Abstract
Macrophages have important roles in the progression of inflammation. Ajania purpurea Shih. is a member of the Ajania Poljakor family that grows in Tibet (China). Extracts from plants in this genus have anti-bacterial and anti-inflammatory properties. However, there are few reports on the activity and mechanism of Ajania purpurea. Here, we confirmed the anti-inflammatory effect of Ajania purpurea Shih. ethanol extract (EAPS) by examining the levels of inflammatory factors in a mouse model of peritonitis and RAW264.7 cells. The main components of EAPS detected by LC-MS analysis included piperine and chlorogenic acid. In particular, in lipopolysaccharide (LPS)-induced RAW264.7 cells, EAPS inhibited the protein expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) in LPS-induced RAW264.7 cells, lowered the levels of nitric oxide (NO) and prostaglandin E2 (PGE2), as well as the release of inflammatory factors such as tumor necrosis factor-alpha (TNF-α) and pro-inflammatory cytokines such as interleukin (IL)-1β and IL-6. In addition, Western blot analysis and immunofluorescence staining verified that EAPS inhibited the activity of the nuclear factor-kappaB (NF-κB) pathway by reducing the nuclear translocation of the p65 subunit. Furthermore, in a mouse model of peritonitis, EAPS inhibited the release of inflammatory factors, as well as the recruitment of immune cells including neutrophils and macrophages. These findings indicated that EAPS suppressed LPS-induced inflammation via inhibiting the NF-κB pathway in RAW264.7 cells and mice with peritonitis. Thus, EAPS may be a viable therapeutic method for the treatment of inflammation and related disorders.
INTRODUCTION
Inflammation can be classified as either acute or chronic depending on the duration of the process.1) Both acute and chronic inflammation may contribute to multiple types of diseases including autoimmune diseases, cancer, diabetes, and arthritis.2)
Toll-like receptors (TLRs) play significant roles in the immune response.3) Lipopolysaccharide (LPS) is the main ligand of TLR4, a surface receptor that activates the classical nuclear factor-kappaB (NF-κB) inflammatory pathway.4) This pathway can produce inflammatory factors like tumor necrosis factor-alpha (TNF-α) and interleukins (ILs).5) Since multiple inflammatory responses in humans are associated with activation of NF-κB, targeting this pathway would be an effective way to alleviate inflammation.6)
Many plants contain various active ingredients, which may be valuable in treating diseases due to their low cost and lack of side effects.7) The Ajania poljakor family includes Ajania nubigena (A. nubigena), Ajania salicifolia (A. salicifolia), Ajania purpurea (A. purpurea) and so on.8) Recent studies demonstrated that TNF-α levels were decreased by A. nubigena extracts in LPS-activated THP-1 cells.9) In addition, quinones and coumarins from A. salicifolia have anti-oxidant properties in vitro, as well as cytotoxicity against Hela and HepG2 cells.10) A. purpurea Shih, a plant belonging to the Ajania poljakor family, mainly grows in the plateau area of the Tibet Autonomous Region.11) Studies have found that Ajania poljaork plants contain triterpenoids, flavonoids, phenols and other chemical components, which have medicinal value such as anti-bacterial and anti-inflammatory activities.8) Therefore, we speculated that A. purpurea also exhibits similar physiological activities.
To further clarify the biological activity of A. purpurea, we examined the anti-inflammatory effects and mechanism of action of an ethanol extract of A. purpurea (EAPS) in mice with LPS-induced peritonitis and a murine macrophage cell line.
MATERIALS AND METHODS
Chemicals and ReagentsPrimary antibodies, including mouse/goat/rabbit anti-cyclooxygenase-2 (COX-2), anti-inducible nitric oxide synthase (iNOS), anti-p65, anti-p-inhibitor of kappaB (IκB)-α, anti-IκB-α, as well as secondary antibodies, were bought from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). Fluorescein isothiocyanate (FITC) labeled immunoglobulin G (IgG) secondary antibody, 4′,6-diamidino-2-phenylindole (DAPI) dye solution and nuclear protein extraction kits were purchased from Solarbio Biotechnology Science, Inc. (Beijing, China). FITC anti-mouse F4/80 antibody, 2′,7-dichlorofluorescein diacetate (DCFH-DA), APC-anti-mouse CD11b antibody, FITC anti-mouse Ly6G antibody, urified anti-mouse CD16/32 antibody and Cell Staining Buffer were obtained from Elabscience Biological Technology Co., Ltd. (Wuhan, China). All other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.).
Preparation of A. purpurea Shih ExtractDried and pulverized aerial parts of Ajania purpurea Shih were subjected to reflux-extraction with 70% ethanol for 1.5 h twice. The extract was then concentrated in a vacuum to produce an EAPS, which was compiled into the Component Bank of Tibetan Medicine (CBTM-E132).
Experimental Animals and TreatmentForty-eight male Kunming mice (8–9 weeks old) were obtained from the Qingdao Institute for Food and Drug Control (Approval No. SYXK (Lu) 2021 0342). The mice were housed at a temperature of 22 ± 2 °C, with a relative humidity of 55 ± 5%, and a day–night cycle of 12 h for 3 d. The male Kunming mice were divided into six groups (n = 8): control (Con); LPS-induced peritonitis mouse (LPS); dexamethasone (Dex, 0.5 mg/kg, intraperitoneally (i.p.)); low-dose-EAPS (L-EAPS, 31.9 mg/kg, intragastrically (i.g.)), middle-dose-EAPS (M-EAPS, 63.9 mg/kg, i.g.), and high-dose-EAPS (H-EAPS,127.9 mg/kg, i.g.). After 14 d of treatment, the mice were microinjected with LPS (1 mg/kg) or sterile saline solution intraperitoneally. After 4 h, mice were humanely killed and the abdominal cavities were washed with heparin-containing sterile phosphate-buffered saline (25 UI/mL).
Ethics Approval and Consent to ParticipateThe Ethical Committee Institutional Animal Care and Use Committee of Qingdao University of Science and Technology approved all related facilities and experimental protocols (Approval No. ACQUST-2021-027).
Flow Cytometry AnalysisThe peritoneal lavage fluids were centrifuged for 15 min. The cell pellets were resuspended in cell staining buffer and washed 1–2 times. Red blood cells were lysed with erythrocyte lysis buffer at 4 °C, centrifuged for 5 min, and washed again 1–2 times. Finally, the cells were resuspended in cell staining buffer at a cell density of 1 × 107 cells/mL. Cells were incubated with purified anti-mouse CD16/32 antibody [2.4G2], followed by incubation with the flow cytometry antibodies APC anti-mouse CD11b [M1/70], FITC anti-mouse Ly6G [1A8], and FITC anti-mouse F4/80 [CI:A3-1]. Finally, the cells were centrifuged for 5 min, resuspended and analysed by flow cytometry.
Cell Culture and Cell Viability AssayRAW264.7 macrophage cells were acquired from the American Type Culture Collection (Rockville, MD, U.S.A.). Cells were incubated in Dulbecco’s modified Eagle’s medium (DMEM; Grand Island, NY, U.S.A.) containing 10% fetal bovine serum (FBS) at 37 °C with 5% CO2. Cells (1 × 104 cells/mL) were cultured with different EAPS concentrations for 24 h in 96-well plates. Then, cells were cultured with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (2.5 mg/mL) for 4 h. After the supernatant was removed, dimethyl sulfoxide (DMSO) (150 µL) was added and the absorbance was read at 490 nm with a microplate reader.
Analysis of Inflammatory Factor ProductionRAW264.7 cells (1 × 105 cells/mL) were cultured with EAPS for 12 h, then LPS treatment for 24 h in 24-well plates. The culture supernatant was collected to examine the production of TNF-α, PGE2, IL-1β and IL-6 using enzyme-linked immunosorbent assay (ELISA) kits (Wuhan, China). The nitrite production in RAW264.7 cells was measured using the Griess reaction. And in vivo experiment, the peritoneal lavage fluid was collected with a syringe and centrifuged to extract the supernatant. The concentrations of TNF-α, IL-1β and IL-6 were detected using ELISA kits.
Western Blot AnalysisCells were washed twice with pre-chilled phosphate buffered saline (PBS) buffer solution, and lysed by adding phenylmethylsulfonyl fluoride (PMSF)-containing radio immunoprecipitation assay (RIPA) lysis buffer. Samples were then centrifuged at 15000 rpm at 4 °C for 20 min. The protein concentration in the whole cell lysates was measured using a Bradford Assay Kit. Cell homogenates were adjusted to the same concentration with loading buffer, then samples were separated on 10% sodium dodecyl sulfate (SDS)-polyacrylamide gels and transferred to a fibrous membrane. Membranes were incubated with specific primary antibodies for 2 h and secondary antibodies for 1.5 h. Protein bands were detected by ECL Western blotting substrate (Amersham Biosciences, Buckinghamshire, U.K.), and quantified using a ChemiDoc Image Analyzer (Tanon4600, China).
Immunofluorescence StainingTo determine the subcellular localization of p65, RAW264.7 macrophages were seeded onto glass cover slips pretreated with tissue cultures. Treated cells were fixed with paraformaldehyde, lysed with Triton X-100, incubated with a primary antibody against p65, followed by incubation with FITC-labelled secondary antibody (Santa Cruz Biotechnology Company, China). Nuclei were stained with DAPI solution. Finally, the cover slip was placed on a glass slide and samples were examined with a fluorescence microscope (CKX53, Olympus, Japan).
Statistical AnalysisAll data were analysed using the GraphPad Prism 7 (GraphPad Software, San Diego, CA, U.S.A.). The two-tailed t-test was used to determine the differences between groups. p < 0.05 was considered to be a significant difference between the groups.
RESULTS
Effects of EAPS on Cell ViabilityThe MTT assay was used to examine the cytotoxic effects of EAPS. As shown in Fig. 1, EAPS had no effect on cells at doses of 25–200 µg/mL. However, a significant decrease in cell viability was observed at 400 µg/mL (p < 0.01). Therefore, for all subsequent experiments, cells were cultured with EAPS at concentrations of 25 to 200 µg/mL.
Effects of EAPS on the Levels of Inflammatory Factors in LPS-Induced RAW264.7 CellsTo determine the effects of EAPS on the production of inflammatory factors, RAW264.7 cells were preconditioned with EAPS (25–200 µg/mL) for 12 h, then treated with LPS (1 µg/mL) for 24 h. As shown in Fig. 2, LPS enhanced the levels of NO, TNF-α, IL-1β, IL-6 and PGE2 (p < 0.01), while EAPS treatment significantly reversed this change (p < 0.01). In addition, levels of gene expression of COX-2 and iNOS were suppressed by treatment with EAPS.
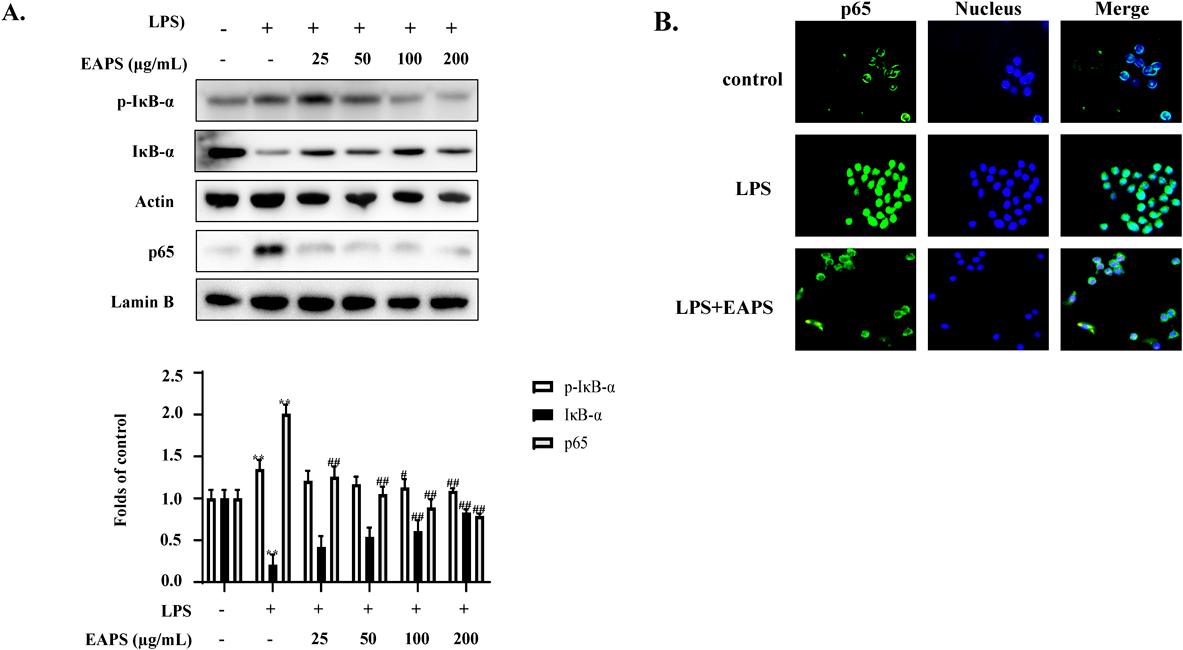
Effects of EAPS on the NF-κB Pathway in LPS-Induced RAW264.7 CellsPro-inflammatory mediators are mainly produced through activation of the NF-κB pathway. Therefore, the effects of EAPS on the expression of NF-κB (p65) pathway components were investigated. IκB-α was found to be phosphorylated in RAW264.7 cells following exposure to LPS, but this effect was significantly reduced by pretreatment with EAPS (25–200 µg/mL) for 12 h (Fig. 3A). In addition, fluorescence microscopy demonstrated that nuclear translocation of p65 was attenuated after treatment with EAPS (Fig. 3B).
Effects of EAPS on Inflammatory Cytokines in an LPS-Induced Mouse Model of PeritonitisTo confirm the anti-inflammatory activity of EAPS, we next examined the effect of EAPS on the expression of TNF-α, IL-6, and IL-1β in a mouse model of peritonitis. As shown in Fig. 4, the production of inflammatory factors was found to be increased in the LPS group, while EAPS treatment significantly reversed these effects (p < 0.01). These findings demonstrated that EAPS can be utilized to effectively treat peritonitis.
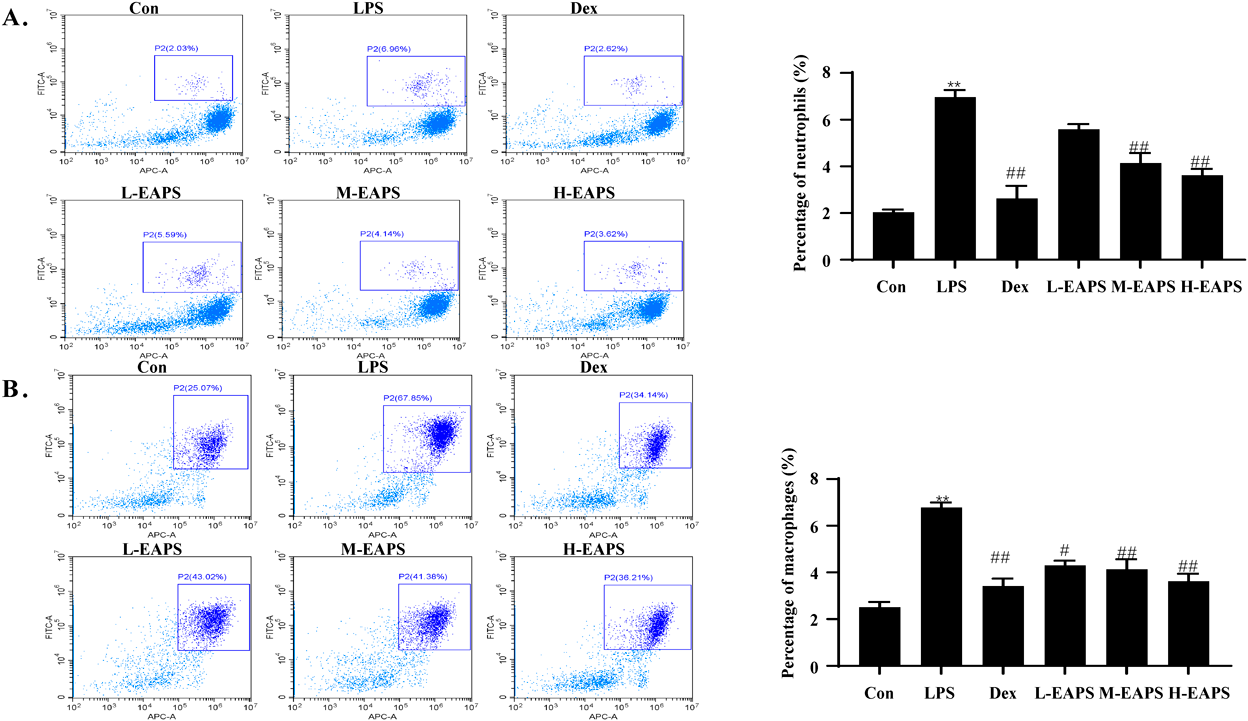
Effects of EAPS on Immune Cells in the Peritoneal Lavage Solution of Mice with PeritonitisChanges in the number and types of immune cells reflect the occurrence and development of inflammation. To determine the effects of EAPS on immune cells, flow cytometry analysis was performed on the peritoneal lavage fluid of mice with LPS-induced peritonitis. As shown in Fig. 5, the percentages of neutrophils and macrophages in LPS-treated mice (LPS group) were significantly higher than those in control mice. After administration, the frequency of neutrophils and macrophages decreased significantly (p < 0.05 or p < 0.01).
DISCUSSION
Inflammation is a series of protective responses to tissue damage.12) This inflammatory response is generally mediated by TLRs on the membrane surface of macrophages and mast cells. Activation of TLRs leads to the production of pro-inflammatory mediators such as inflammatory cytokines and chemokines, followed by neutrophils, which accumulate at the site of tissue damage to eliminate invasive material.13,14) Therefore, we measured the levels of macrophages and neutrophils in the peritonitis mouse peritoneal lavage fluid to assess the anti-inflammatory properties of EAPS. Here, we found that treatment with EAPS significantly reduced the levels of macrophages and neutrophils in an LPS-induced mouse model of peritonitis.
Although NO has the ability to eradicate bacterial infections in vivo,15) overproduction causes inflammation.16) Thus, we investigated the effect of EAPS on iNOS, which is one of the most significant enzymes involved in the synthesis of NO. In addition, COX are key enzymes that catalyze the conversion of arachidonic acid into prostanoids.17) COX-2 is induced by a number of stimuli including pro-inflammatory cytokines, and results in PGE2 synthesis, which is linked to inflammation and carcinogenesis.18) In this study, we confirmed that EAPS suppressed the levels of these two pro-inflammatory mediators and their inducing enzymes in RAW264.7 cells.
LPS is a bacterial endotoxin that can bind to TLR4 on the macrophage membrane, resulting in a configuration change of the latter, and activation of the downstream factor MyD88, followed by activation of the NF-κB complex.19) IκB-α is phosphorylated and dissociated from the NF-κB complex, which is degraded in the cytoplasm. Finally, free NF-κB (p65) exposes its nuclear localization sequence, resulting in nuclear translocation, where it binds to specific target genes including TNF-α, IL-6 and other inflammatory factors to initiate their expression.5) In this study, we confirmed that EAPS may reduce inflammation by decreasing nuclear translocation of p65.
Ajania poljakor plants have been traditionally used as antiseptics and antioxidants.8,20) Here, we report for the first time that A. purpurea inhibits the NF-κB pathway to reduce inflammation both in vivo and in vitro. Based on our LC-MS analysis (Supplementary Table 1, Supplementary Fig. 1), the active ingredients found in EAPS probably included 7-hydroxycoumarin, neochlorogenic acid, nootkatone, pinolenic acid, piperine and chlorogenic acid. In previous studies, 7-hydroxycoumarin attenuates the inflammatory response in UVB-induced HDFa cells by reducing the NF-κB protein expression.21) Neochlorogenic acid inhibits NF-κB pathway by reducing the phosphorylation of IKKα/β and IκBα to alleviate LPS-induced macrophage inflammation.22) Nootkatone attenuates inflammation caused by the diesel exhaust particles-induced lung damage in mice by reducing the activation of p65,23) and pinolenic acid alleviated TPA-induced skin inflammation in mice by decreasing the p38 and c-Jun N-terminal kinase (JNK) phosphorylation.24) In addition, pepperine reduces the inflammatory response of LPS-induced RAW264.7 cells via NF-κB pathway.25) Chlorogenic acid can significantly reduce tissue inflammation and apoptosis to alleviate dextran sodium sulfate-stimulated ulcerative colitis in mice via mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK)/ JNK pathway.26) Consistent with these reports, our study suggests that A. purpurea has an inhibitory effect on inflammation. However, since only the ethanol extract of Ajania purpurea Shih. was tested in this study, future studies will examine the correlation between the key components and inflammation.
CONCLUSION
Taken together, our findings suggest that EAPS decreased the levels of pro-inflammatory enzymes and cytokine production by suppressing the NF-κB pathway, resulting in suppression of the LPS-induced inflammatory response in RAW264.7 cells and a mouse model of peritonitis. These results provide a pharmacological basis and possible mechanism for the anti-inflammatory effect of EAPS and indicate that EAPS is a potential therapeutic agent for the treatment of inflammation and related disorders.
Acknowledgments
The work was supported by National Natural Science Foundation of China (82074578, 81960775, 81960781) and Shandong Province Natural Science Fundation (ZR2021LZY032).
Conflict of Interest
The authors declare no conflict of interest.
Supplementary Materials
This article contains supplementary materials.
REFERENCES
- 1) Germolec DR, Shipkowski KA, Frawley RP, Evans E. Markers of inflammation. Methods Mol. Biol., 1803, 57–79 (2018).
- 2) Yeung YT, Aziz F, Guerrero-Castilla A, Arguelles S. Signaling pathways in inflammation and anti-inflammatory therapies. Curr. Pharm. Des., 24, 1449–1484 (2018).
- 3) Fitzgerald KA, Kagan JC. Toll-like receptors and the control of immunity. Cell, 180, 1044–1066 (2020).
- 4) Kitajima T, Muroi M, Yamashita N, Tanamoto K. Toll-like receptors required for dermatophagoides farinae to activate NF-κB. Biol. Pharm. Bull., 37, 74–80 (2014).
- 5) Speranskii AI, Kostyuk SV, Kalashnikova EA, Veiko NN. Enrichment of extracellular DNA from the cultivation medium of human peripheral blood mononuclears with genomic CpG rich fragments results in increased cell production of IL-6 and TNF-a via activation of the NF-kB signaling pathway. Biomed. Khim., 62, 331–340 (2016).
- 6) Li J, Kim KW, Oh H, Kim YC. Anti-inflammatory effects of Sanhuang–Siwu–Tang in lipopolysaccharide-stimulated RAW264.7 Macrophages and BV2 Microglial Cells. Biol. Pharm. Bull., 44, 535–543 (2021).
- 7) Arulselvan P, Fard MT, Tan WS, Gothai S, Fakurazi S, Norhaizan ME, Kumar SS. Role of antioxidants and natural products in inflammation. Oxid. Med. Cell. Longev., 2016, 5276130 (2016).
- 8) Cui X, De J. Research advance on chemical constituents and bioactivities of volatile oil from Ajania poljak. Northwest Pharmaceutical Journal, 36, 159–162 (2021).
- 9) Wangchuk P, Keller PA, Pyne SG, Taweechotipatr M. Inhibition of TNF-α production in LPS-activated THP-1 monocytic cells by the crude extracts of seven Bhutanese medicinal plants. J. Ethnopharmacol., 148, 1013–1017 (2013).
- 10) Wu HR, Zhang W, Pang XY, Gong Y, Obulqasim XM, Li HF, Zhu Y. Quinones and coumarins from Ajania salicifolia and their radical scavenging and cytotoxic activity. J. Asian Nat. Prod. Res., 17, 1196–1203 (2015).
- 11) Wu X, Hong G, Zhao H. Establishment of efficient regeneration system from calli of the stems and leaves of Ajania purpurea Shih. Acta Agriculturae Zhejiangensis, 26, 335–338 (2014).
- 12) Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB. Inflammation and cancer. Ann. Afr. Med., 18, 121–126 (2019).
- 13) Taguchi T, Mukai K. Innate immunity signalling and membrane trafficking. Curr. Opin. Cell Biol., 59, 1–7 (2019).
- 14) Liew PX, Kubes P. The Neutrophil’s role during health and disease. Physiol. Rev., 99, 1223–1248 (2019).
- 15) Malone-Povolny MJ, Maloney SE, Schoenfisch MH. Nitric oxide therapy for diabetic wound healing. Adv. Healthc. Mater., 8, 1801210 (2019).
- 16) Guzik TJ, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol., 54, 469–487 (2003).
- 17) Yu T, Lao X, Zheng H. Influencing COX-2 activity by COX related pathways in inflammation and cancer. Mini Rev. Med. Chem., 16, 1230–1243 (2016).
- 18) Tyagi A, Kamal MA, Poddar NK. Integrated pathways of COX-2 and mTOR: roles in cell sensing and Alzheimer’s disease. Front. Neurosci., 14, 693 (2020).
- 19) Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat. Rev. Immunol., 2, 725–734 (2002).
- 20) Li Y, Yan SS, Wang JJ, Li LY, Zhang J, Wang K, Liang JY. Insecticidal activities and chemical composition of the essential oils of Ajania nitida and Ajania nematoloba from China. J. Oleo Sci., 67, 1571–1577 (2018).
- 21) Karthikeyan R, Kanimozhi G, Prasad NR, Agilan B, Ganesan M, Mohana S, Srithar G. 7-Hydroxycoumarin prevents UVB-induced activation of NF-κB and subsequent overexpression of matrix metalloproteinases and inflammatory markers in human dermal fibroblast cells. J. Photochem. Photobiol. B, 161, 170–176 (2016).
- 22) Park SY, Jin ML, Yi EH, Kim Y, Park G. Neochlorogenic acid inhibits against LPS-activated inflammatory responses through up-regulation of Nrf2/HO-1 and involving AMPK pathway. Environ. Toxicol. Pharmacol., 62, 1–10 (2018).
- 23) Nemmar A, Al-Salam S, Beegam S, Yuvaraju P, Hamadi N, Ali BH. In vivo protective effects of nootkatone against particles-induced lung injury caused by diesel exhaust is mediated via the NF-κB pathway. Nutrients, 10, 263 (2018).
- 24) Chen SJ, Huang WC, Shen HJ, Chen RY, Chang H, Ho YS, Tsai PJ, Chuang LT. Investigation of modulatory effect of pinolenic acid (PNA) on inflammatory responses in human THP-1 macrophage-like cell and mouse models. Inflammation, 43, 518–531 (2020).
- 25) Ying X, Yu K, Chen X, Chen H, Hong J, Cheng S, Peng L. Piperine inhibits LPS induced expression of inflammatory mediators in RAW 264.7 cells. Cell. Immunol., 285, 49–54 (2013).
- 26) Gao W, Wang C, Yu L, Sheng T, Wu Z, Wang X, Zhang D, Lin Y, Gong Y. Chlorogenic acid attenuates dextran sodium sulfate-induced ulcerative colitis in mice through MAPK/ERK/JNK pathway. Biomed. Res. Int., 2019, 6769789 (2019).