatment day 3. (B) Bar graph shows the mean score of alloknesis at VH or SDS treatment day 3 (n = 6). * p < 0.05, ** p < 0.01 vs. VH (unpaired t test (A) and Mann–Whitney U test (B)).
To investigate the distribution of nerves excited by SDS treatment in the SDH, we first conducted immunohistochemical staining for c-Fos18) and IB4 (a marker of the ventral part of L1 and dorsal part of L2)19) in sections lumbar 4-5 (Fig. 3A). While there was no change in the number of c-Fos-immunoreactive cells in L3-6, SDS treatment significantly increased the number of c-Fos-immunoreactive cells in L1-2 of the SDH compared to that by vehicle treatment (Figs. 3A, B).
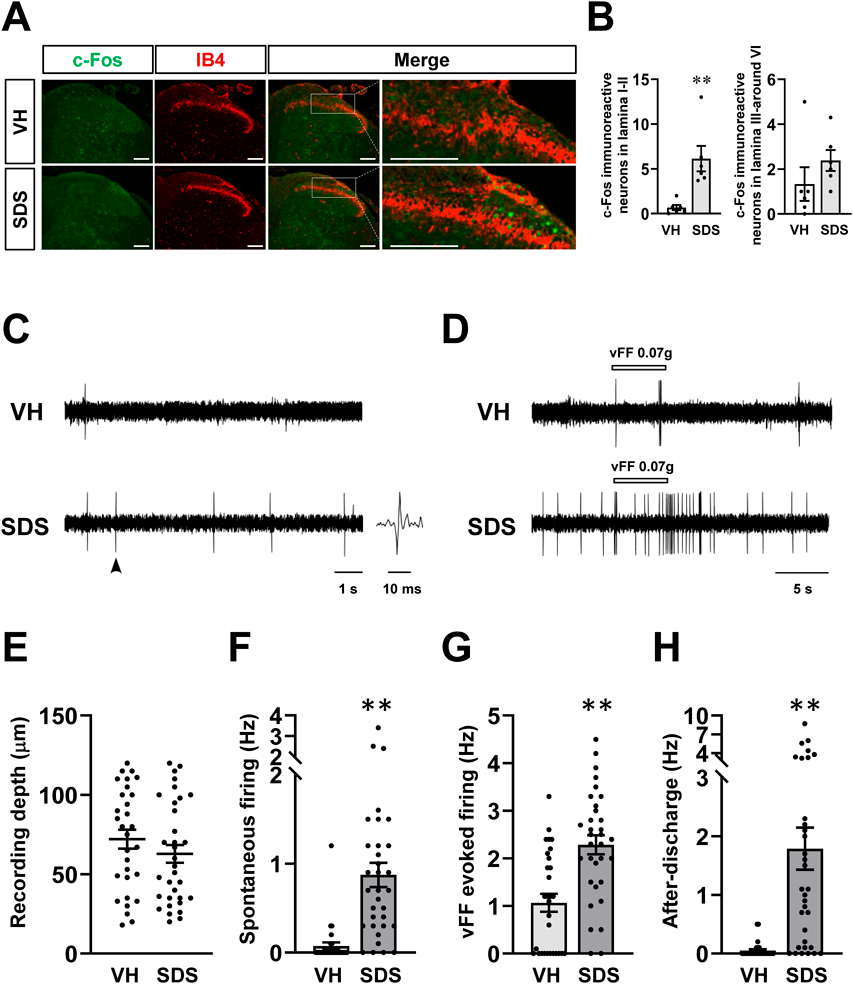
(A) Typical photomicrograph images showing immunohistochemical staining for c-Fos (green) and IB4 (red) in superficial SDH (ipsilateral side) of thoracolumbar segments (scale bar = 100 µm). (B) The number of c-Fos immunoreactive neurons in L1-2 (left, n = 6 mice per group) and L3-6 (right, n = 6 mice per group) of SDH (ipsilateral side). Each small circle represents one mouse. ** p < 0.01 vs. VH (Mann–Whitney U test). (C, D) Typical traces of spontaneous single neuronal firing (C), vFF-evoked firing and subsequent afterdischarges (D) in superficial SDH neurons of VH- and SDS-treated mice. Arrowheads showing typical trace of spike. (E) Summary showing recording depth in superficial SDH neurons. (F–H) Summary showing the frequency of spontaneous single neuronal firing (F), vFF-evoked firing (G), and afterdischarges (H) in superficial SDH neurons. Each small circle represents a single neuron (n = 30 neurons/6 6 VH group and 33 neurons/6 SDS group). ** p < 0.01 vs. VH (Mann–Whitney U test).
The number of c-Fos-immunoreactive cells in L1-2 of the SDH in SDS treated mice was higher than that of vehicle treatment (Figs. 3A, B). Therefore, we recorded the spontaneous and vFF-evoked single neuronal firing in the L1-2 of vehicle- and SDS-treated group. The frequency of spontaneous neuronal firing in superficial SDH neurons in SDS-treated group was significantly increased, compared to vehicle-treated group (Figs. 3C, F). An innoxious stimulus to the receptive field on the skin using a vFF13,17) also evoked higher neural firing frequencies in SDS-treated group (Figs. 3D, G). Moreover, conspicuous afterdischarge firing was observed in all SDS-treated group, and the response was recorded in many superficial SDH neurons following the termination of the vFF stimulus, but not in vehicle-treated group (Figs. 3D, H). Recordings were preformed from superficial SDH neurons and there was no significant in the average depth from the surface of the spinal cord (Fig. 3E).
Correlation between Firing Frequency of Afterdischarge in Single Neuron and the Representative Recording DepthWhen plotting individual data of the frequency of afterdischarge and recording depth, there was a right- and downward shift of data in the SDS-treated group compared with data in the VH-treated controls. However, the correlations for this parameter were significant only in the SDS-treated group, but not in the VH-treated group (Fig. 4).

The correlations in the VH-treated group are not significant (A, n = 30 neurons/6 VH group, r =−0.199, p = 0.29), but significant negative correlations are detected in the SDS-treated group (B, n = 33 neurons/6 SDS group, r = 0.488, p < 0.01).
SDS exposure is a standard method used in many experiments to induce dermatitis and dryness in mice’s skin, but its effectiveness is dependent on the frequency and duration of SDS exposure.5) In the present study, the once-daily application of 10% SDS gradually induced skin irritant responses such as dermatitis, skin dryness, and cutaneous barrier disruption over a three-day treatment period. Repetitive application of 10% SDS elicited spontaneous scratching (an index of itch) and brought skin sensation into the state of “alloknesis.” In addition to skin inflammation, repetitive SDS application to the skin surface significantly increased both spontaneous and vFF-evoked neural firing in the SDH. Taken together, these findings suggest that repetitive application of strong detergents, such as SDS, can trigger skin hypersensitivity through changes in the skin, peripheral nerves, and hyperexcitability of sensory and spinal neurons.
Repeated application of SDS also increased the expression of c-Fos-positive neurons18) in the superficial SDH (L1-2), but not in the deep SDH (L3-6). The C fibers that transmit the itching sensation terminate in the superficial SDH.20) In addition, several itch stimuli, such as allergens, increase c-Fos-positive neurons in the superficial SDH.21) Therefore, enhanced neuronal activity in the superficial SDH might be involved in itching and cutaneous hypersensitivity in SDS-treated mice.
We identified neurons in the superficial SDH that exhibited high rates of spontaneous neural firing, which we postulated to be due to ongoing pruriceptive input from the SDS-induced itch. Both skin-derived and spinal factors may contribute to SDS-induced neuronal hyperactivity in the spinal cord. A variety of factors released from keratinocytes, such as histamine, may excite elongated peripheral nerve fibers. Our previous study showed that SDS increased the epidermal histamine concentration.7) Intradermal injection of histamine also increased c-Fos-positive neurons21) and firing13,22) in the superficial SDH. Activated astrocytes release itch-sensing mediators, such as nitric oxide23) and prostaglandin E2,24) which induce neuronal firing.25,26) Therefore, multiple factors from the skin and spinal cord may promote spontaneous firing of SDH neurons in SDS-treated mice.
In addition to spontaneous neural activity, 0.07 g vFF (innoxious mechanical stimulation)-evoked firing was enhanced in the superficial SDH of SDS-treated mice. In vivo electrophysiological recordings in the superficial SDH also revealed a striking afterdischarge of SDS-treated, but not vehicle-treated, mice. This observation might be particularly important in chronic hypersensitivity, which is responsible for conferred sensory input in the absence of actual stimulation. Mechanical cutaneous stimulation-induced afterdischarges in the SDH are responsible for the ectopic afferent activity of peripheral nerve fibers persisting beyond the stimulus.27) This ectopic afferent activity is associated with the induction of central nerve sensitization.28) As our study did not investigate the mechanisms underlying mechanical cutaneous stimulation-induced afterdischarges, future studies are needed to clarify them. A possible mechanism involves changes in SDH cells (neurons and glial cells, especially astrocytes) rather than in the primary afferents, which might be involved in afterdischarges. Shiratori-Hayashi et al. reported that lipocalin-2 (LCN2) released from spinal astrocytes contributes to chronic itching and sustained potentiation of itching.29) LCN2 acts on neurons and astrocytes, triggering the production of nitric oxide and some cytokines.30) However, whether spinal LCN2 is involved in the enhanced neural activity in superficial SDH remains unclear. Therefore, the underlying mechanisms should be investigated in future studies. In conclusion, we demonstrated that repeated SDS treatment induced changes in the peripheral nerves and skin increased spontaneous firing and innoxious mechanical stimulus-evoked firing, and accelerated afterdischarges in superficial SDH neurons. Collectively, these changes may explain spinal transmission and modulation of skin hypersensitivity induced by SDS.
This work was supported by JSPS KAKENHI (Grant Nos. 19K09323 and 22K09020) to D.U. and was partially supported by the Adaptable and Seamless Technology Transfer Program through Target-driven R&D (A-STEP) from the Japan Science and Technology Agency (JST) (to D.U.; JPMJTM20DN). This study was also supported in part by Hoyu Co., Ltd. (Nagakute, Aichi, Japan).
Yoshihiro Inami and Miki Fukushima are employed by Hoyu Co., Ltd., who financially supported this research.