2022 Volume 45 Issue 3 Pages 250-259
2022 Volume 45 Issue 3 Pages 250-259
As a member of transcription factor E-Twenty Six (ETS) family, ETS variant 6 (ETV6) plays significant role in hematopoiesis and embryonic development. ETV6 dysexpression also involved in the occurrence, development and progression of cancers and leukemia. In current work, we hypothesized that ETV6 plays a role in erythroid differentiation of chronic myeloid leukemia (CML). We found the protein expression level of ETV6 was significantly upregulated during hemin-induced erythroid differentiation of K562 cells. Moreover, overexpression of ETV6 inhibited erythroid differentiation in hemin-induced K562 cells with decreased numbers of benzidine-positive cells and decreased expression levels of erythroid differentiation specific markers glycophorin (GPA), CD71, hemoglobin A (HBA), α-globin, γ-globin and ε-globin. Conversely, ETV6 knockdown promoted erythroid differentiation in hemin-induced K562 cells. Furthermore, ETV6 expression level slightly positively with the proliferation capacity of K562 cells treated with hemin. Mechanistically, ETV6 overexpression inhibited fibrosarcoma/mitogen activated extracellular signal-regulated kinase/extracellular regulated protein kinase (Raf/MEK/ERK) pathway, ETV6 knockdown activated the Raf/MEK/ERK pathway. Collectively, the current work demonstrates that ETV6 plays an inhibitory role in the regulation of K562 cell erythroid differentiation via Raf/MEK/ERK pathway, it would be a potentially therapeutic target for dyserythropoiesis.
Hematopoiesis is a dynamic complex precisely modulated multi-step process involving hematopoietic stem/progenitor cell differentiation of early erythroid progenitors and hematopoietic stem cell (HSC) self-renewal.1,2) Erythropoiesis is significant part of hematopoiesis.1–4) Regular erythropoiesis generates approximately 1011 new red blood cells (RBCs) each day through the commitment of HSC into erythroid progenitors.5,6) During erythropoiesis, hemoglobin A (HBA) synthesis increases from early progenitors to mature enucleated erythrocytes, which composed of 4 globin chains, and glycophorins A (GPA) is specifically expressed in human erythrocytes.7,8) Dysregulation of erythropoiesis process regulatory networks will lead to hematologic disorders. Understanding the molecular regulatory mechanism of erythropoiesis can lead to identification and characterization of new regulatory factors and development of novel approaches for treatment of leukemia.
Chronic myeloid leukemia (CML) is a hematopoietic malignancy characterized by the B-cell receptor/V-abl Abelson murine leukemia viral oncogene (BCR/ABL) fusion gene located within Philadelphia (Ph) chromosome [t(9;22)(q34;q11)].9,10) The BCR-ABL fusion protein is the hallmark of CML with constitutively tyrosine kinase activity which can potentially regulate several signaling pathways, leading to aberrant cell differentiation, growth, apoptosis, drug resistance and metastasis.11–13) Differentiation and maturation disorders are considered to be common characteristic of leukemia cells, inducing leukemia cell differentiation has become research hotspot in basic medical and clinical translation.14) K562 cell is obtained from pleural effusion of a CML woman patient,15,16) it carries the Philadelphia chromosome marker and behaves like undifferentiated early pluripotent hematopoietic progenitors which can be chemically induced to differentiate to more erythroid character, therefore, K562 cells have been widely used as model for studying hematological differentiation.17,18)
As a member of the transcription factor E-Twenty Six (ETS) family, ETS variant 6 (ETV6), known also as translocation ETS leukaemia (TEL).19) ETV6 is a modular protein composed of a C-terminus DNA-binding ETS domain and an N-terminus helix-loop-helix (HLH) domain.20) The ETS domain is necessary for specifically binding to DNA region rich in purine.19–21) The HLH domain mediates homogenous dimerization and heterogenous dimerization, it also frequently induces constitutive activation of tyrosine kinase activity.20,21) ETV6 is originally involvement in chromosomal translocation linked with hematologic and various cancers, a lot of chromosomal translocation oncogenes have been identified, such as ETV6-platelet-derived growth factor receptor beta (ETV6-PDGFRβ), ETV6-ABL, ETV6-runt-related transcription factor 1 gene (ETV6-RUXN1), ETV6-neurotrophic tyrosine kinase receptor type 3 (ETV6-NTRK3), ETV6-ecotropic virus integration site 1 (ETV6-EVI-1), ETV6-janus Kinase 2 (ETV6-JAK2), etc.22,23) As a transcriptional repressor, ETV6 is biologically important in embryonic development and hematopoietic regulation.24–26) Meanwhile, ETV6 missense mutations and translocations are universally detected in cancers.27–32) However, the exact role and mechanism of ETV6 in erythropoiesis of CML remain unclear.
In this article, we investigate the contribution of ETV6 to erythroid differentiation of K562 cells. Recent studies have shown that ETV6 involved in erythroid differentiation of CML as a negative erythroid regulator. ETV6 was down-regulated during hemin-induced erythroid differentiation of K562 cells. Moreover, ETV6 expression negatively regulated the erythroid differentiation of K562 cells induced by hemin. Furthermore, mechanistic studies revealed that ETV6 regulated K562 cell erythroid differentiation through the rapidly accelerated fibrosarcoma/mitogen activated extracellular signal-regulated kinase/kinase/extracellular regulated protein kinases (Raf/MEK/ERK) pathway. ETV6 may serve as a potential therapeutic target molecule for CML disease.
Human CML K562 cell lines were grown in RPMI-1640 medium (Gibco, U.S.A.) with 15% fetal bovine serum (FBS, TransGen Biotech, Beijing, China) and 1% streptomycin/penicillin (Gibco) at 37 °C with 5% CO2. For erythroid differentiation, K562 cells were treated with 40 µM Hemin (Sigma, St. Louis, MO, U.S.A.).
Benzidine Staining AssayK562 cells treated with 40 µM hemin (Sigma) for 0, 12, 24, 36 and 48 h before harvesting, and washed with ice-cold phosphate buffered saline (PBS), then 9 µL cell suspension was mixtured with 1 µL benzidine dihydrochloride solution containing H2O2 and imaged by an upright light microscope (Olympus, Tokyo, Japan). The benzidine-positive cells represented more mature erythroid cells and the percentage of benzidine-positive cells was calculated. Benzidine-positive cells were stained blue, while benzidine-negative cells were light yellow.
Plasmid Construction, Small Interfering RNA (siRNA) Design and Transient TransfectionFor ETV6 overexpression in K562 cells, the cDNA of ETV6 was amplified by PCR using the following primers: F 5′-TAGCTAGCGCCACCATGTCTGAGACTCCTGCTC-3′ and R 5′-GCCGCGCTTCGAATCAGCATTCATCTTCTTGG-3′, then inserted into the pMD19-T and PCDH-EF1-MCS-T2A-Puro vectors at the NheI and BstBI sites, respectively. All constructed plasmids were verified by sequencing. The empty vector PCDH-EF1-MCS-T2A-Puro was used as control and PCDH-EF1-MCS-T2A-Puro-ETV6 recombinant expression vector was used for overexpressing ETV6 in K562 cells.
We knockdown ETV6 in K562 cells by si-ETV6 targeting the si-ETV6-1 (5′-CAATATAGGTCTCAGAAATCC-3′), si-ETV6-2 (5′-GCATTAAGCAGGAACGAAT-3′), si-ETV6-3 (5′-GGGATTACGTCTATCAGTT-3′) and one non-targeting sequence 5′-TTCTCCGAACGTGTCACGT-3′ was used as negative control (NC).
Briefly, 4 × 105 cells/well in RPMI-1640 with 15% FBS were seeded into 6-well plate overnight, and the 6 µL si-NC, 6 µL si-ETV6 mixtures, 3 µg PCDH-EF1-MCS-T2A-Puro and 3 µg PCDH-EF1-MCS-T2A-Puro-ETV6 were transiently transfected into K562 cells using 5 µL Lipofectamine™ 2000 (Invitrogen, U.S.A.) respectively. After 48 h, the transfected cells were harvested for subsequent assays.
RNA Extraction and Quantitative Real-Time RT-PCR (qRT-PCR) AssayTrizol™ reagent (TransGen Biotech) used to extract the total RNA from K562 cells according to the manufacturer’s protocol, the cDNA was reversely transcribed by EasyScript® One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen Biotech). qRT-PCR rection was then performed by TransStart Tip Green qPCR SuperMix (TransGen Biotech) with Step-one Real-time PCR System (Life, U.S.A.) according to the manufacturer’s instructions. The relative mRNA expression levels of targeting genes were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The specific sequences of primers are showed in Table 1.
| Gene | Primer sequence | |
|---|---|---|
| α-Globin | F | 5′-GTCAACTTCAAGCTCCTAAGC-3′ |
| R | 5′-TGGACAAGTTCCTGGCTTCTG-3′ | |
| γ-Globin | F | 5′-GCAGCTTGTCACAGTGCAGTTC-3′ |
| R | 5′-TGGCAAGAAGGTGCTGACTTC-3′ | |
| ε-Globin | F | 5′-CCAGACAGAGAGGCAGCAGC-3′ |
| R | 5′-TCCAGGGGTAAACAACGAGG-3′ | |
| GPA | F | 5′-GACAAATGATACGCACAAACGG-3′ |
| R | 5′-TCCAATAACACCAGCCATCAC-3′ | |
| HBA | F | 5′-TGGAGGGTGGAGACGTCCTG-3′ |
| R | 5′-TCCATCCCCTCCTCCCGCCCCTGCCTTTTC-3′ | |
| GAPDH | F | 5′-ACCACAGTCCATGCCATCACT-3′ |
| R | 5′-CAGGTCCACCACTGACACGTT-3′ |
Radio immunoprecipitation assay (RIPA) lysis buffer was used to extract cell total protein, the supernatant was obtained by centrifugation with 12000 rpm for 30 min at 4 °C. Protein concentration was measured by Bradford assay, fractionated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membrane, blocked with 5% nonfat milk (BD Biosciences, Franklin Lakes, NJ, U.S.A.) at room temperature (r.t.) for 3 h and then incubated with specific antibodies at appropriate dilution [(ETV6: 1 : 500, ABclonal, China), (GAPDH: 1 : 10000, Sanying, China), (MEK: 1 : 500, Sanying, China), (ERK: 1 : 500, Sanying, China), (p-ERK: 1 : 500, Cell Signaling, U.S.A.), (p-Raf: 1 : 500, Cell Signaling, U.S.A.), (p-MEK: 1 : 500, Cell Signaling, U.S.A.), (Ras: 1 : 500, Cell Signaling, Danvers, MA, U.S.A.), (Raf: 1 : 500, Cell Signaling)] at 4 °C overnight. After washing with TBST for 3 times, the membrane was incubated with horseradish peroxidase (HRP) linked secondary antibodies at r.t. for 3 h. The protein blots were visualized using ECL (Advansta, U.S.A.) and quantified by Bio-Rad ChemiDoc™ MP system (Bio-Rad, Hercules, CA, U.S.A.).
Cell Flow CytometryThe influence of ETV6 on erythroid surface markers CD71 and GPA of hemin-induced K562 cells was detected by flow cytometry assay. The cell was harvested and dissolved in 100 µL PBS, labeled with CD71-fluorescein isothiocyanate (FITC) and PE-GPA (BD Biosciences) at r.t. for 30 min in darkness according to the manufacturer’s protocol, then the samples were analyzed for fluorescence emission using FACScalibur (BD Biosciences).
Cell Proliferation AssayThe effect of ETV6 on K562 cells proliferation ability during hemin-induced erythroid differentiation was measured using Cell Counting Kit-8 (CCK-8) (Abbkine, China) assay. CCK-8 allows sensitive colorimetric assays for the determination of the number of viable cells in cell proliferation and cytotoxicity assays. CCK-8 kit contains [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium monosodium salt] (WST-8), WST-8 can be reduced by dehydrogenase of cell mitochondria to a water-soluble yellow formazen dye in the presence of an electron carrier, the amount of the formazan dye is directly proportional to the number of viable cells, the viable cell number can be determined using the absorbance at 450 nm. The 5 × 103 cells/well cells in 100 µL complete medium were seeded into 96-well plate, incubated at 37 °C with 5% CO2 for 0, 24, 48, 72 h, respectively, then incubated with 10 µL CCK-8 at 37 °C for 3 h. The absorbances for each ample were measured using a microplate reader at 450 nm (Thermo, U.S.A.).
Statistical AnalysisThe data was presented as mean ± standard deviation (S.D.) of at least triplicate independent experiments. Statistical differences between the two groups were performed with unpaired Student’s t-test using GraphPad Prism 5.0 software. *, **, ***, **** represent p < 0.05, p < 0.01, p < 0.001 and p < 0.0001, respectively. p < 0.05 was indicated statistically significant difference.
To address the role of ETV6 in erythroid differentiation, we first investigated the expression pattern change of ETV6 during hemin-induced erythroid differentiation of K562 cells. After treatment with hemin, K562 cells can be differentiated into erythroid cells, K562 cells showed significant increases in the number of benzidine-positive cells in a time-dependent manner after treatment with 40 µM hemin. The benzidine-positive rates of K562 cells were 0.7, 4.2 (p = 0.0021), 23.1 (p < 0.0001), 35.7 (p < 0.0001), and 54.5% (p = 0.0002) after treatment with hemin for 0, 12, 24, 36, and 48 h, respectively (Fig. 1A), indicating hemin successfully induced the erythroid differentiation of K562 cells. Meanwhile, ETV6 protein expression level significantly changed during hemin-induced erythroid differentiation of K562 cells (Fig. 1B). The protein expression levels of ETV6 in K562 cells induced by hemin for 0, 12, 24, 36, 48 h were decreased by 17.3 (p = 0.0492), 29.8 (p = 0.0278), 29.5 (p = 0.0011) and 48.0% (p = 0.0003), respectively. Moreover, we further analyzed the correlation between ETV6 expression level changes and percentage of positive cells, there exist a negative correlation between the percentage of erythroid differentiation positive cells and ETV6 expression level changes (R2 = 0.9713, p = 0.0021, Fig. 1C), the higher the erythroid differentiation benzidine-positive rate of K562 cells, the lower the ETV6 expression level. Taken together, the expression level of ETV6 is decreased during hemin-induced erythroid differentiation of K562 cells, suggesting an underlying effect of ETV6 in erythropoiesis.
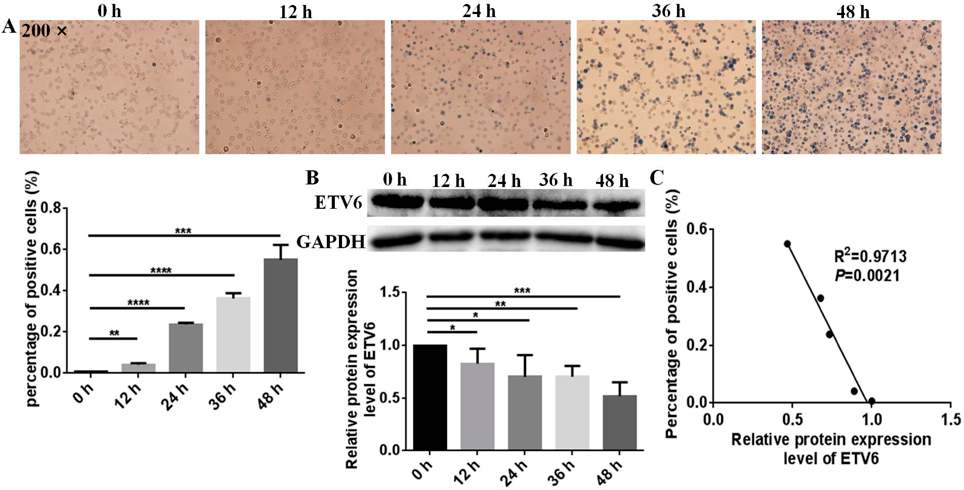
(A) Benzidine staining measured the benzidine-positive K562 cells. (B) WB measured the ETV6 protein expression level change during hemin-induced erythroid differentiation of K562 cells. (C) The correlation between percentage of benzidine-positive cells and ETV6 protein expression level changes.
We successfully constructed the PCDH-ETV6 recombinant plasmid to further explore the potential role of ETV6 in hemin-induced erythropoiesis, then the PCDH-ETV6 expression plasmid was transiently transfected into K562 cells to overexpress exogenous ETV6. ETV6 protein level was increased by 52.6% in K562-PCDH-ETV6 cells than in K562-PCDH cells (p = 0.0012, Fig. 2A), suggesting ETV6 was successfully overexpressed in K562 cells.

(A) ETV6 was overexpressed in K562 cells by PCDH-ETV6 recombinant expression plasmid. (B) The benzidine-positive cells were stained in K562-PCDH and K562-PCDH-ETV6 cells. (C) α-Globin, γ-globin, ε-globin, GPA and HBA mRNA expression levels were measured in K562-PCDH and K562-PCDH-ETV6 cells by qRT-PCR. (D) The GPA+/CD71+ double positive was detected in K562-PCDH and K562-PCDH-ETV6 cells by flow cytometry.
After treatment with hemin, compared to K562-PCDH cells, K562-PCDH-ETV6 cells exhibited decreased numbers of benzidine-positive cells. The benzidine-positive rate of K562-PCDH-ETV6 cells was decreased by 25.7% (p = 0.0016) compared with K562-PCDH cells (Fig. 2B). Meanwhile, qRT-PCR assay showed that the erythroid genes α-globin, γ-globin, ε-globin, GPA and HBA mRNA expression levels also decreased by 49.5 (p < 0.0001), 33.5 (p = 0.0018), 30.9 (p = 0.0051), 25.1 (p = 0.0123) and 25.6% (p = 0.0086) in K562-PCDH-ETV6 cells compared to K562-PCDH cells (Fig. 2C). We further detected the erythroid differentiation markers CD71, GPA in K562-PCDH-ETV6 and K562-PCDH cells induced by hemin using flow cytometry assay. Compared to K562-PCDH cells, the GPA+/CD71+ double positive of K562-PCDH-ETV6 cells was decreased by 15.0% (p = 0.0474) (Fig. 2D). Based on the above results, we demonstrate that ETV6 overexpression inhibits hemin-induced erythroid differentiation of K562 cells.
ETV6 Knockdown Promoted Hemin-Induced Erythroid Differentiation of K562 CellsThe si-ETV6 mixtures were transiently transfected into K562 cells to knockdown endogenous ETV6 expression to further investigate the effect of ETV6 on erythropoiesis. ETV6 protein level was decreased by 47.9% in K562-si-ETV6 cells than in K562-si-NC cells (p = 0.0022, Fig. 3A), suggesting ETV6 was successfully knockdown in K562 cells.

(A) ETV6 was down-regulated in K562 cells by si-ETV6. (B) The benzidine-positive cells were stained in K562-si-NC and K562-si-ETV6 cells. (C) α-Globin, γ-globin, ε-globin, GPA and HBA mRNA expression levels were measured in K562-si-NC and K562-si-ETV6 cells by qRT-PCR. (D) The GPA+/CD71+ double positive was detected in K562-si-NC and K562-si-ETV6 cells by flow cytometry.
After treatment with hemin, compared to K562-si-NC cells, K562-si-ETV6 cells showed higher numbers of benzidine-positive cells. The benzidine-positive rate of K562-si-ETV6 cells was increased by 26.9% (p = 0.0017) compared with K562-si-NC cells (Fig. 3B). Meanwhile, qRT-PCR assay showed that the erythroid genes α-globin, γ-globin, GPA, HBA and ε-globin expression levels also increased by 44.8 (p = 0.0166), 31.9 (p < 0.0001), 23.8 (p = 0.0183), 112.3 (p = 0.0142) and 38.5% (p = 0.0135) in K562-si-ETV6 cells compared to K562-si-NC cells (Fig. 3C).
We further measured the erythroid differentiation markers CD71, GPA in K562-si-ETV6 and K562-si-NC cells treated with hemin by flow cytometry analysis. The GPA+/CD71+ double positive of K562-si-ETV6 cells was increased by 14.4% (p = 0.0272) compared to K562-si-NC cells (Fig. 3D). Based on the above results, we further demonstrate that ETV6 knockdown promotes hemin-induced erythroid differentiation of K562 cells.
ETV6 Inhibited Proliferation Capacity of K562 Cells during Hemin-Induced Erythroid DifferentiationMeanwhile, we measured the effect of ETV6 on K562 cells growth capacity during erythroid differentiation by CCK8 assay. We found that ETV6 overexpression slightly promoted the proliferation ability of K562 cells (Fig. 4A). The proliferation abilities of K562-PCDH-ETV6 cells were increased by approx. 13.0 (p = 0.0003), 24.2 (p < 0.0001) and 15.9% (p = 0.0004) compared to K562-PCDH cells at 24, 48 and 72 h. Consequently, ETV6 knockdown slightly decreased the proliferation ability of K562 cells (Fig. 4B). ETV6 knockdown decreased proliferation abilities of K562-si-ETV6 cells by approx. 6.6 (p = 0.0012), 20.6 (p = 0.0001) and 22.0% (p < 0.0001) compared to K562-si-NC cells at 24, 48 and 72 h, respectively. The above results demonstrate that ETV6 expression level positively with the proliferation capacity of K562 cells, while ETV6 expression level negatively with the erythroid differentiation ability of K562 cells.

(A) ETV6 overexpression promoted K562 cells proliferation by CCK8 assay. (B) ETV6 knockdown inhibited K562 cells proliferation by CCK8 assay.
Raf/MEK/ERK pathway has been reported associated with erythroid differentiation. Therefore, we speculated ETV6 regulates hemin-induced erythroid differentiation of K562 cells through the Raf/MEK/ERK pathway. ETV6 overexpression inhibited the Raf/MEK/ERK pathway. Compared to K562-PCDH cells, the protein expression levels of p-Raf, p-MEK and p-ERK were downregulated by 43.0 (p = 0.0149), 36.6 (p = 0.0066) and 45.6 (p = 0.0382) in K562-PCDH-ETV6 cells (Fig. 5A), meanwhile, there were no changed for Ras, Raf, MEK and ERK expression (Fig. 5A). Consequently, ETV6 knockdown activated the Raf/MEK/ERK pathway. Compared to K562-si-NC, the protein expression levels of p-Raf, p-MEK and p-ERK were upregulated by 18.8 (p = 0.0058), 61.5 (p = 0.0119), and 37.9% (p = 0.0178) in K562-si-ETV6 cells (Fig. 5B), meanwhile, there were no changed for Ras, Raf, MEK and ERK (Fig. 5B). Raf/MEK/ERK pathway may be contribute to ETV6 regulated-erythroid differentiation.
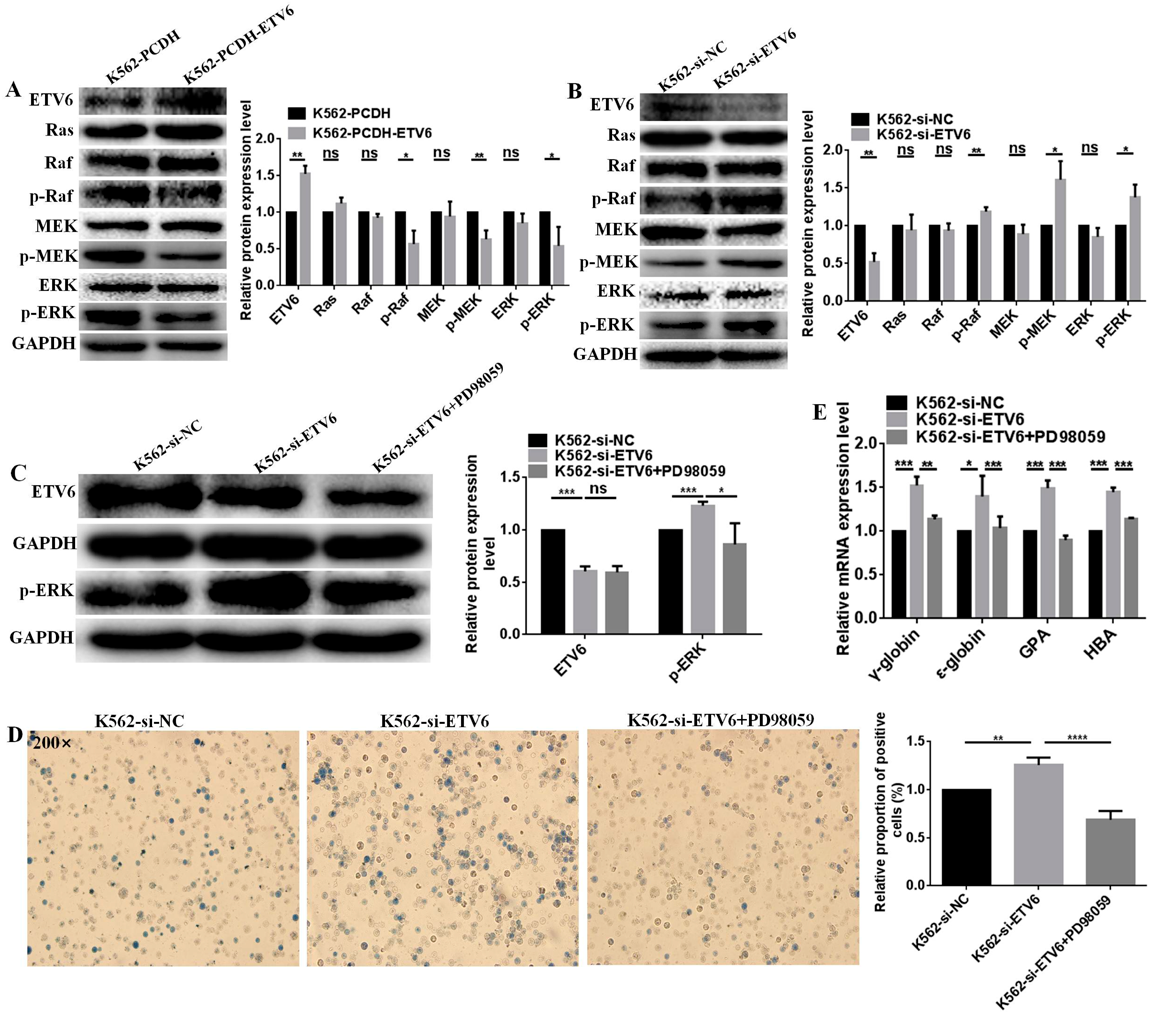
(A) ETV6 overexpression inhibited the Raf/MEK/ERK pathway. The protein expression level comparisons of Ras, Raf, p-Raf, MEK, p-MEK, ERK, p-ERK in K562-PCDH-ETV6 and K562-PCDH cells. (B) ETV6 knockdown activated the Raf/MEK/ERK pathway. The protein expression comparisons of Ras, Raf, p-Raf, MEK, p-MEK, ERK, p-ERK in K562-si-ETV6 and K562-si-NC cells. (C) The Raf/MEK/ERK pathway was successfully blocked by PD98059. WB measured the expression levels change of ETV6 and p-ERK in K562-si-ETV6 cells after treatment with PD98059 for 48 h. (D) Benzidine staining measured the benzidine-positive rate change in K562-si-ETV6 cells after treatment with PD98059 for 48 h. (E) qRT-PCR measured the expression levels change of erythroid differentiation markers γ-globin, ε-globin, GPA and HBA in K562-si-ETV6 cells after treatment with PD98059 for 48 h.
The association of Raf/MEK/ERK pathway with ETV6-regulated erythropoiesis was verified by PD98059. Previously, we screened the appropriate concentration of PD98059 which could block Raf/MEK/ERK pathway in K562 cells, 20 µM PD98059 slightly affected proliferation ability and viability of K562 cells, but it could block Raf/MEK/ERK pathway of K562 cells in a certain extent. Therefore, we chosed 20 µM PD98059 for the following study in this article. After treatment with 20 µM PD98059, p-ERK protein expression was downregulated by 29.9% (p = 0.0446) in K562-si-ETV6 cells, while there was no changed for ETV6 expression (Fig. 5C), indicating 20 µM PD98059 successfully blocked the Raf/MEK/ERK pathway.
Moreover, PD98059 significantly counteracted the acceleration effect of ETV6 knockdown on erythropoiesis potential. The benzidine-positive cell rates and the expression level changes of erythroid differentiation related markers were measured after blocking the Raf/MEK/ERK pathway by 20 µM PD98059. The benzidine-positive rate of K562-si-ETV6 cells was decreased by 45.3% (p < 0.0001, Fig. 5D) after treatment with PD98059. The mRNA expression levels of γ-globin, ε-globin, GPA and HBA were decreased by 52.3 (p = 0.0009), 39.7 (p = 0.0451), 49.3 (p = 0.0007), and 44.8% (p = 0.0001) in K562-si-ETV6 cells after treatment with PD98059 (Fig. 5E), respectively. The above results demonstrate that ETV6 regulates hemin-induced erythroid differentiation of K562 cells via Raf/MEK/ERK pathway.
Erythropoiesis is a stepwise cellular process going from committed erythroid progenitors to mature red cells which happens in human red bone marrow after kidneys responses to low levels of oxygen by releasing erythropoietin.33–36) The dynamic process is mediated by a balance of intrinsic and extrinsic factors which closely mediate erythroid-specific gene expression networks, and disruption of the dynamic process leads to leukemia. The treatment of CML with tyrosine kinase inhibitors (TKIs) targeting BCR-ABL have effectively improved the survival and prognosis of CML patients, however, about 30% of CML patients have become TKIs resistance.37–39) Therefore, it is urgent to seek more efficient treatment strategies to overcome the problem. Further study of the molecular mechanisms regulating the CML differentiation will help to find new therapeutic biomarkers and boost the therapeutic efficacy of CML patients.
ETV6 has been described as a critical transcriptional regulator involved in hematopoiesis, hematological malignancies, embryogenesis, tumorigenesis, vascular development.40,41) ETV6 often forms fusion proteins through chromosomal translocations, more than fifty translocations have been identified and thirty ETV6 partner genes have been molecularly characterized.42) Previously, we found ETV6 was overexpressed in CML patients compared with normal samples, we speculated ETV6 may be associate with erythroid differentiation of K562 cells, so in this work we investigated the expression pattern of ETV6 during hemin-induced erythroid differentiation in K562 cells and also investigated the exact role of ETV6 on erythroid differentiation of K562 cells. The current work demonstrated that ETV6 is a negative regulator of erythroid differentiation.
Hemin is an iron-containing porphyrin which is involved in oxygen delivery and used to treat acute porphyria and thalasssemia intermedia, and is also a relatively strong inducer for heme biosynthesis of K562 cell erythroid differentiation.43) In current work, the protein expression level of ETV6 was decreased during hemin-induced erythroid differentiation of K562 cells (Fig. 1B), indicating ETV6 might play crucial role in erythropoiesis of K562 cells. It has been reported that hemin stimulates transcriptional and translational processes, and involved in a post-transcriptional regulation mechanism.44) I think hemin could change the ETV6 mRNA expression. The aim of this study was to confirm whether the expression level of ETV6 changed during hemin-induced erythroid differentiation, we found the protein expression level of ETV6 was decreased during erythroid differentiation, so we did not measure the change of ETV6 mRNA expression in a time course by RT-PCR. The mechanism of erythroid differentiation of erythroid stem cells is a highly regulated complex process. Human erythroleukaemic cell, K562, which is analogous to erytroid stem cells, is often used to investigate these processes. K562 cells can be induced to erythroid differentiate by promoting globin gene expression.45) The molecular mechanism of ETV6 is down-regulated during hemin-induced erythroid differentiation is unknown, we speculated that the hemin induces the globin gene expression in K562 cells at the transcription level, then globin inhibits transcription of ETV6, lead to its expression level decreases.
To test the hypothesis, the PCDH-ETV6 expression plasmid and si-ETV6 mixtures were transiently transfected into K562 cells to overexpress exogenous and to knockdown endogenous ETV6. The results showed that ETV6 upregulation inhibited hemin-induced erythroid differentiation of K562 cells with decreased numbers of benzidine-positive cells and decreased expression levels of α-globin, γ-globin, ε-globin, GPA, CD71 and HBA (Fig. 2), conversely, knockdown of ETV6 in K562 cells promoted erythroid differentiation (Fig. 3). Moreover, cell proliferation and erythroid differentiation are opposite processes, so we measured the effect of ETV6 deregulation on proliferation capacity of K562 cells, the results showed that ETV6 expression level positively with the proliferation capacity of K562 cells during hemin-induced erythropoiesis (Fig. 4), the results further illustrated ETV6 negatively regulate erythroid differentiation of K562 cells. The current work illustrates that ETV6 is a mediator for erythroid differentiation of K562 cells.
The potential exact role of ETV6 in erythroid differentiation is controversial. ETV6 was reported to suppress or enhance the erythrocytic program different systems, however, some literatures showed that ETV6 play a non-essential role in erythropoiesis.46,47) ETV6 is essential for maintaining hematopoietic stem cells in the bone marrow. Eguchi-Ishimae et al. established transgenic mice and embryonic stem (ES) cells that express ETV6 specifically in the erythroid-committed cells driven by Gata1 promoter. Forced expression of ETV6 in the erythroid-committed cells resulted in higher HB levels in the mice and promoted expansion of erythroid progenitor following erythropoietin (EPO) stimulation in vitro.48) Moreover, ETV6 accelerated erythroid differentiation of mouse erythroleukemia (MEL) cells induced by hexamethylene bisacetamide (HMBA) or dimethylsulfoxide (DMSO).49) Meanwhile, ETV6 accelerated erythroid differentiation upon EPO treatment and inhibited megakaryocytic maturation upon thrombopoietin (TPO) of acute megakaryoblastic leukemia UT-7/GM cells, however, expression of endogenous ETV6 proteins decreased upon both EPO and TPO treatments in UT-7/GM cells.50) Furthermore, ETV6 was down-regulated during 12-O-tetradecanoyl-pholbol-13-acetate (TPA)-induced megakaryocytic and hemin-induced erythroid differentiation of K562 cells, meanwhile, ETV6 overexpression inhibited TPA-induced megakaryocytic differentiation, however, ETV6 overexpression had no obvious effect on hemin-induced erythroid differentiation of K562 cells.51) The phenomenon may be attributed to the effect of ETV6 on terminal erythroid differentiation of K562 cells is small, because the author measured the effect of ETV6 overexpression on erythroid differentiation of K562 cells after treatment with hemin for 96 h. Consistent with previous results, we also found that ETV6 expression level was down-regulated during hemin-induced erythroid differentiation of K562 cells, and ETV6 overexpression or downregulation inhibited or promoted erythroid differentiation of K562 cells after treatment with hemin for 48 h. ETV6 might specifically function either as a erythroid differentiation suppressor or a erythroid differentiation promoter candidate for certain leukemia depending on the particular type of cells or depending on different inducer for cell erythroid differentiation. Thus, while intriguing, an intrinsic role for ETV6 in the erythroid lineage remains somewhat uncertain, the exact functional role of ETV6 involved in erythropoiesis need further confirmation.
No research has studied the potential mechanism of ETV6 regulates erythroid differentiation of K562 cells, in current work, we primarily investigated the regulatory mechanism of ETV6-associated erythroid differentiation. Raf/MEK/ERK pathway involved in erythropoiesis which primarily prevents apoptosis and promotes differentiation, growth of hematopoietic cells.52–54) ERK was activated and the expression level of G protein upregulated during hemin-induced differentiation of K562 cells.55) Meanwhile, Annexin1 mediates erythroid differentiation via ERK pathway.56) Actiation of Raf/MEK/ERK pathway increased HSCs engraftment and inhibited erythroid differentiation of haematopoietic stem cells.57) In current work, ETV6 regulated the Raf/MEK/ERK pathway-related molecules protein expression (Fig. 5), we hypothesized that ETV6 might regulates hemin-induced erythropoiesis of K562 cells through Raf/MEK/ERK pathway. We further verified the potential involvement of Raf/MEK/ERK in hemin-induced erythroid differentiation of K562 cells. After blocking the Raf/MEK/ERK pathway by a specific ERK inhibitor PD98059, the benzidine-positive rates of K562 cells decreased (Fig. 5D), meanwhile, the expression levels of γ-globin, ε-globin, GPA and HBA were also decreased (Fig. 5E). Our results first demonstrate that ETV6 regulates hemin-induced erythroid differentiation of K562 cell through the Raf/MEK/ERK pathway.
The mechanism of ETV6 inhibits the phosphorylation of Raf in K562 cells is unknown. Previously, our Co-immunoprecipitation (Co-IP) assay showed that ANXA11 directly binds to ETV6 to positively regulate its expression, meanwhile, chromatin immunoprecipitation (ChIP) assay showed that ETV6 negatively regulates miR-4521 expression by directly binding to its DNA promoter region GCGGAAGT, forming a ANXA11-ETV6-miR-4521 axis to regulate malignant behaviours of K562 cells (unpublished). Moreover, our previously work found that miR-4521 regulates development and progression of K562 cells (unpublished) and clear cell renal cell carcinoma (ccRCC)58) by targeting FAM129A-3′-UTR at the 3232-3238 site. We speculated that ETV6 directly binds to the DNA promoter region of miR-4521 to suppress its expression, then miR-451 downregulation promotes FAM129A expression by targeting FAM129A-3′-UTR, then FAM129A overexpression potentially inhibits hemin-induced erythroid differentiation of K562 cells by inactivating ERK pathway. Downstream information transmission of Ras is a cascade of protein kinases and amplification processes, Ras/Raf/MEK/ERK pathway plays an important role in cell signal conversion and amplification.59) The Ras-dependent Raf/MEK/ERK pathway plays important role in erythroid differentiation. Acting as molecular switches, the Ras respond to external signals by exchanging guanosine-5′-triphosphate (GTP) for bound GDP, and the activated GTP bound Ras then recruits, by direct interaction, Raf to the plasma membrane where the catalytic activity of the kinase becomes activated. Subsequently, Raf stimulates the MEK kinases by phosphorylation, which then phosphorylate ERK on both serine and tyrosine residues to activate their kinase activity.60) The previous reports showed that the expression level of Ras was not changed but the activity of Ras was activated in the Ras/Raf/MEK/ERK pathway, our results showed that the protein expression level of Ras was not altered by ETV6 downregulation or overexpression, however, ETV6 regulated the Raf/MEK/ERK pathway, according to the literature, we think ETV6 could directly binds to the DNA promoter region of miR-4521 to promotes FAM129A expression, then FAM129A overexpression potentially inactivated Ras, and inactivating Raf/MEK/ERK pathway. In current work, we preliminary study ETV6 regulates hemin-induced erythroid differentiation of K562 cell through the Raf/MEK/ERK pathway, next, we will further investigate the exact mechanism of ANXA11-ETV6-miR-4521 axis regulates erythroid differentiation via Raf/MEK/ERK pathway.
Taken together, in this study, our findings point to ETV6 as a biomarker associating with erythropoiesis of CML. The discovery could be necessary for the development of novel cell gene therapies for CML diseases. Our data establish a functional and molecular mechanism for ETV6 in hematopoiesis.
This work was supported by Grants from the National Natural Science Foundation of China (81672737, 81272186, 31900517), Natural Science Foundation of Liaoning (20181550168, LZ2020045).
ZL and CG performed most of the experiments; XL performed the erythroid differentiation assay; CG, SL and MZS designed and guided the experiments; ZL and CG performed data processing and statistical analysis; CG wrote the manuscript; MZS revised the manuscript; CG, SL and MZS finalized the manuscript.
The authors declare no conflict of interest.