2022 Volume 45 Issue 2 Pages 184-193
2022 Volume 45 Issue 2 Pages 184-193
Bendimidazole anthelmintics (BAs) have gained interest for their anticancer activity. The anticancer activity is mediated via multiple intracellular changes, which are not consistent under different conditions even in the same cells. We investigated the anticancer activity of fenbendazole (FZ, one of BAs) under two different growth conditions. The growth rate of H4IIE cells was dose-dependently decreased by FZ only in actively growing cells but not in fully confluent quiescent cells. Apoptosis-associated changes were also induced by FZ in actively growing cells. Markers of autophagy were not changed by FZ. The number of cells was markedly increased in sub-G1 phase but decreased in S- and G2/M phases by FZ. FZ up-regulated p21 (an inhibitor of cyclin-CDK) but suppressed the expression of cell cycle-promoting proteins (cyclin D1 and cyclin B1). FZ did not affect integrin αV or n-cadherin expression as well as cell migration. Glycolytic changes (glucose consumption and lactate production) and the generation of reactive oxygen species (ROS) were not affected by FZ. Although the activity of mitogen-activated protein kinases (MAPKs) was altered by FZ, the inhibition of MAPKs did not affect the pro-apoptotic activity of FZ. Taken together, FZ selectively suppressed the growth of cells via p21-mediated cell cycle arrest at G1/S and G2/M, and resulted in apoptosis only in actively growing cells but not in quiescent cells. Glucose metabolism, ROS generation, and MAPKs are unlikely targets of FZ at least in H4IIE rat hepatocellular carcinoma cells used in this study.
Fenbendazole (FZ), one of benzimidazole anthelmintics (BAs), is a veterinary medicine extensively used to control internal parasites. BAs include albendazole, fenbendazole, mebendazole, and many other derivatives. BAs have been used for several decades without adverse effects since their introduction in the 1960 s,1) providing a basis for safety in humans. BAs are potent and selective inhibitors of β-tubulin, and interfere with microtubule formation during mitosis.2,3) Accordingly, BAs have been studied as anticancer agents to disrupt tubulin polymerization for drug repurposing safely and cost-effectively. Conventional chemotherapy and radiation therapy are associated with severe toxicity in normal cells within the human body and often exhibit resistance against these therapies.4) Drug repurposing can be used to identify new uses of conventional, approved drugs to overcome drawbacks and risks as well as decrease the cost of clinical trials. Hepatocellular carcinoma (HCC) is one of the most serious types of cancer. HCC is the third leading cause of cancer-associated death.5) Compared with other types of cancer, fewer effective agents are available for chemotherapy in HCC,6) and recurrent relapse and drug resistance are obstacles to HCC treatment.7) We previously reported a drug repurposing study involving metformin, the first-line antidiabetic drug, which induces oxidative stress-mediated apoptosis in HCC.8)
The anticancer activity of BAs is mediated by multiple factors, such as loss of cell viability, suppression of cancer cell migration and invasion, apoptosis and autophagy, cell-cycle arrest, and impaired glucose metabolism.9) BAs are known to be effective in paclitaxel- and doxorubicin-resistant cancer cells.10) A recent study showed that albendazole (AZ) can be used to overcome tumor multidrug resistance and metastasis.11) Mebendazole augments sensitivity to soranifenib (an oral multi-kinase inhibitor used in the therapy of liver cancer) by targeting mitogen-activated protein kinase (MAPK) and bcl-2 signaling in HCC.12) BAs are mostly non-toxic to normal human cells but highly sensitive to various cancer cells.9) However, some cancer cells are less sensitive to BAs and differences exist in the IC50s of BAs even in the same cell lines.9) In order to elucidate the variable efficacy of BAs as potent anticancer agents, the exact microenvironments should be investigated in drug response studies in vitro. Multiple factors such as the duration of drug treatment, nutritional composition within the culture media, and density of cultured cells play a role in accurate clinical decision-making, but are overlooked occasionally. Anticancer activity of FZ among BAs is unclear. However, FZ is still approved to treat internal parasites only in veterinary animals, but not in humans. Additionally, evidence supporting the anticancer activity of FZ is rare even in in vitro studies. The present study investigated the anticancer activity of FZ and the related biological mechanisms under different culture conditions, especially under different densities of cultured cells. Our results revealed that FZ induces intracellular changes to promote apoptotic cell death via distinct mechanism depending on different stages of cell growth.
H4IIE cells were obtained from the Korean Cell Line Bank (Seoul, Korea) and maintained in Dulbecco’s minimal essential medium (DMEM, 5.5 mM glucose) with 10% fetal bovine serum (FBS). Initially, H4IIE cells were plated on culture dishes at different densities depending on the purpose of each experiment.
MaterialsFBS was obtained from Life Technologies, Inc. (Rockville, MD, U.S.A.). FZ and albendazole (AZ) were purchased from Cayman Chemcals (Ann Arbor, MI, U.S.A.) and other reagents were acquired from Sigma-Aldrich Chemical Corp. (Sigma, St. Louis, MO, U.S.A.) unless otherwise specified. Polyclonal and monoclonal antibodies against β-actin, Bad, beclin-1, bcl-2, (cleaved) caspase-3, histone H3, c-Jun N-terminal kinase (JNK), LC3A, p38 MAPK, phospho-bad, phospho-histone H3, phospho-JNK, phospho-p38 MAPK, and poly ADP ribose polymerase (PARP) were purchased from Cell Signaling Technology (Danvers, MA, U.S.A.). Antibodies against extracellular signal-regulated kinase (ERK), p21, p53, cyclin A, cyclin B1, cyclinD1, integrin αV, n-cadherin, phosph-ERK and horseradish peroxidase-conjugated secondary antibodies were supplied by Santa Cruz Biotech (Santa Cruz, CA, U.S.A.). Electrophoresis reagents were ordered from Invitrogen (Carlsbad, CA, U.S.A.).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) AssayCell viability was analyzed using the MTT assay as previously described.13) Briefly, H4IIE cells were incubated in D-phosphate-buffered saline (PBS) containing MTT (0.5 mg/mL) for 30 min at 37 °C. The cells were washed with D-PBS, and the blue-colored formazan product was subsequently solubilized in 0.5 mL of 2-propanol for 20 min. The absorbance of the converted dye was measured at a wavelength of 570 nm.
Measurement of Glucose Consumption and Lactate ReleaseLactate concentration in the medium was measured using a lactic acid assay kit supplied by Megazyme (Wicklow, Ireland). Glucose concentration in the medium was measured with a glucose assay reagent (Asan Pharm, Whaseong, Korea) based on the glucose oxidase method. The amount of glucose in the culture medium was subtracted from that of DMEM to calculate the glucose consumption.14)
Reactive Oxygen Species (ROS) MeasurementROS generation within cells was measured with 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA).15) H4IIE cells were grown in 24-well culture plates and treated with reagents, followed by the addition of H2DCFDA (10 μM) to each well and further incubation for 30 min. The supernatant was removed from each well. The cell monolayers were dispersed with trypsin–ethylenediaminetetraacetic acid (EDTA) solution. Each aliquot of cell suspension was transferred to a black 96-well plate. The fluorescence intensity of DCF was measured at 485 nm/535 nm to measure the excitation/emission with a multi-well fluorescence reader (Spectrafluor, Tecan, Austria).
Western Blotting AnalysisAfter treatment, the cells were lysed in an ice-cold lysis buffer (50 mM Tris–HCl, 1% nonidet P-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM sodium orthovanadate, 1 mM NaF, 1 mM phenylmethylsulfonyl fluoride, 1 mM aprotinin, 1 mM leupeptin, and 1 mM pepstatin A). Equal amounts (10–20 mg) of protein were separated via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 4–12% polyacrylamide gels and transferred onto polyvinylidene difluoride (PVDF) membranes. The membranes were incubated in blocking buffer (5% nonfat dry milk in Tris-buffered saline (TBS) − 0.1% Tween-20 (TBS-T)) for 1 h at room temperature, followed by probing the membranes with different primary antibodies (at dilutions of 1 : 1000–1 : 2000). After a series of washes, the membranes were further incubated with the respective horseradish peroxidase (HRP)-conjugated secondary antibodies at a dilution of 1 : 10000. The signal was detected via enhanced chemiluminescence (ECL) detection system (Intron, Seongnam, Korea).
H33342 and Acridine Orange StainingThe degree of nuclear chromatin condensation (a marker of apoptosis) was determined using a cell membrane-permeable DNA-specific fluorescent dye (bisBenzimide H33342 trihydrochloride, H33342). Subsequently, the cells were incubated with H33342 (1 mg/mL) for 15 min. The cells were analyzed under a fluorescent microscope (IX70, Olympus, Tokyo, Japan) and imaged using a digital camera (DP-70, Olympus). Acidic intracellular vesicles, a marker of the onset of autophagy, were visualized using acridine orange (AO) staining. After treatment, the cells were washed twice with D-PBS and stained in 2% acetone in D-PBS containing AO (1 μg/mL) for 15 min at room temperature. AO-stained cells were also observed and imaged under the fluorescent microscope.
Cell Migration AssayCell migration assay was performed as previously described.16) Confluent cell monolayer, previously incubated overnight in a serum-free culture medium to induce quiescence, was scrapped by a fine micropipette tip and further incubated in the growth medium containing 10% FBS. Photographs were taken at 0 and 48 h, and the distance between the two edges was measured.
Cell Cycle AnalysisFlow cytometry analyses were performed to measure cell cycle distribution and apoptosis. Cells were plated at a density of 2.5 × 104 cell/cm2 and serum-starved overnight to induce G0/G1 synchronization.17,18) After further incubation in a fresh culture medium containing 10% FBS for 24 h, the detached cells were removed and the adherent cells were obtained via trypsin–EDTA treatment. Detached and adherent cells were mixed and briefly centrifuged. Cell pellets were rinsed with ice-cold PBS and fixed with ice-cold 70% ethanol for 30 min. Cells were stained with a solution (50 μg/mL propidium iodide, 50 μg/mL ribonuclease (RNase) A in PBS for 30 min at room temperature in the dark. Cell cycle analysis was performed using a flow cytometer (LSRFortessa, BD Bioscience, NJ, U.S.A.).
Statistical AnalysisThe experimental results are presented as the mean ± standard error. The significance of the differences among groups was determined using Student’s t-test. p < 0.05 was considered statistically significant.
Although several studies showed the anticancer activity of BAs in vitro, the experimental conditions were not described precisely, such as the density of cultured cells and the nutritional features of the culture media. In fact, the rate of cell proliferation differed significantly depending on the time course from the initial (rapidly growing) to the late (near confluent, stationary) phases after the attachment of seeded cells to the culture plate. Based on this background, we compared the effect of FZ/AZ on the growth rate using actively growing cells and confluent cells. Initially, we measured the number of H4IIE cells on the culture plates. The density of H4IIE cells at confluence was approximately 1 × 105 cells/cm2. Two different cell groups seeded at different densities (2.5 × 104 cells/cm2, 25% confluent and 1 × 105 cells/cm2, fully confluent) were used (Fig. 1A). When cells were seeded at a lower density, FZ/AZ dose-dependently suppressed the cell growth, showing maximal suppression (52.1 and 48.6% of the control by 1.25 μM FZ and AZ, respectively) after 48 h treatment (Fig. 1B). In the same experiments, the levels of protein markers representing the onset of apoptosis (cleaved caspase-3, cleaved PARP, phospho-histone H3, and p53) were elevated by FZ (Fig. 1B). However, FZ and AZ did not suppress the cell growth additively, suggesting that they share common pathway(s) to suppress the growth of H4IIE cells (Fig. 1C). If FZ alone and AZ alone have distinct pathways, FZ plus AZ should suppress cell growth more than FA or AZ alone. Next, confluent cells (1 × 105 cells/cm2) were also treated with FZ/AZ in serum-free culture media or normal growth media (10% FBS) for 48 h. FZ/AZ decreased the number of viable cells only by 10–20% in serum-free media (Fig. 1D) and FZ decreased the number of viable cells by 14.3% in serum-containing media (Fig. 1E). But in 25% confluent cells (2.5 × 104 cells/cm2), FZ decreased the number of viable cells by 44.1% in serum-containing media (Fig. 1F). These results suggest that FZ/AZ effectively suppressed the growth of cells and induced apoptosis only in actively growing cells, but not efficiently in slowly-growing and highly confluent cells.
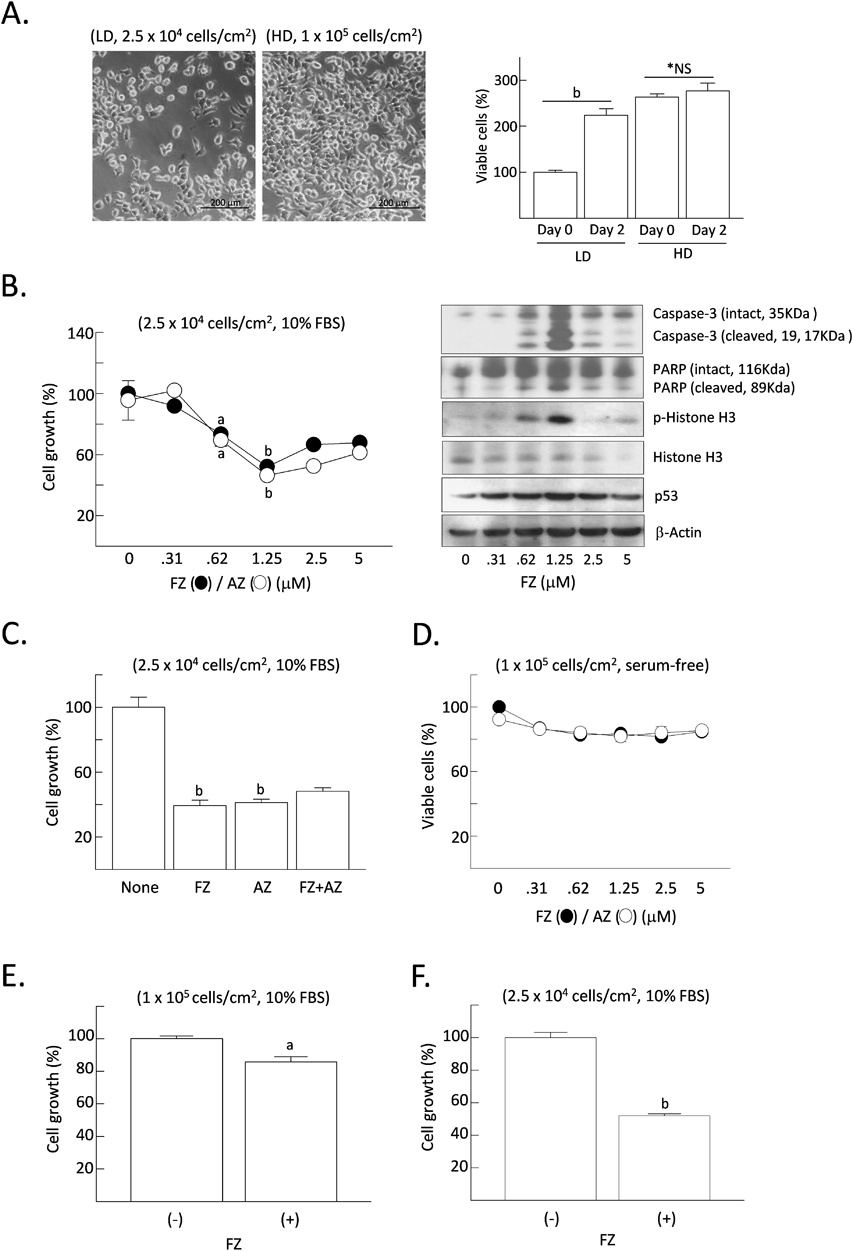
H4IIE cells were plated on culture dishes with low- or high density (2.5 × 104 or 1 × 105 cells/cm2, respectively) and maintained for 12 h for attachment (Day 0). Cells were further grown in culture medium containing 10% FBS with different doses of fenbendazole/albendazole for 48 h (A, B, C, E, F). Otherwise, nearly confluent H4IIE cells (1 × 105 cells/cm2) were incubated in serum-free culture medium with different doses of fenbendazole/albendazole for 48 h (D). The relative rate of cell growth or cell viability was calculated from the results of MTT assay. Results are expressed as the mean ± standard error of the mean (S.E.M.) (n = 4). Statistical analyses were performed using the Student’s t-test. *NS, not significant vs. control (Day 0). ap < 0.05, bp < 0.01 vs. the control (non-treated group). LD, low density; HD, high density; FZ, fenbendazole (1.25 μM); AZ, albendazole (1.25 μM).
Based on the results that FZ/AZ suppresses the growth of actively growing H4IIE cells, we investigated the effect of FZ/AZ on apoptosis and autophagy, two different intracellular pathways determining the fate of cultured cells. Growing cells (2.5 × 104 cells/cm2) were incubated in culture media containing FZ/AZ and 10% FBS for 24 h. They were then analyzed via phase-contrast or fluorescent microscopy to identify the morphological changes associated with apoptosis or autophagy (Fig. 2A). The phase-contrast microscopy revealed a visible increase in the number of detached and nonviable cells and the fluorescent H33342 staining showed an increase in dense and fragmented apoptotic bodies following FZ/AZ treatment. Among cells incubated without FZ/AZ, several mitotic cells were apparent after H33342 staining. However, the formation of autolysosomes, a progressive feature of autophagy, was unchanged by FZ/AZ. Based on these morphological changes after FZ/AZ treatment, specific protein markers of apoptosis (Fig. 2B) or autophagy (Fig. 2C) were identified. In cells actively growing in serum-containing culture media, FZ/AZ elevated the levels of pro-apoptotic protein markers (cleaved caspase-3, cleaved PARP, phospho-bad, phospho-histone H3), whereas the levels of bcl-2, an anti-apoptotic marker declined. Under serum-free conditions, the protein levels were not affected by FZ/AZ. The levels of cleaved caspase-3 were elevated by serum depletion as expected; however, the basal levels were restored by FZ/AZ. Levels of beclin-1 and LC3A, two key indicators of autophagy, were unchanged by FZ/AZ.

Growing cells (2.5 × 104 cells/cm2) were treated with reagents in the culture media with or without 10% FBS for 24 h. Nuclear chromatin condensation was visualized with H33342 and the formation of autolysosomes was detected with acridine orange (A). Protein levels were analyzed by Western blot (B–C, a representative of duplicated experiments). FZ (1.25 μM); AZ (1.25 μM).
The effect of FZ on the progression of cell cycle was analyzed with a flow cytometer. FZ markedly increased the number of cells residing in sub-G1 phase but decreased the cells in G1 and S phases. Although not significant, the number of cells in G2/M phase was slightly decreased (Fig. 3A). Levels of p21, a potent tight-binding inhibitor of CDKs, were elevated, whereas levels of cyclin D1 mediating G1/S progression and cyclin B1, a mitotic promoter, declined following FZ treatment (Fig. 3A). These results suggest that FZ obviously interfered with the G2-to-M phase transition as well as in G1-to-S phase of cell cycle and promoted the entry of cells into subG1, the apoptotic phase. Inhibition of cell migration is one of the characteristics of anticancer drug activity. Thus, we investigated whether FZ affected the migration of H4IIE cells after disrupting the monolayer of confluent cells. When migration width was measured as the distance between two opposite edges of cell monolayer and expressed as 1 unit in the control group, the migration width induced by FZ was negative (−2.2 units), indicating the death of cells adjacent to the scratched edges and detachment from the monolayer. Markers of cell migration (integrin αV and n-cadherin) were not altered by FZ at all. These results suggest that FZ interferes with the progression of cell cycle, thereby promoting apoptosis. However, it does not affect the migration of H4IIE HCC cells used in the experiments.

Growing cells (2.5 × 104 cells/cm2) were serum-starved overnight to induce G0/G1 synchronization, followed by further incubation in a fresh culture medium containing 10% FBS for 24 h. The percentages of cells at different stages of cell cycle were calculated using flow cytometry analysis (A). Fully-grown cells were evaluated using cell migration assay (B). Results are expressed as the mean ± S.E.M. (n = 3) except for Western blots (each figure is a representative of duplicated experiments). Statistical analyses were performed using Student’s t-test. ap < 0.05, bp < 0.01 vs. the control (non-treated group). FZ (1.25 μM).
In general, metabolic alterations such as enhanced glucose uptake and glycolytic activity are observed in cancer cells.19) Although a number of studies seek to develop potential anticancer remedies targeting the dysregulated glucose metabolism in cancer cells, a successful attempt has yet to be reported. A recent study found that FZ effectively interferes with multiple steps in glucose metabolism including glucose uptake, expression of glucose transporters (GLUTs) and hexokinase in human non-small cell lung carcinoma (NSCLC) cells.20) In our experiments, FZ did not affect glucose consumption in actively growing cells or confluent cells (Fig. 4). Excess ROS contribute to apoptotic death in cancer cells although moderate levels of ROS mediate tumor promotion and progression.21) Some derivatives of BAs inhibited tumor growth and promoted apoptosis via ROS-dependent signaling pathways in HCC22) and human colon cancer cells.23) However, we found no increase in ROS by FZ under any growth conditions in H4IIE HCC cells (Fig. 4).

Growing cells (2.5 × 104 cells/cm2) (A) or fully grown cells (1 × 105 cells/cm2) (B) were treated with FZ in 10% FBS-containing media or serum-free media, respectively. Concentrations of glucose/lactate in the culture media, and intracellular ROS content along with cell viability after 24 h of FZ treatment was measured. The cell viability was measured by MTT assay. Results are expressed as mean ± S.E.M. (n = 4). Statistical analyses were performed using the Student’s t-test. ap < 0.05 vs. the control (untreated group). FZ (1.25 μM).
Mitogen-activated protein kinase kinase (MEK)-ERK signaling, one of MAPK signaling cascades, is activated in various cancer cells including HCC.24) Mebendazole, one of BAs, exerts antitumor activity by targeting MAPK and bcl-2 signaling pathways in murine HCC.12) We investigated whether the inhibition of MEK-ERK by FZ is essential to induce cytotoxicity in H4IIE cells. ERK activity was decreased by FZ after treatment for 1 h but was not affected after 24 h in growing cells (Fig. 5A). Although ERK activity was decreased to basal levels by treatment with U0126, the number of viable cells was not decreased by U0126 (Fig. 5B). FZ-induced cytotoxicity was not further increased by the addition of U0126. These results imply that the decreased ERK activity is not responsible for the growth suppression of H4IIE cells. Accordingly, the effect of FZ on the activity of p38MAPK and JNK, two other members of MAPKs, was investigated (Fig. 5C). The activity of p38MAPK and JNK was induced after 24 h treatment with FZ. However, it remained unchanged after 1 h. The inhibition of p38MAPK and JNK did not protect cells from death, suggesting that they do not play a role in FZ-induced cell death. Inhibition of other kinases with various inhibitors, such as H89 against protein kinase A, GF109203x against protein kinase C, KT5823 against protein kinase G, and compound C against AMP-activated protein kinase, together with FZ, did not alter FZ-induced cell death (Fig. 5D).

Growing cells (2.5 × 104 cells/cm2) were treated with FZ and various signaling inhibitors in fresh growth media (10% FBS). Inhibitors of signal transduction were added to the cultured cells 30 min prior to FZ treatment. Levels of phosphorylated MAPKs were measured after treatment for 1 and 24 h. Cell viability was measured after treatment for 48 h. Results are expressed as the mean ± S.E.M. (n = 4) except for western blots (A–C, a representative of duplicated experiments). Statistical analyses were performed using the Student’s t-test. *NS, not significant vs. the control (FZ-alone). FZ (1.25 μM); U, U0126 (20 μM); SP, SP600125 (20 μM); SB, SB202190 (20 μM); H89 (5 mM); PKCi, PKC inhibitor (GF109203×, 500 nM); KT (500 nM); CC, compound C (20 μM).
FZ was one of the BAs approved for use only in animal species, but not in humans.25) FZ, like other BAs, targets microtubules within the cells of parasites. BAs including FZ exhibit microtubule-targeting activity to induce mitotic arrest, followed by apoptotic death of cancer cells. Although numerous microtubule-targeting anticancer drugs have been developed and widely used in the treatment of human cancers, they are limited by serious and painful adverse effects involving both normal as well as cancer cells.26) Because most animals tolerate FZ very well without significant adverse effects, FZ has attracted the interest of researchers developing new anticancer drugs with increased safety and limited toxicity as a cost-effective and timely strategy to overcome the challenges encountered in conventional drug development process. Besides microtubule-targeting activity, FZ exhibits several cytotoxic effects such as proteosomal interference27) and inhibition of glucose metabolism in cancer cells.19) However, whether the cytotoxic effects of FZ occur under different growth conditions and nutritional composition of culture media or the density of growing cells requires investigation. A previous study divided the growth period (4 d) of H4IIE cells into three phases after seeding cells on the culture plates: initial lag phase (1 d), active growth phase (2 d), and late stationary phase (1 d).28) Possible mechanisms associated with the anticancer activity of a compound may include inhibition of cell growth, induction of cell death, or both. In the actively growing phase, FZ inhibited the active growth of H4IIE cells as well as induced changes in pro-apoptotic proteins. However, in the late stationary phase (1 × 105 cells/cm2), the anti-proliferative effect of FZ was not comparable to that of the actively growing phase. Under serum-free conditions, the decrease in the number of surviving cells represents induction of apoptosis but not the inhibition of cell growth because cells cannot maintain mitotic division in a culture environment lacking growth factors in the serum. Under serum-free conditions, FZ did not significantly decrease the number of viable cells, indicating that apoptosis-inducing activity of FZ was highly selective only in cells in active growth phase but not stationary phase. The Western blot results supported the findings. Many proteins involved in the onset of apoptosis (PARP, phospho-bad, bcl-2, phospho-histone H3) were unaffected by FZ under serum-free conditions, whereas they were evidently up- or down-regulated in apoptosis only under serum-containing conditions. The relationship between tumor cell density and the cytotoxic effects of certain anticancer agents is designated as the inoculum effect.29) The precise nature of inoculum effect is not clear. One hypothesis is that the inoculum effect might be a result of the acidification of culture medium due to the high cell density. However, this hypothesis is still disputed because the cytotoxic effect of anticancer drugs is not influenced by the pH of the culture medium.30) Another hypothesis suggests that the content of anticancer agents was insufficient under high cell density. In fact, limited amounts of specific anticancer drugs cannot interact with all cellular binding sites when cells at high densities are exposed to drugs.29) Our results also support such a hypothesis, because FZ is cytotoxic to H4IIE cells only in actively growing cells with lower densities, but less toxic at high cell densities regardless of the growth factors in the serum. The cytotoxic effect of FZ in H4IIE cells might be mediated via multiple events, including the arrest of cell cycle progression, onset of apoptosis or autophagy, interference with cancer cell energy metabolism, and oxidative damage. In H4IIE cells, FZ clearly elicited several cytotoxic responses, such as the generation of apoptotic bodies and the disappearance of mitotic spindle, and the elevation of pro-apoptotic proteins only in actively growing cells with low densities. Thus, the onset of apoptosis is a key mechanism underlying the anticancer activity of FZ. The role of autophagy in tumor promotion or suppression in cancer is still debated. Autophagy protects cells from carcinogenesis but promotes tumor progression in advanced stages of HCC.31) If this hypothesis is correct, FZ should suppress the autophagy-associated changes in H4IIE cells. However, FZ failed to affect autophagy of actively growing H4IIE cells indicating that autophagy is not associated with the cytotoxic activity of FZ.
Most studies suggest that BAs arrest cell cycle at G2/M by increasing the levels of phospho-histone H3, cyclin B1, and p27Kip1 in several cancer cells.9,32–34) Similar to other BAs, FZ also interfered with cell cycle of actively growing H4IIE cells in our study. FZ stimulated the expression of p21 (an inhibitor of cyclin-CDK) but suppressed the expression of cyclin D1 as well as cyclin B1. p21 mediates the induction of S and G2/M cell cycle arrest in human hepatoma cell lines.35) Our results suggest that FZ simultaneously induces both p21/cyclin D-mediated cell cycle arrest at G1/S phase and p21/cyclin B-mediated arrest at G2/M checkpoints concurrently. However, the degree of FZ-mediated cell cycle arrest was significant and more pronounced at G1/S than G2/M transition. To our knowledge, this finding is inconsistent with previous studies suggesting that the FZ-induced cell cycle arrest occurs mainly at G2/M and not at G1/S checkpoint. A marked increase in the number of apoptotic cells belonging to the sub-G1 population appears to be the result of apoptotic transition of cells in G1 and G2 phases for extended duration. The antitumor potential of BAs may include the inhibition of cancer cell migration as reported previously.36,37) Unlikely other BAs, FZ did not reduce the cell migration distance but rather increased the cell dead space in our study. Integrin and cadherin coordinately regulate contact-mediated inhibition of cell migration.38) Thus, the down-regulation of integrin and cadherin releases cells from contact inhibition and thereby induces the migration of cancer cells (metastasis). But FZ did not affect the expression of integrin or cadherin, implying that cancer cell migration was not a major target of FZ in H4IIE cells.
According to the Warburg effect, the rate of glucose consumption and lactate production is dramatically increased in the presence of oxygen and fully functioning mitochondria.19) Accordingly, numerous drug compounds and therapeutic strategies target glucose metabolism in cancer treatment. A recent study reported that FZ effectively inhibited glucose uptake and the expression of GLUT transporters as well as hexokinase, a key glycolytic enzyme in human lung cancer cells.20) That study is based on the “Warburg’s effect,” which hypothesizes that most (not all) cancer cells utilize inefficient aerobic glycolysis to produce energy for survival due to their mitochondrial dysfunctions. However, some results of that study were questionable. First, glucose uptake was clearly suppressed by 1 μM FZ for 4 h. However, lactate production was not suppressed under the same conditions. Second, expression of hexokinase (HK) II was dramatically abolished by treatment with 1 μM FZ for 24 h. However, relative HK activity was still robust (70% or higher compared to the non-treated control). Third, 1 μM FZ failed to reduce HK activity (from in vitro HK assay using purified HK II). Thus, the residual 70% of HK II activity after 24 h treatment with 1 μM FZ was still enough to metabolize glucose. Fourth, that study lacked direct evidence showing that the suppression of glucose turnover could lead to suppression of cell growth. Thus, FZ-induced changes in glucose metabolism might not be a key factor in the growth suppression of lung cancer cells. We investigated whether FZ affected glucose metabolism in H4IIE cells. Neither basal glucose consumption nor lactate release was suppressed by FZ in actively growing cells or fully confluent stationary cells over a 24-h period. Our previous study suggested that ROS generation was stimulated by excessive glucose consumption and lactate release in H4IIE cells.7) Intracellular levels of ROS remained unchanged under any conditions in FZ-treated cells for 24 h. In summary, the pro-apoptotic activity of FZ is not mediated via glucose metabolism or ROS-induced oxidative stress.
BAs inhibit the epidermal growth factor receptor39) and disrupt microtubule polymerization. Although the anticancer activities of BAs are mediated via induction of apoptosis, inhibition of cell cycle progression, anti-angiogenesis, and the blockade of glucose transport and mitochondrial respiration,9) the intracellular signaling pathways and anticancer mechanisms are unknown. A recent study reported that mebendazole, one of BAs, targets MAPK and bcl-2 signaling in murine HCC.12) The MAPK pathway, often recognized as the stepwise activation of RAS/RAF/MEK/ERK signaling proteins, is activated in numerous human cancer cells including HCC.24,40,41) Our data showed that levels of activated (phosphorylated) ERK were marginally decreased after short-term (1 h) treatment of actively growing H4IIE cells with FZ. If ERK activity is responsible for maintaining mitotic proliferation, pharmacological inhibition of ERK plus FZ should additively decrease the number of viable cells. However, the addition of U0126 (ERK inhibitor) plus FZ did not further diminish the number of viable cells treated with FZ alone, whereas ERK activity was additively suppressed by the combination of FZ and ERK inhibitor. These results suggest that the suppression of ERK per se is not sufficient in FZ-induced cytotoxicity of H4IIE cells. Although the Raf-Mek-ERK pathway can promote the proliferation or survival of many cancer cells, other evidences also suggest a cytotoxic effect of ERK. ERK-p53 apoptotic pathway can induce G2/M arrest and apoptosis.42) ROS-mediated p38/ERK signaling is known to induce apoptosis and cell cycle arrest in human HCC.43) Thus, depending on the cell type and stimulus, ERK activity can mediate anti-proliferative events such as apoptosis.44) In our study, the inhibition of other MAPK members (JNK and p38MAPK) did not affect the FZ-induced cytotoxicity. FZ-induced cytotoxicity was not affected by the inhibition of other protein kinases (protein kinase A (PKA), PKC, PKG, AMP-activated protein kinase (AMPK)) in H4IIE cells. Further experimental evidence is needed to identify FZ-induced changes in intracellular signaling pathways.
Taken together, we investigated the action mechanisms underlying FZ-induced cytotoxicity in H4IIE HCC cells in defined culture conditions with different densities and the presence or absence of serum. Our key finding is that the cytotoxicity of FZ occurs exclusively in actively growing cells with low densities. FZ arrests the entry into S and M phases concurrently by up-regulating p21, and down-regulating cyclin D and cyclin B, resulting in apoptosis. These mechanisms do not entail autophagy and cell migration is not a target of FZ. FZ also does not alter glucose metabolism or ROS generation. Although ERK activity is marginally decreased by FZ, it does not play an important role in FZ-induced cytotoxicity of H4IIE cells. Although our experiments exclude a number of candidates, further studies are needed to elucidate the precise mechanism of FZ action, as a potential anticancer agent.
This work was supported by a research Grant from the Jeju National University Hospital Research Fund of Jeju National University in 2020.
The author declares no conflict of interest.