Abstract
Oxaliplatin (OXA) is used in chemotherapy for various cancer types and is associated with acute and chronic neurotoxicity. However, a preventive strategy for OXA-induced peripheral neuropathy (OIPN) and its underlying mechanism remain unclear. We examined the effects of renin-angiotensin-aldosterone system inhibitors (RAASIs) on OIPN by performing a retrospective multicenter study and an in vitro assay. We retrospectively evaluated electronic medical records of 976 patients who underwent one or more courses of OXA-containing regimens at Ehime, Okayama, and Tokushima University Hospitals. The primary endpoint was the incidence of OIPN during or after OXA administration. The effects of RAASIs and OXA on the neurite length in PC12 cells were determined. The combined administration of an OXA-containing regimen and RAASI significantly inhibited the cumulative incidence grade-2 or higher OIPN (log-rank test; p = 0.0001). RAASIs markedly suppressed the development of both acute and chronic OIPN (multivariate analysis; p = 0.017 and p = 0.011). In an in vitro assay, 10 µM OXA suppressed the neurite length; treatment with 1 µM aliskiren, spironolactone, 10 µM candesartan, and enalapril significantly restored neurite length to the control level. Moreover, 1 µM SCH772984 (a selective inhibitor of extracellular signal-regulated kinase, ERK1/2) and 500 µM SQ22536 (a cell-permeable adenylate cyclase (AC) inhibitor) markedly abolished neurite-extending effects of candesartan and enalapril. These results indicate that RAASIs possess preventive or therapeutic effects in acute and chronic OIPN, candesartan and enalapril may increase in the activity of ERK1/2 and AC in PC12 cells.
INTRODUCTION
Oxaliplatin (OXA) is used in the standard treatment of colorectal, gastric, and pancreatic cancers, either in adjuvant settings or metastatic disease.1–3) OXA-induced peripheral neuropathy (OIPN) presents as acute cold-aggravated neurotoxicity and dose-dependent cumulative neuronal degeneration, characterized by transient and rapid onset neurological symptoms, as well as sensory ataxia, in most patients treated with 750–850 mg/m2.4) Although OIPN gradually recovers after treatment completion, it takes several months to years for complete recovery to be achieved, causing a marked deterioration in the patient’s QOL.5) Several clinical trials have reported that pregabalin and duloxetine are effective for treating OIPN.6,7) In contrast, the inhibitory effects of goshajinkigan, a Japanese traditional medicine, vitamin E, calcium gluconate/magnesium sulfate, pregabalin, and antihyperalgesic drugs on chemotherapy-induced peripheral neuropathy (CIPN) are insufficient.8–13) Accordingly, an effective and preventive strategy for CIPN or OIPN needs to be established.7,11)
Previous reports have demonstrated that OXA and oxalate (OXA metabolite) damage sensory nerve cell bodies of the dorsal root ganglion, inducing peripheral neuropathy.14) Hobara et al. have revealed that selective stimulation of angiotensin II (Ang II) type 2 receptor (AT2R) with Ang II facilitates reinnervation of mesenteric perivascular calcitonin gene-related peptide (CGRP)-containing nerves, the primary sensory nerve, injured by topical application of phenol in rats.15) Furthermore, our previous study demonstrated that long-term administration of temocapril (an angiotensin-converting enzyme inhibitor, ACEI) or losartan (an Ang II receptor blocker, ARB) markedly ameliorated the age-related decrease in CGRP nerve density observed in spontaneous hypertensive rats. These reports strongly suggest that ACEIs and ARBs are highly likely to inhibit OIPN via long-term suppression of renin-angiotensin system (RAS), AT1R and/or a selective stimulation of AT2R. Indeed, our previous single-center study has shown that RAS inhibitors (RASIs) confer protection against the onset of OIPN.16) However, our sample size was limited, and our study was restricted to RASIs without aldosterone and renin antagonists. Moreover, the preventive effects of renin-angiotensin-aldosterone system inhibitors (RAASIs) on acute and chronic OIPN separately and its underlying mechanism remain unknown. Therefore, in the present study, we examined whether RAASIs have the preventive effects on acute and chronic OIPN in a multicenter investigation and evaluated the neurite-extending mechanism of RAASIs in vitro using rat adrenal pheochromocytoma PC12 cells, a commonly used neuronal cell model.
MATERIALS AND METHODS
Study ParticipantsWe retrospectively evaluated electronic medical records of patients (aged ≥20 years) who underwent one or more courses of an OXA-containing regimen at Ehime, Okayama, and Tokushima University Hospitals between May 2009 and December 2016. All patient records were identified and analyzed. We extracted the necessary clinical information regarding physical and hematological examinations, grade evaluation of OIPN, medical and pharmacotherapy history with treatment cycles or dosage of OXA, the presence or absence of dose reduction, interruption or molecular targeted drugs, regimen type, regular use of combined drugs with RAASIs, calcium channel blockers, use of supportive drugs such as goshajinkigan, vitamin B12, pregabalin, gabapentin, an extract from inflammatory rabbit skin inoculated with vaccinia virus, antiepileptic drugs, antidepressant drugs, duloxetine, mexiletine, opioids, non-steroidal anti-inflammatory drugs, and acetaminophen. Patients meeting any of the criteria shown in Supplementary Fig. S1 were excluded from this study.
Definition of Acute and Chronic OIPNIn the present study, OIPN included paresthesias, dysesthesias, cold hypersensitivity, spontaneously numbness and pain around the limbs and lips, distal amyotrophy and muscle weakness, and decreased tendon reflex. We defined acute OIPN as a condition characterized by onset from immediately after OXA treatment to the second day and after administering a cumulative dose of OXA less than 540 mg/m2. We also considered chronic OIPN to involve a cumulative OXA dosage exceeding 540 mg/m2.17)
EndpointThe primary endpoint was the incidence of severe peripheral neuropathy during or at any time after OXA administration.18) OIPN was defined as grade 2 or higher according to the Common Terminology Criteria for Adverse Events, version 5.0.
Cell Culture and Drug TreatmentPC12 cell line was obtained from the RIKEN BioResource Center (Ibaraki, Japan). Undifferentiated cells were cultured in RPMI 1640 medium (Nacalai Tesque Inc., Kyoto, Japan) supplemented with 10% horse serum (Life Technologies NZ Ltd., Auckland, New Zealand), 5% fetal bovine serum (Life Technologies Corporation, Grand Island, NY, U.S.A.), and 1% penicillin-streptomycin (Nacalai Tesque), under 95% air and 5% CO2 at 37 °C. PC12 cells (1 × 103 cells/well) were seeded in 96-well plates and cultured for 24 h; then, the serum-free medium was replaced, supplemented with 100 ng/mL pro-NGF (nerve growth factor; Alomone Labs, Jerusalem, Israel). After 48 h of pro-NGF treatment, differentiated cells exhibiting an increased neurite outgrowth from cell bodies were cultured in serum-free medium containing 0.1, 1, and 10 µM OXA (Wako Pure Chemical Corporation, Osaka, Japan), 1 and 10 µM RAASIs with candesartan cilexetil, enalapril maleate, aliskiren hemifumarate (Wako Pure Chemical Corporation), and spironolactone (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan), 10 and 100 µM duloxetine hydrochloride (Tokyo Chemical), 1 µM SCH 772984 (Cayman Chemical Company, Ann Arbor, MI, U.S.A.), or 500 µM SQ 22536 (Tokyo Chemical) for 2 d. Changes in the length of neurites were obtained with a phase-contrast microscope (CKX41, Olympus Corporation, Tokyo, Japan) equipped with a digital camera (Olympus E330-ADU12X) and expressed as the ratio to that of the control group.
All drugs, except candesartan cilexetil, spironolactone, and SCH 772984, were dissolved in RPMI 1640 medium. Candesartan cilexetil, spironolactone, and SCH 772984 were dissolved in 10 mM dimethyl sulfoxide (DMSO) and diluted with RPMI 1640 medium to a final concentration.
Statistical AnalysisCox proportional-hazards regression analysis was performed to evaluate risk factors associated with the cumulative dosage of OXA and the onset of acute or chronic OIPN. The cumulative dosage of OXA and the onset of acute or chronic OIPN were estimated using the Kaplan–Meier method and analyzed by the log-rank test. Categorical variables were expressed as numbers and compared using the χ2 test or Fisher’s exact test between RAASIs and non-RAASIs groups.
All in vitro assay data are expressed as the mean ± standard error of the mean (S.E.M.) of at least three observations. All statistical analyses were performed using one-way ANOVA followed by Student’s t-test, Tukey’s test, or Dunnett’s test to determine significance. Statistical significance was set at p < 0.05. Statistical analyses were performed using JMP 14.2 (SAS Institute Inc., NC, U.S.A.).
Ethical ConsiderationThe study protocol was approved by the ethics committee of Matsuyama University, Ehime, Okayama, and Tokushima University Hospitals (Approval Nos. 21005, 1806003, 1910-007, and 3275, respectively). The study was conducted in accordance with the principles of the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects by the Ministry of Education, Culture, Sports, Science and Technology, and the Ministry of Health, Labour, and Welfare of Japan. Japanese law does not require individual informed consent from participants in a non-invasive observational trial, as in the present study. Therefore, we used our official website as an opt-out method rather than acquiring written or oral informed consent.
RESULTS
Basic Characteristics of the PatientsThis study included 641 patients treated with an OXA-containing regimen at three hospitals; the breakdown of 335 patients excluded from the study is shown in Supplementary Fig. S1. As shown in Supplementary Table S1, baseline patient characteristics in RAASIs and non-RAASIs groups have multiple biases, which include age (p < 0.001), sex (p < 0.01), body mass index (BMI; p < 0.001), estimated glomerular filtration rate (eGFR; p < 0.001), concomitant diabetes mellitus (p < 0.001), and concomitant use of calcium channel blockers (p < 0.001).
OIPNBased on the univariate Cox proportional-hazards model analysis, we observed that the reduced risk of OIPN was undoubtedly related to the combined administration of RAASIs (crude hazards ratio (HR): 0.50, 95% confidence interval (CI): 0.34–0.71, p < 0.0001), calcium channel blockers (crude HR: 0.57, 95% CI: 0.40–0.79, p = 0.0005), and concomitant diabetes mellitus (crude HR: 0.53, 95% CI: 0.36–0.76, p = 0.0003) (Table 1). In the multivariate Cox proportional-hazards model analysis, the combination of RAASIs and diabetes mellitus significantly suppressed the onset of OIPN (adjusted HR: 0.64, 95% CI: 0.42–0.95, p = 0.027 and adjusted HR: 0.61, 95% CI: 0.41–0.89, p = 0.008, respectively) (Table 1). As shown in Fig. 1, the combination of an OXA-containing regimen and RAASIs markedly inhibited the cumulative incidence of grade 2 or higher OIPN (log-rank test; p = 0.0001).
Table 1. Cox Proportional-Hazards Model Analysis
| | n | Event | Censored | Univariate analysis | Multivariate analysis |
|---|
| Crude HR (95% CI) | Adjusted HR (95% CI) |
|---|
| RAASIs, n | Yes | 131 | 32 | 99 | 0.50 (0.34–0.71) | 0.64 (0.42–0.95) |
| No | 510 | 245 | 265 |
| Age (year) | 0.81 (0.43–1.57) | 1.13 (0.58–2.26) |
| Sex, n | Female | 264 | 121 | 143 | 1.12 (0.88–1.42) | 1.04 (0.81–1.32) |
| Male | 377 | 156 | 221 |
| BMI (kg/m2) | 0.54 (0.15–1.86) | |
| eGFR (mL/min/1.73 m2) | 0.75 (0.27–2.06) | |
| Diabetes mellitus, n | Yes | 108 | 32 | 76 | 0.53 (0.36–0.76) | 0.61 (0.41–0.89) |
| No | 533 | 245 | 288 |
| Supportive drugs, n | Yes | 133 | 78 | 55 | 1.54 (1.18–1.99) | 1.50 (1.14–1.96) |
| No | 508 | 199 | 309 |
| Calcium channel blockers, n | Yes | 133 | 40 | 93 | 0.57 (0.40–0.79) | 0.76 (0.52–1.08) |
| No | 508 | 237 | 271 |
| Analgesic drugs/analgesic adjuvant, n | Yes | 240 | 11 | 128 | 1.17 (0.92–1.49) | |
| No | 401 | 165 | 236 |
Abbreviations: BMI, body mass index; CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio; RAASIs, renin-angiotensin-aldosterone system inhibitors.
Among 641 patients, 489 were assessed for acute OIPN treated with OXA (<540 mg/m2). The basic characteristics of patients with acute OIPN differed significantly in terms of age (p < 0.001), sex (p < 0.05), BMI (p < 0.001), eGFR (p < 0.001), concomitant diabetes mellitus (p < 0.001), and co-administration of calcium channel blockers (p < 0.001) between RAASIs (n = 101) and non-RAASIs group (n = 388) (Supplementary Table S2). RAASIs used in the patients with acute OIPN included olmesartan (median dose [interquartile range]: 20 mg [20–20], number of prescriptions: n = 18), valsartan (80 mg [80–80], n = 16), spironolactone (25 mg [25–25], n = 16), candesartan (8 mg [4–8], n = 15), losartan (50 mg [43.8–50], n = 10), azilsartan (20 mg [20–20], n = 10), telmisartan (40 mg [25–40], n = 9), enalapril (5 mg [4.4–5], n = 5), imidapril (5 mg [5–5], n = 4), and canrenoate (100 mg [100–100], n = 1). Patients with combined use of doble (losartan and spironolactone, n = 1) or triple (candesartan, enalapril and spironolactone, n = 1) drug are included.
The univariate analysis revealed a significant relationship between reduced OIPN and the regular usage of RAASIs (crude HR: 0.45, 95% CI: 0.29–0.67, p < 0.0001), calcium channel blockers (crude HR: 0.54, 95% CI: 0.36–0.79, p = 0.001), and concomitant diabetes mellitus (crude HR: 0.57, 95% CI: 0.36–0.86, p = 0.007) (Table 2). Additionally, the multivariate analysis clarified that the combination of RAASIs and concomitant diabetes mellitus markedly suppressed the onset of acute OIPN (adjusted HR: 0.59, 95% CI: 0.36–0.91, p = 0.017 and adjusted HR: 0.64, 95% CI: 0.40–0.99, p = 0.044, respectively) (Table 2).
Table 2. Cox Proportional-Hazards Model Analysis: Acute OIPN
| | n | Event | Censored | Univariate analysis | Multivariate analysis |
|---|
| Crude HR (95% CI) | Adjusted HR (95% CI) |
|---|
| RAASIs, n | Yes | 101 | 26 | 75 | 0.45 (0.29–0.67) | 0.59 (0.36–0.91) |
| No | 388 | 190 | 198 |
| Age (year) | 0.51 (0.25–1.03) | 0.80 (0.39–1.68) |
| Sex, n | Female | 201 | 98 | 103 | 1.23 (0.94–1.60) | 1.15 (0.88–1.52) |
| Male | 288 | 118 | 170 |
| BMI (kg/m2) | 0.44 (0.17–1.10) | |
| eGFR (mL/min/1.73 m2) | 1.74 (0.57–5.17) | |
| Diabetes mellitus, n | Yes | 74 | 23 | 51 | 0.57 (0.36–0.86) | 0.64 (0.40–0.99) |
| No | 415 | 193 | 222 |
| Supportive drugs, n | Yes | 101 | 64 | 37 | 1.69 (1.25–2.25) | 1.76 (1.30–2.35) |
| No | 388 | 152 | 236 |
| Calcium channel blockers, n | Yes | 94 | 29 | 65 | 0.54 (0.36–0.79) | 0.71 (0.46–1.08) |
| No | 395 | 187 | 208 |
| Analgesic drugs/analgesic adjuvant, n | Yes | 190 | 91 | 99 | 1.14 (0.86–1.49) | |
| No | 299 | 125 | 174 |
BMI, body mass index; CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio; RAAS, renin-angiotensin-aldosterone system inhibitors; OIPN, oxaliplatin-induced peripheral neuropathy.
Among 641 patients, the development of chronic OIPN induced by OXA treatment (≥540 mg/m2) was assessed in 152 patients. As shown in Supplementary Table S3, basic patient characteristics, including age (p < 0.05), concomitant diabetes mellitus (p < 0.001), and concomitant use of calcium channel blockers (p < 0.001), differed significantly between the RAASIs (n = 30) and non-RAASIs groups (n = 122). RAASIs used in the patients with chronic OIPN included olmesartan (median dose [interquartile range]: 20 mg [20–20], number of prescriptions: n = 8), valsartan (40 mg [40–80], n = 6), telmisartan (40 mg [30–40], n = 4), irbesartan (100 mg [100–100], n = 3), enalapril (5 mg [5–5], n = 3), spironolactone (not detected, n = 3), candesartan (2 mg [2–2], n = 2), losartan (not detected, n = 1), azilsartan (20 mg [20–20], n = 1). Patients with combined use of doble drug (valsartan and spironolactone, n = 1) are included. The co-administration of RAASIs and OXA significantly suppressed chronic OIPN in both univariate and multivariate analyses (crude HR: 0.44, 95% CI: 0.17–0.94, p = 0.033 and adjusted HR: 0.37, 95% CI: 0.14–0.81, p = 0.011), as shown in Table 3.
Table 3. Cox Proportional-Hazards Model Analysis: Chronic OIPN
| | n | Event | Censored | Univariate analysis | Multivariate analysis |
|---|
| Crude HR (95% CI) | Adjusted HR (95% CI) |
|---|
| RAASIs, n | Yes | 30 | 6 | 24 | 0.44 (0.17–0.94) | 0.37 (0.14–0.81) |
| No | 122 | 55 | 67 |
| Age (year) | 2.04 (0.53–8.62) | 3.54 (0.86–6.02) |
| Sex, n | Female | 63 | 2 | 40 | 0.83 (0.49–1.39) | 0.73 (0.42–1.23) |
| Male | 89 | 38 | 51 |
| BMI (kg/m2) | 7.31 (0.83–41.97) | |
| eGFR (mL/min/1.73 m2) | 0.58 (0.08–3.92) | |
| Diabetes mellitus, n | Yes | 34 | 9 | 25 | 0.59 (0.27–1.13) | |
| No | 118 | 52 | 66 |
| Supportive drugs, n | Yes | 32 | 14 | 18 | 1.19 (0.63–2.12) | |
| No | 120 | 47 | 73 |
| Calcium channel blockers, n | Yes | 39 | 11 | 28 | 0.77 (0.38–1.43) | |
| No | 113 | 50 | 63 |
| Analgesic drugs/analgesic adjuvant, n | Yes | 50 | 21 | 29 | 0.86 (0.50–1.44) | |
| No | 102 | 40 | 62 |
Abbreviations: BMI, body mass index; CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio; RAAS, renin-angiotensin-aldosterone system inhibitors; OIPN, oxaliplatin-induced peripheral neuropathy.
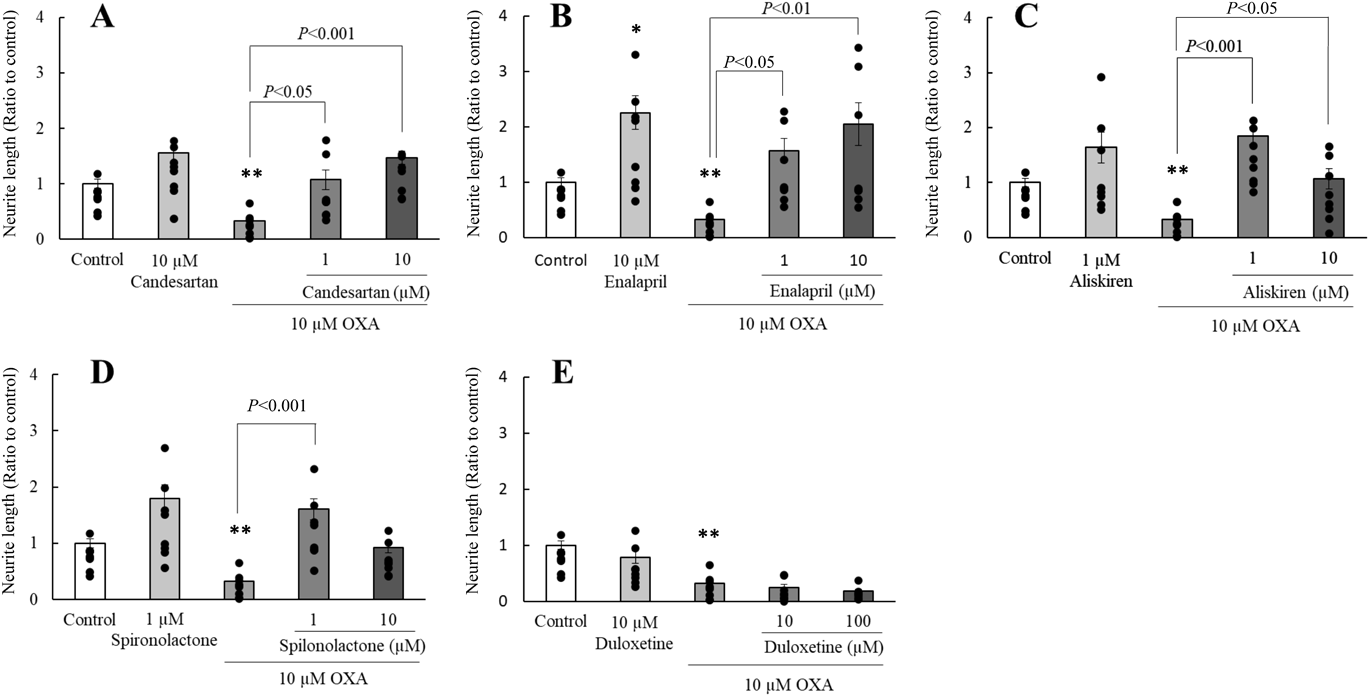
In differentiated PC12 cells, treatment with OXA (0.1–10 µM) dose-dependently decreased the elongation of neurite outgrowth, with significant differences (p < 0.01) observed in the 10 µM OXA-treated group when compared with that in the control group. Candesartan and enalapril at 1 and 10 µM and 10 µM enalapril markedly ameliorated the neurodegeneration induced by 10 µM OXA (Figs. 2A, B). In addition, 1 and 10 µM aliskiren and 1 µM spironolactone significantly improved the reduced neurite outgrowth induced by 10 µM OXA (Figs. 2C, D); moreover, no dose-dependent relationship was observed. However, 10 and 100 µM duloxetine did not impact the OXA-induced decrease in neurite outgrowth (Fig. 2E).
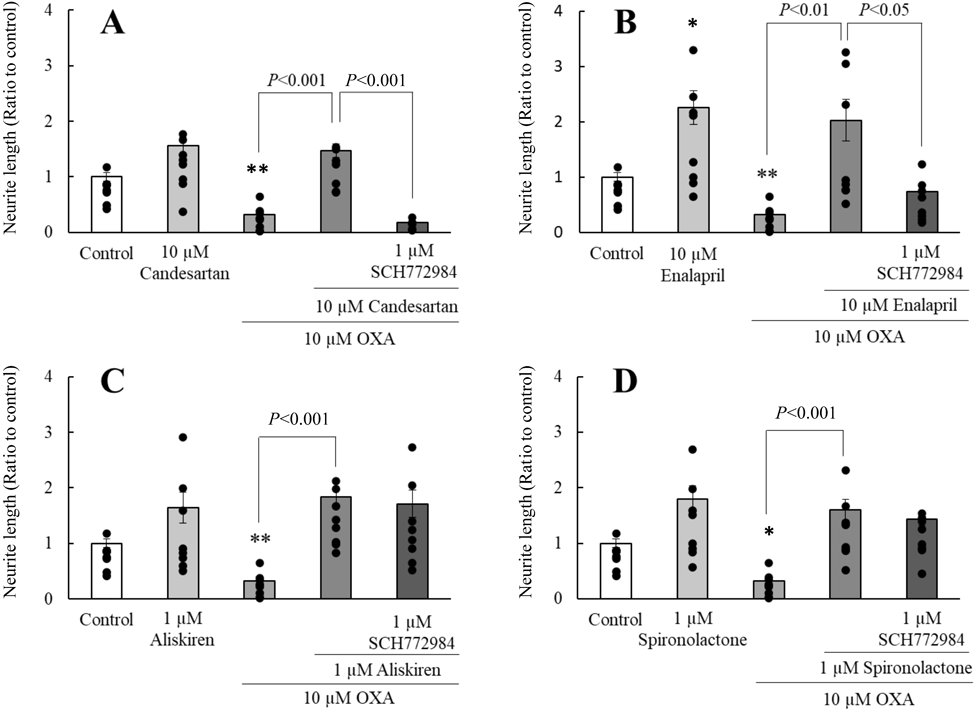
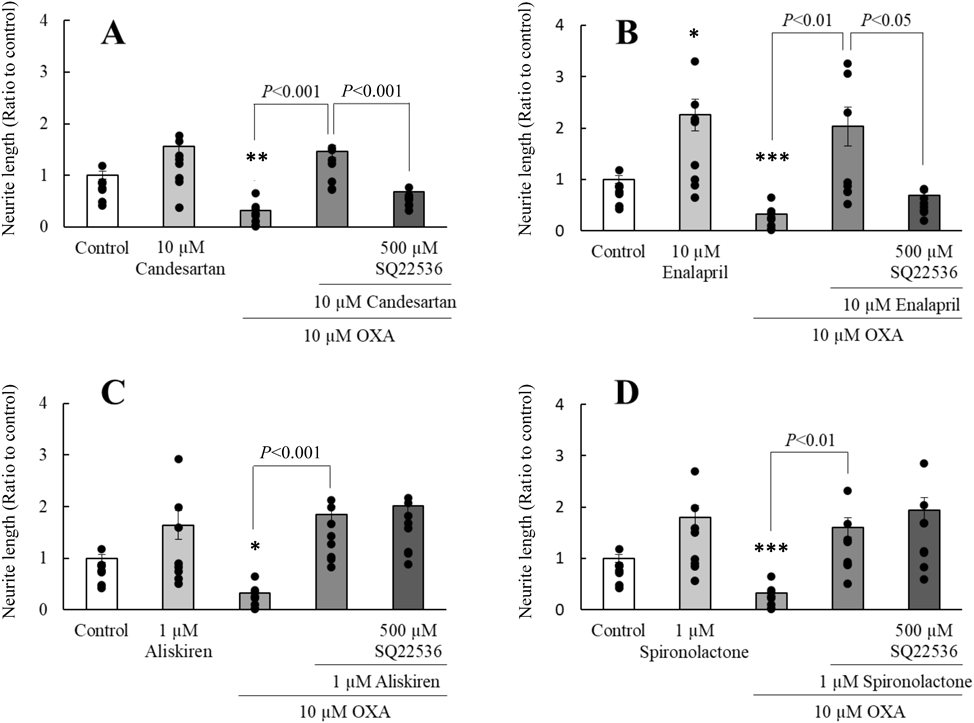
Effect of ERK1/2 or AC Inhibitor on RAASIs-Induced Neurite-extending EffectsNotably, 1 µM SCH 772984, a selective ERK1/2 inhibitor, markedly inhibited neurite-extending effects of candesartan and enalapril on neurite outgrowth in PC12 cells to the levels observed in the OXA-treated group (Figs. 3A, B). In addition, the neurite-extending effects of candesartan and enalapril were significantly inhibited following treatment with 500 µM SQ 22536, an AC inhibitor (Figs. 4A, B). However, both SCH 772984 and SQ 22536 did not alter the neurite-extending effects of aliskiren and spironolactone (Figs. 3C, D, 4C, D).
DISCUSSION
The present study revealed that combined treatment with an OXA-containing regimen and RAASIs significantly prevented the development of both acute and chronic OIPN, as assessed in a retrospective multicenter study. Moreover, we determined for the first time that the mechanism of neurite-extending effects afforded by RAASIs in PC12 cells. Acute neurotoxicity following OXA therapy reportedly occurs in 85–95% of patients and consists of cold intolerance and throat discomfort and largely abates after a few days.19,20) On the other hand, chronic OIPN is generally more problematic and dose-limiting,19) its development usually occurs when the cumulative dosage exceeds 540 mg/m2.17,20) It has been reported that an OXA dosage of 1170 mg/m2 or higher can result in grade-3 OIPN in 50% of patients.21) Pachman et al. have reported that the severity of acute OIPN is associated with that of chronic symptoms.22) Recent clinical studies have reported that goshajinkigan, a traditional Japanese herbal medicine, and the antidepressant duloxetine may improve OIPN.7,23) However, breakthrough therapy and prevention of especially acute OIPN remain elusive.16)
In the present study, we defined a boundary concentration of 540 mg/m2 between acute and chronic OIPN.17,20) Moreover, we demonstrated that OXA induced cumulative dose-dependent and marked grade-2 or higher OIPN, resulting in occurrence rates of 76 and 24% for acute and chronic OIPN, respectively, thus revealing novel evidence that RAASIs markedly suppressed the occurrence of acute OIPN from a relatively early stage of OXA therapy and chronic OIPN. In our previous study, however, we did not examine the preventive effects of RAASIs on acute and chronic OIPN separately.16)
OIPN is frequently associated with discontinuation of chemotherapy, as well as a change or dosage reduction of anticancer drugs, posing a serious challenge to clinicians, interfering with cancer treatment, and resulting in a reduced patient QOL.24) Previous reports have indicated that risk factors for developing acute and chronic OIPN include cumulative dose, young age, low body weight, body surface area >2.0, persistent neuropathy in the past cycle, and surgery.25,26) The present multivariate analysis for acute OIPN reveals that regular administration of RAASIs and diabetic complications markedly suppressed OIPN, indicating a strong interaction between diabetes mellitus and the RAASIs group, as RASIs,16) except for aldosterone and renin antagonists, are the first-line antihypertensive drugs for hypertensive patients with diabetes mellitus. However, diabetic neuropathy and diabetes may have less relevance to the present results because the neuropathy caused by diabetes mellitus is excluded in this study, and there is no correlation between incidence or duration of OIPN and diabetes.16,27) Moreover, a prescription rate (43.2%) of RAASIs in diabetics is higher than that of non-diabetics (16.6%), which may be the cause of decreasing acute OIPN. The present study also demonstrated another novelty that the co-administration of RAASIs and OXA significantly also suppressed chronic OIPN in both univariate and multivariate analyses. We investigated various types and usage rates of RAASIs, including olmesartan (19.3%), valsartan (16.3%), spironolactone (14.1%), candesartan (12.6%), telmisartan (9.6%), azilsartan (8.1%), losartan (8.1%), enalapril (5.9%), imidapril (3.0%), irbesartan (2.2%), and canrenoate (0.7%). Most RAASIs have ARBs and ACEIs, as in our previous investigation16); however, the present study is the first report to reveal the involvement of aldosterone antagonists with spironolactone and canrenoate.
To investigate the inhibitory effects and mechanism of RAASIs with high-frequency usage, as shown above, we examined PC12 cells, which have been widely used in both neurobiological and neurotoxicological studies as a model of neuronal differentiation.28,29) Treatment with OXA dose-dependently reduced neurite outgrowth in differentiated PC12 cells; however, RAASIs, including candesartan, enalapril, aliskiren, and spironolactone, significantly improved the decreased neurite outgrowth following OXA treatment. Therefore, it is strongly suggested that these medications may afford protection against OXA-induced neuronal damage. In the present vitro assay, non-protein binding OXA may induce neuronal damage, because we used serum-free medium to examine neuroprotective effects of RAASIs on OXA-induced neuronal damage. Although OXA has a high plasma protein binding rate, this study revealed OXA significantly induced neuronal damage in PC12 cells, suggesting that there is no effect of protein binding OXA under this condition. Moreover, the concentration (0.1–10 µM) of OXA we assessed in vitro assay is a similar level of patient plasma concentration.30)
It has been reported that OXA-induced neurotoxicity in PC12 cells may be associated with oxidative stress and reduced intracellular calcium concentrations.28,29) Scuteri et al. have reported that prolonged exposure to OXA inhibits ERK1/2 and c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) activity in dorsal root ganglia (DRG) neurons, which mediate neuronal apoptosis, and that high dose NGF (100 µM) increases neuronal survival by restoring the phosphorylation of ERK1/2 and JNK/SAPK.31) Our present results are in accordance with this observation; however, this is the first report to document that the neurite-extending mechanisms of candesartan and enalapril mediate an increased activation of ERK1/2 and AC in PC12 cells with a significant decrease in neurite outgrowth caused by OXA. In rodents, ARBs ameliorated stress-induced disorders, anxiety, and depression, decreased brain inflammation, amyloid-β neurotoxicity, and traumatic brain injury.32) Direct anti-inflammatory and protective effects of ARBs, as demonstrated in cultured microglia, cerebrovascular endothelial cells, neurons, and human circulating monocytes, could be attributed to AT1 receptor (AT1R) blockade, as well as activation of peroxisome-proliferator-activated receptor γ.32) Our previous research has demonstrated that the treatment of type 2 diabetic rats with candesartan significantly ameliorates the decrease in neurite elongation via AT2R in DRG cells.33) Endogenous AT2R, but not AT1R, is predominantly expressed in PC12 cells.34) AT1R also activates ERK1/2 in rat vascular smooth muscle cells and AT1R-transfected PC12 cells.35) Therefore, in the present study, the neurite-extending effects of candesartan and enalapril may be not involved in ATR stimulation. Nostramo et al. confirmed the presence of (pro)renin receptor protein in PC12 cells, which has been reported to be localized primarily in the Golgi and endoplasmic reticulum in NGF-induced PC12 cells.36) However, there has been no definitive role of RAS system to control the neurite outgrowth in PC12 cells.
On the other hand, the neurite-extending effects of aliskiren and spironolactone is not involved in the increased activation of ERK1/2 and AC. Aliskiren reportedly confers protection against the development and progression of diabetic neuropathy through antioxidant effects, including enhanced superoxide dismutase and glutathione peroxidase activity.37) To our knowledge, the neurite-extending effects of spironolactone on OXA-induced neuronal damage in PC12 cells has not been elucidated yet. Hence, it is necessary to examine the neurite-extending mechanisms of aliskiren and spironolactone, and whether RAS system changes caused by the treatment with RAASIs are involved in the improvement of OXA-induced neurotoxicity under the same conditions.
In conclusion, our findings indicate that RAASIs have preventive effects on both acute and chronic OIPN and can strongly facilitate the development of drug repositioning research and new drugs for OIPN.
Acknowledgments
We would like to thank the Nagai Memorial Research Scholarship from the Pharmaceutical Society of Japan (Uchida M).
Author Contributions
MU and ST wrote the manuscript. MU, ST, H. Kawazoe, and ST, H. Kawasaki designed the observational study and in vitro assay, respectively. MU, SU, TN, HN, AT, HA, YZ, KI, YK, TS, MT and MU, ST, KT, HN, KS contributed to data collection and interpretation, analyzing the data, respectively. All authors have reviewed and approved the final version of the manuscript.
Conflict of Interest
The authors declare no conflict of interest.
Supplementary Materials
This article contains supplementary materials.
REFERENCES
- 1) Cassidy J, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzén F, Saltz L. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J. Clin. Oncol., 26, 2006–2012 (2008).
- 2) Yamada Y, Higuchi K, Nishikawa K, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann. Oncol., 26, 141–148 (2015).
- 3) Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med., 364, 1817–1825 (2011).
- 4) Bennett BK, Park SB, Lin CS, Friedlander ML, Kiernan MC, Goldstein D. Impact of oxaliplatin-induced neuropathy: a patient perspective. Support. Care Cancer, 20, 2959–2967 (2012).
- 5) André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J, Tabah-Fisch I, de Gramont A. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N. Engl. J. Med., 350, 2343–2351 (2004).
- 6) Saif MW, Syrigos K, Kaley K, Isufi I. Role of pregabalin in treatment of oxaliplatin-induced sensory neuropathy. Anticancer Res., 30, 2927–2933 (2010).
- 7) Smith EML, Pang H, Cirrincione C, Fleishman S, Paskett ED, Ahles T, Bressler LR, Fadul CE, Knox C, Le-Lindqwister N, Gilman PB, Shapiro CL, Alliance for Clinical Trials in Oncology. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA, 309, 1359–1367 (2013).
- 8) Oki E, Emi Y, Kojima H, Higashijima J, Kato T, Miyake Y, Kon M, Ogata Y, Takahashi K, Ishida H, Saeki H, Sakaguchi Y, Yamanaka T, Kono T, Tomita N, Baba H, Shirabe K, Kakeji Y, Maehara Y. Preventive effect of Goshajinkigan on peripheral neurotoxicity of FOLFOX therapy (GENIUS trial): a placebo-controlled, double-blind, randomized phase III study. Int. J. Clin. Oncol., 20, 767–775 (2015).
- 9) Schloss JM, Colosimo M, Airey C, Masci P, Linnane AW, Vitetta L. A randomised, placebo-controlled trial assessing the efficacy of an oral B group vitamin in preventing the development of chemotherapy-induced peripheral neuropathy (CIPN). Support. Care Cancer, 25, 195–204 (2017).
- 10) Loprinzi CL, Qin R, Dakhil SR, Fehrenbacher L, Flynn KA, Atherton P, Seisler D, Qamar R, Lewis GC, Grothey A. Phase III randomized, placebo-controlled, double-blind study of intravenous calcium and magnesium to prevent oxaliplatin-induced sensory neurotoxicity (N08CB/Alliance). J. Clin. Oncol., 32, 997–1005 (2014).
- 11) de Andrade DC, Jacobsen Teixeira M, Galhardoni R, et al. Pregabalin for the prevention of oxaliplatin-induced painful neuropathy: a randomized, double-blind trial. Oncologist, 22, 1154–e105 (2017).
- 12) Shinde SS, Seisler D, Soori G, Atherton PJ, Pachman DR, Lafky J, Ruddy KJ, Loprinzi CL. Can pregabalin prevent paclitaxel-associated neuropathy? An ACCRU pilot trial. Support. Care Cancer, 24, 547–553 (2016).
- 13) Kautio AL, Haanpää M, Leminen A, Kalso E, Kautiainen H, Saarto T. Amitriptyline in the prevention of chemotherapy-induced neuropathic symptoms. Anticancer Res., 29, 2601–2606 (2009).
- 14) Adelsberger H, Quasthoff S, Grosskreutz J, Lepier A, Eckel F, Lersch C. The chemotherapeutic oxaliplatin alters voltage-gated Na (+) channel kinetics on rat sensory neurons. Eur. J. Pharmacol., 406, 25–32 (2000).
- 15) Hobara N, Goda M, Yoshida N, Takatori S, Kitamura Y, Mio M, Kawasaki H. Angiotensin II type 2 receptors facilitate reinnervation of phenol-lesioned vascular calcitonin gene-related peptide-containing nerves in rat mesenteric arteries. Neuroscience, 150, 730–741 (2007).
- 16) Uchida M, Kawazoe H, Takatori S, Namba H, Uozumi R, Tanaka A, Kawasaki H, Araki H. Preventive effects of renin-angiotensin system inhibitors on oxaliplatin-induced peripheral neuropathy: a retrospective observational study. Clin. Ther., 40, 1214–1222.e1 (2018).
- 17) Cersosimo RJ. Oxaliplatin-associated neuropathy: a review. Ann. Pharmacother., 39, 128–135 (2005).
- 18) Inoue N, Ishida H, Sano M, Kishino T, Okada N, Kumamoto K, Ishibashi K. Discrepancy between the NCI-CTCAE and DEB-NTC scales in the evaluation of oxaliplatin-related neurotoxicity in patients with metastatic colorectal cancer. Int. J. Clin. Oncol., 17, 341–347 (2012).
- 19) Grothey A, Nikcevich DA, Sloan JA, Kugler JW, Silberstein PT, Dentchev T, Wender DB, Novotny PJ, Chitaley U, Alberts SR, Loprinzi CL. Intravenous calcium and magnesium for oxaliplatin-induced sensory neurotoxicity in adjuvant colon cancer: NCCTG N04C7. J. Clin. Oncol., 29, 421–427 (2011).
- 20) Argyriou AA, Cavaletti G, Briani C, Velasco R, Bruna J, Campagnolo M, Alberti P, Bergamo F, Cortinovis D, Cazzaniga M, Santos C, Papadimitriou K, Kalofonos HP. Clinical pattern and associations of oxaliplatin acute neurotoxicity: a prospective study in 170 patients with colorectal cancer. Cancer, 119, 438–444 (2013).
- 21) Ewertz M, Qvortrup C, Eckhoff L. Chemotherapy-induced peripheral neuropathy in patients treated with taxanes and platinum derivatives. Acta Oncol., 54, 587–591 (2015).
- 22) Pachman DR, Qin R, Seisler DK, Smith EML, Beutler AS, Ta LE, Lafky JM, Wagner-Johnston ND, Ruddy KJ, Dakhil S, Staff NP, Grothey A, Loprinzi CL. Clinical course of oxaliplatin-induced neuropathy: results from the randomized phase III trial N08CB (Alliance). J. Clin. Oncol., 33, 3416–3422 (2015).
- 23) Yoshida N, Hosokawa T, Ishikawa T, Yagi N, Kokura S, Naito Y, Nakanishi M, Kokuba Y, Otsuji E, Kuroboshi H, Taniwaki M, Taguchi T, Hosoi H, Nakamura T, Miki T. Efficacy of goshajinkigan for oxaliplatin-induced peripheral neuropathy in colorectal cancer patients. J. Oncol., 2013, 139740 (2013).
- 24) Selvy M, Pereira B, Kerckhove N, et al. Long-term prevalence of sensory chemotherapy-induced peripheral neuropathy for 5 years after adjuvant FOLFOX chemotherapy to treat colorectal cancer: a multicenter cross-sectional study. J. Clin. Med., 9, 2400 (2020).
- 25) Gornet JM, Savier E, Lokiec F, Cvitkovic E, Misset JL, Goldwasser F. Exacerbation of oxaliplatin neurosensory toxicity following surgery. Ann. Oncol., 13, 1315–1318 (2002).
- 26) Alejandro LM, Behrendt CE, Chen K, Openshaw H, Shibata S. Predicting acute and persistent neuropathy associated with oxaliplatin. Am. J. Clin. Oncol., 36, 331–337 (2013).
- 27) Vincenzi B, Frezza MA, Schiavon G, Spoto C, Silvestris N, Addeo R, Catalano V, Graziano F, Santini D, Tonini G. Identification of clinical predictive factors of oxaliplatin-induced chronic peripheral neuropathy in colorectal cancer patients treated with adjuvant Folfox IV. Support. Care Cancer, 21, 1313–1319 (2013).
- 28) Kawashiri T, Shimizu S, Shigematsu N, Kobayashi D, Shimazoe T. Donepezil ameliorates oxaliplatin-induced peripheral neuropathy via a neuroprotective effect. J. Pharmacol. Sci., 140, 291–294 (2019).
- 29) Takeshita M, Banno Y, Nakamura M, Otsuka M, Teramachi H, Tsuchiya T, Itoh Y. The pivotal role of intracellular calcium in oxaliplatin-induced inhibition of neurite outgrowth but not cell death in differentiated PC12 cells. Chem. Res. Toxicol., 24, 1845–1852 (2011).
- 30) Ito H, Yamaguchi H, Fujikawa A, Tanaka N, Furugen A, Miyamori K, Takahashi N, Ogura J, Kobayashi M, Yamada T, Mano N, Iseki K. A full validated hydrophilic interaction liquid chromatography-tandem mass spectrometric method for the quantification of oxaliplatin in human plasma ultrafiltrates. J. Pharm. Biomed. Anal., 71, 99–103 (2012).
- 31) Scuteri A, Galimberti A, Ravasi M, Pasini S, Donzelli E, Cavaletti G, Tredici G. NGF protects dorsal root ganglion neurons from oxaliplatin by modulating JNK/Sapk and ERK1/2. Neurosci. Lett., 486, 141–145 (2010).
- 32) Saavedra JM. Angiotensin II AT(1) receptor blockers as treatments for inflammatory brain disorders. Clin. Sci. (Lond.), 123, 567–590 (2012).
- 33) Hashikawa-Hobara N, Hashikawa N, Inoue Y, Sanda H, Zamami Y, Takatori S, Kawasaki H. Candesartan cilexetil improves angiotensin II type 2 receptor-mediated neurite outgrowth via the PI3K-Akt pathway in fructose-induced insulin-resistance rats. Diabetes, 61, 925–932 (2012).
- 34) Saito M, Shinohara Y, Sasaki H, Netsu Y, Yoshida M, Nakahata N. Type 1 angiotensin receptor (AT1-R)-mediated decrease in type 2 angiotensin receptor mRNA level is dependent on Gq and extracellular signal-regulated kinase 1//2 in AT1-R-transfected PC12 cells. J. Neuroendocrinol., 20, 299–308 (2008).
- 35) Ishida M, Ishida T, Thomas SM, Berk BC. Activation of extracellular signal-regulated kinases (ERK1/2) by angiotensin II is dependent on c-Src in vascular smooth muscle cells. Circ. Res., 82, 7–12 (1998).
- 36) Nostramo R, Serova L, Laukova M, Tillinger A, Peddu C, Sabban EL. Regulation of nonclassical renin-angiotensin system receptor gene expression in the adrenal medulla by acute and repeated immobilization stress. Am. J. Physiol. Regul. Integr. Comp. Physiol., 308, R517–R529 (2015).
- 37) Alkhudhayri S, Sajini R, Alharbi B, Qabbani J, Al-Hindi Y, Fairaq A, Yousef A. Investigating the beneficial effect of aliskiren in attenuating neuropathic pain in diabetic Sprague-Dawley rats. Endocrinol. Diabetes Metab., 4, e00209 (2020).