Abstract
Ultrasonic vocalization (USVs) is a promising tool to measure behavioral anxiety in rodents as USV recording is noninvasive, behaviorally relevant, ethological, and reproducible. Studies reporting the effects of stress-induced USVs in adult mice remain limited and debated. We investigated the conditions under which mice emit aversive USVs and evaluated the effects of psychiatric drugs on stress-induced USVs. Male C57BL/6J mice were used. USVs during entire stress sessions were recorded according to their frequency. To investigate the effect of psychiatric drugs on USVs, the number of USVs under cold-restraint stress conditions before and after drug administration was compared. Immediately after stress exposure, blood samples were collected and plasma corticosterone levels were measured. The combination of cold and restraint stress conditions significantly increased the USV numbers and plasma corticosterone levels compared with each stress alone. A benzodiazepine anxiolytic (midazolam) and δ-opioid receptor agonist putative anxiolytic (KNT-127) significantly reduced the stress-induced USV number and plasma corticosterone levels; however, a monoaminergic antidepressant (duloxetine) and N-methyl-D-aspartic acid receptor antagonist antidepressant (ketamine) did not reduce the USV numbers. No changes were noted in the USV numbers after repeated exposure to cold-restraint stress on days 1 and 3. The suppressive effect of midazolam on day 3 was comparable to that on day 1. Stress-induced USV may be used as a quantitative measure of anxiety to systematically assess the effects of anxiolytics. Therefore, cold-restraint stress-induced USVs may be used as a novel tool to measure rodent anxiety and as a useful anxiolytic-screening system.
INTRODUCTION
Ultrasonic vocalization (USV) is a promising tool to measure behavioral anxiety in rodents. This approach is advantageous as USV recording is noninvasive, behaviorally relevant, ethological, and reproducible. Considering the benefits of using transgenic mouse models in preclinical basic research, this model is garnering growing scientific interest.1–3) Rats were the first rodents used in basic preclinical USV studies; however, studies reporting the effects of stress-induced USVs in adult mice are limited and remain debated.4)
Mice constitute an important model of vocal behavior and social communication, with studies focusing on the brain’s communication system and use of vocalizations as biomarkers for animal health and disease status.5–7) Although several studies have investigated mouse USVs emitted by isolated pups or males in mating contexts, studies on behavioral contexts other than mating and vocalization categories are limited.8)
Given that anxiety disorders remain poorly understood and inadequately treated, the development of in vivo models for evaluating novel treatment approaches is desired in preclinical settings. Various models of anxiety have been developed to assess the “approach–avoidance behavior” of rats and mice.9) Many of these models were originally developed and validated based on their ability to predict the effects of anxiolytics in rats and have since been adapted for their use in mice, although the rat results have been translated into mouse versions with varying success. Moreover, such tests require a large-scale apparatus that matches the laboratory’s experimental environment as well as complicated operations by skilled researchers to obtain reproducibility. This study, therefore, investigated the conditions under which mice emit aversive USVs and evaluated the effects of psychiatric drugs on stress-induced USVs.
MATERIALS AND METHODS
AnimalsMale C57BL/6J mice (12–14 weeks old, purchased from Tokyo Laboratory Animals Science, Tokyo, Japan) were used for behavioral and biochemical experiments. All animals had free access to food and water in an animal room with a stable temperature (23 ± 1 °C) and relative humidity (55 ± 5%) under 12/12-h light–dark cycles (lights were automatically switched on at 8:00 a.m.). All experimental protocols were approved by the Institutional Animal Care and Use Committee at the Tokyo University of Science (Approval No. Y-20020), and the procedures were conducted in accordance with the guidelines of the National Institute of Health and Japan Neuroscience Society.
DrugsKNT-127 was synthesized at the University of Tsukuba (Tsukuba, Ibaraki, Japan). The purity (≥95%) of KNT-127 was determined using analytical HPLC. Midazolam, duloxetine, ketamine, and haloperidol were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). Haloperidol was dissolved in 0.08% lactic acid, and the other drugs were dissolved in normal saline at the concentrations indicated in Results. Ketamine was administered intraperitoneally. The other drugs and vehicle (saline) were administered subcutaneously (s.c.) at a volume of 0.1 mL/10 g of body weight. The administration doses of each drug—KNT-127,10) midazolam,11) ketamine,12) haloperidol,13) and duloxetine14)—were determined based on the previous rodent studies regarding anxiety-related behavioral analyses.
Stress ExposureThe mice were exposed to cold stress in a soundproof box (20 × 20 × 33 cm, LabDesign, Ibaraki, Japan) maintained at 4–8 °C under 500 lux illuminations. For the first stress exposure session, each mouse was placed in a small chamber (7.5 × 7.5 × 9.5 cm) inside the soundproof box for 10 min. Immediately after, a drug was administered, and it was then placed in the home cage for 30 min. After 30 min since the first stress exposure, the mouse was re-exposed to cold stress for 10 min—second stress exposure.
The mice were exposed to restraint stress using a modification of the previously described methods.15) For first stress exposure, each mouse was restrained in a plastic snug-fit apparatus (2.5 cm in diameter and 7.5 cm in length) and fixed in a soundproof box maintained at 22–23 °C under 500 lux illuminations for 10 min. Immediately after, saline was administered to the mouse, which was then placed in its home cage for 30 min. After 30 min since the first stress exposure, the mouse was re-exposed to restraint stress for 10 min—second stress exposure.
The mice were exposed to cold-restraint stress as described in this paragraph. On day 1, which was the day before the experiments, a 10-min training session was conducted (Fig. 1). On day 2, i.e., the day of experiment, each mouse was restrained in the previously described plastic snug-fit apparatus in the soundproof box (4–8 °C, 500 lux). After the first stress exposure, drugs were administered, and the mouse was placed in its home cage for 30 min. After 30 min since the first stress exposure, the mouse was re-exposed to cold-restraint stress for 10 min—second stress exposure (Fig. 1).
For repeated stress exposure, the mice were exposed to stress twice, 48 h apart. The number of USVs induced by cold-restraint stress, before and after drug administration, was compared on day 1 following the method described above and assessed following the same method on day 3. On day 2 and for the rest of the experiment, the mice were left untreated and were maintained in their home cages.
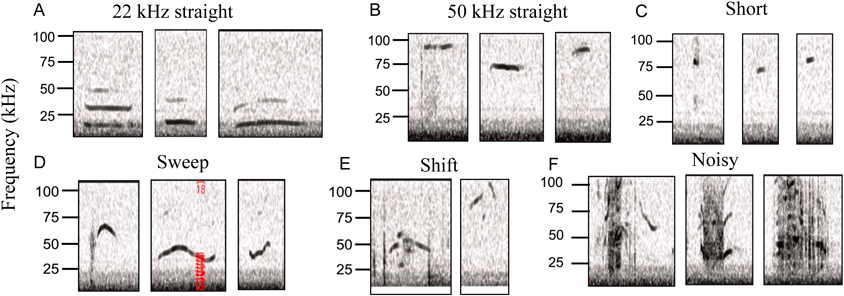
USV Recording and AnalysisUSV acoustic signals were recorded using an ultrasonic condenser microphone (CM16/CAMPA; Avisoft Bioacoustics, Berlin, Germany). The ultrasound microphone was positioned approximately 5 cm in front of the animal’s mouth and connected via an UltraSoundGate 416H USB audio device (Avisoft Bioacoustics e.K. Germany) to a PC, where the acoustic data were recorded with a sampling rate of 250 kHz (16-bit format; recording range 0–250 kHz) by RECORDER USGH. For USV analysis, the recordings were converted into high-resolution spectrograms (frequency resolution 488 Hz; time resolution 0.512 ms) through fast Fourier transformation (256 fast Fourier transform (FFT) length, 100% frame, flat top window, and 75% time–window overlap) using SASLab Pro software 5.2.09 (Avisoft Bioacoustics). Calls with frequencies of >15 kHz were defined as aversive USVs and were counted by trained investigators in five categories shown in Fig. 2: 22 kHz straight (syllables in 18–33 kHz with <5 kHz frequency modulation), 50 kHz straight (syllables in 33–100 kHz with <5 kHz frequency modulation), short (<10 ms regardless of the shape), sweep (syllables with >5 kHz frequency modulation), shift (syllables separated by ≥1 frequency jump/s without time separation), or noisy (syllables that do not fit any of the other four categories because of background noise or the lack of clearly defined spectrographic features). USV classification was performed using the modified version of a previously described indicator.8,16)
Locomotor Activity MeasurementLocomotor activity was assessed using a modified version of previously described procedures.10) Locomotor activity was monitored through an activity sensor unit for mice (Supermex PAT.P, Muromachi Kikai Co., Ltd., Tokyo, Japan). Each mouse was placed into the apparatus under normal lighting conditions. Animals were administered with identical concentrations of the drug mentioned in Results. The activity was measured 30 min after drug administration for 10 min.
Hormonal AssessmentImmediately after the second stress exposure, blood sample was collected from each mouse’s facial or mandibular vein into tubes containing heparin, as previously reported.17) The tubes were stored on ice. Subsequently, the samples were centrifuged for 15 min at 15000 rpm at 4 °C. Plasma was separated immediately, aliquoted for use in the corticosterone assay, and stored at −30 °C until assayed. Plasma corticosterone levels were measured in duplicate with a corticosterone enzyme-linked immunosorbent assay kit (Cayman Chemical, MI, U.S.A.; Catalog No. 501320) according to the manufacturer’s instructions.
Data AnalysisAll data are presented as the mean ± standard error of the mean. The data were analyzed using one-way ANOVA for comparisons among three or more groups. If ANOVA results were significant, Dunnett’s multiple comparison tests were performed. For statistical analysis, we used GraphPad Prism7 (GraphPad Software Inc., U.S.A.) For tests between two groups with correspondence, t-tests were used. p-Values of <0.05 were considered significant. Smirnov–Grubb’s test was performed on the results of all behavioral tests, and outliers were rejected. Both the t- and Smirnov–Grubb’s tests were performed based on calculations performed in Microsoft Excel.
RESULTS
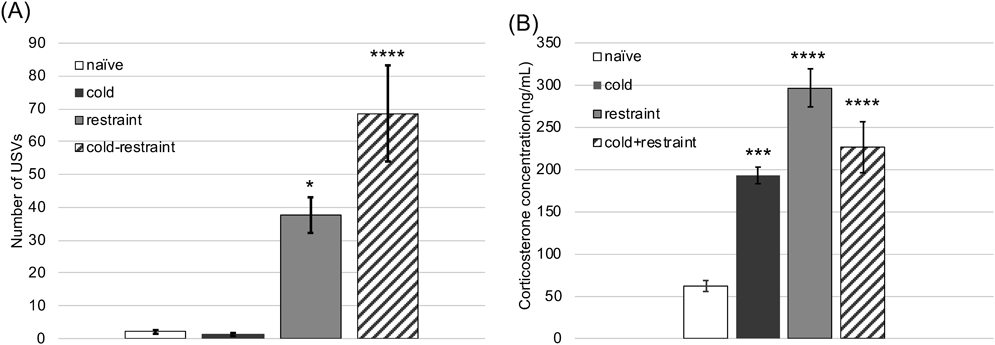
Effects of Stress Exposure on USV Number and Plasma Corticosterone Levels in MiceTo obtain aversive (15–100 Hz) definite USVs of mice exposed to stress, we first explored the effects of the stress paradigm. No effects were observed on the USV number of mice after cold stress exposure, whereas restraint stress exposure resulted in slightly and a significant increase in the USV number (Fig. 3A). Combination of cold stress with restraint stress (cold-restraint stress) showed a significant increase in the USV number compared with each stress alone (Fig. 3A). Plasma corticosterone levels in mice were significantly increased after all stress exposure conditions (cold, restraint, and combination; Fig. 3B).
Effects of Psychotropic Drug Administration on Mice USV NumberTo determine whether mice emit aversive USVs and the emotional state under which they do so after cold-restraint stress exposure, we examined the effects of the administration of several types of psychotropic drugs on USV numbers.
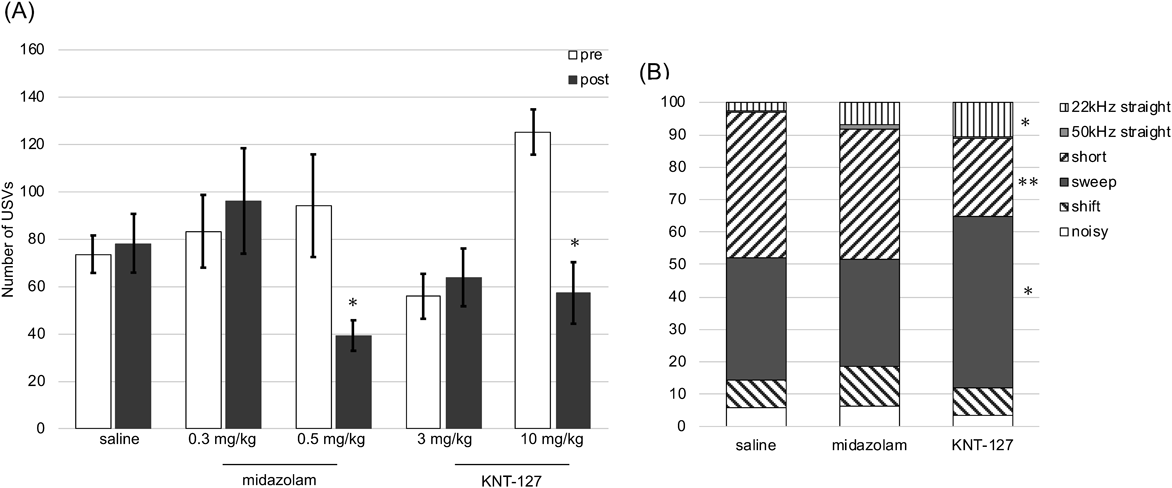
No significant differences were noted in the USV number between the first and second stress exposures in saline-treated mice (Figs. 4A, B). A benzodiazepine anxiolytic, midazolam (0.5 mg/kg, s.c.), significantly decreased the USV number of mice in the second stress exposure compared with that in the first stress exposure (Fig. 4A). Similarly, a putative novel anxiolytic and the selective δ-opioid receptor (DOP) agonist KNT-127 (10 mg/kg, s.c.) significantly decreased the USV number in the second stress exposure (Fig. 4A).
We observed no significant effects on the USV classification of each spectrogram (22 kHz straight, 50 kHz straight, short, sweep, and noisy) between the saline- and midazolam-treated mice. However, KNT-127 administration resulted in an increase in the 22 kHz straight and sweep spectrograms but a decrease in the probability of short spectrograms (Fig. 4B).
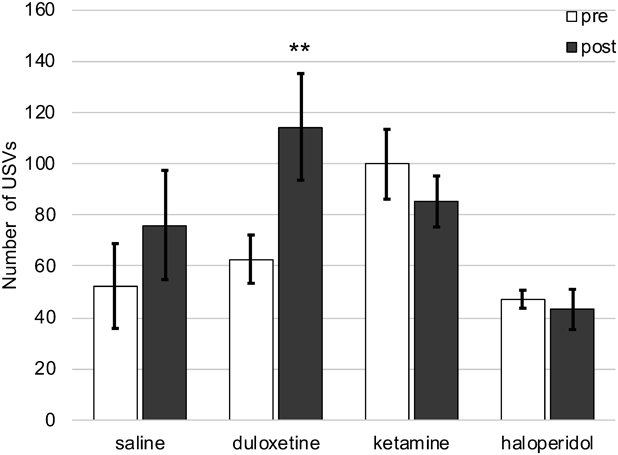
The monoaminergic antidepressant duloxetine (15 mg/kg, s.c.) significantly increased the USV number in the second stress exposure compared with that in the first stress exposure. Both the N-methyl-D-aspartic acid receptor antagonist ketamine (15 mg/kg, s.c.) and the typical antipsychotic drug, haloperidol (0.1 mg/kg, s.c.) showed no changes in the USV number between the first and second stress exposures (Fig. 5).
Effect of Drugs on Locomotor ActivityTo clarify whether the decrease in USVs was because of a sedative effect, we measured mice locomotor activity 30 min after drug administration for 10 min. Figure 6 shows the total locomotor activity in the last 10 min. The administration of midazolam, KNT-127, duloxetine, and haloperidol did not influence locomotor activity.
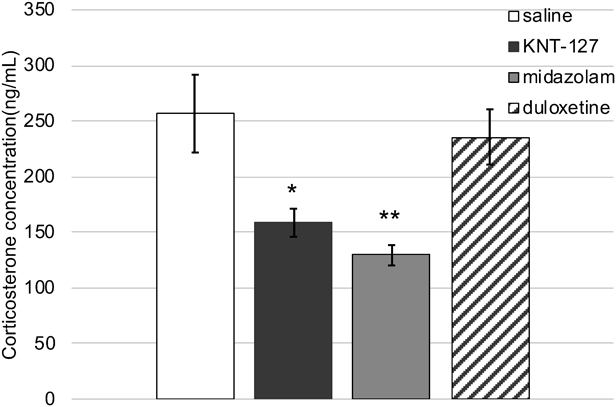
Effect of Drugs on Plasma Corticosterone LevelsTo clarify the emotional state of mice administrated with psychotropic drugs (midazolam and KNT-127), we then examined the plasma corticosterone levels, which are the known indicators of stress exposure, 30 min after drug administration in the second stress exposure. The cold-restraint stress-induced increase in the plasma corticosterone levels was significantly reduced by KNT-127 (10 mg/kg, s.c.) and midazolam (0.5 mg/kg, s.c.) treatments (Fig. 7). On the other hand, duloxetine (15 mg/kg, s.c.) exerted no effects on the plasma corticosterone levels after cold-restraint stress exposure (Fig. 7).
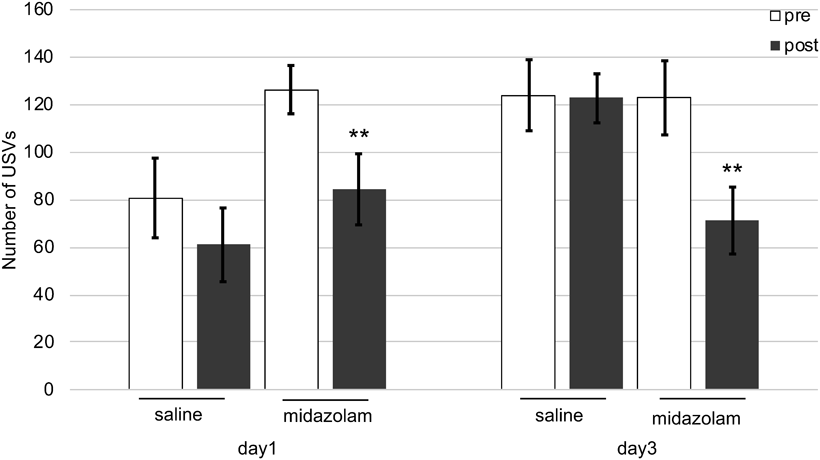
Repeated Stress ExposureTo check if the cold-restraint stress-induced paradigm can be performed more than once, the experiment was repeated 48 h after the test on day 1. In both tests on days 1 and 3, no significant differences were noted in the number of cold-restraint stress-induced USVs before and after drug administration in the saline group (Fig. 8). On the other hand, midazolam (0.5 mg/kg, s.c.) significantly reduced the USV number in mice after stress exposure compared with that before stress exposure on days 1 and 3 (Fig. 8).
DISCUSSION
The combination of cold and restraint stress conditions induced a significant increase in the USV number compared with each stress alone and raised the plasma corticosterone levels. Unlike antidepressants, a benzodiazepine anxiolytic (midazolam) and a DOP agonist (KNT-127) significantly reduced the number of stress-induced USVs and levels of plasma corticosterone in adult male mice. Thus, we suggest that cold-restraint stress increases the USV number, which can indicate the level of anxiety in adult mice.
Mice USVs in the Stress Exposure ParadigmMost studies on mouse vocal behavior have focused on higher frequency USVs that are emitted not only by male mice during courtship and mating18) but also by male and female mice during other social interactions.19,20) Furthermore, USVs are the most commonly emitted vocalizations by mouse pups.19,21,22) Thus, studies reporting the effects of stress-induced USVs in adult mice are remain scarce and debated; however, the results reported in previous studies were in line with our results. The present study indicated that aversive USVs within the range of 15–100 Hz were slightly elevated in adult mice after the single restraint stress exposure. Several studies indicated that restraint stress increased mouse USVs in the range of 30–48 kHz.8,23) Chabout et al. reported that mice exposed to restraint stress produced some USVs within the range of 15–100 Hz.23) These previous results are consistent with our present results, indicating that single restraint stress results in mice producing USV calls for less time. Interestingly, we found that the combination of cold stress with restraint stress increased the USV number, but restraint or cold stress alone resulted in mice producing USV calls for less time.
Corticosterone is a useful endogenous stress biomarker. In rodents, stress and anxiety cause corticosterone secretion via the activation of the hypothalamic–pituitary–adrenocortical axis.24) Thus, plasma corticosterone levels can provide complementary information about anxiety-like behaviors in rodents.25) The present study showed that cold-restraint stress exposure significantly increased the plasma corticosterone levels in mice, which were similar to the levels following exposure to each stress alone. These results suggest that the present paradigms exposed the mice to sufficient stress to induce anxiety-like behaviors. On the other hand, USVs were observed mainly in the mice exposed to cold-restraint combination stress but not much in those exposed to each stress alone. We propose that USVs in mice may depend on the stress exposure type rather than the stress intensity. Furthermore, the cold-restraint stress exposure paradigm may be reproducible as a simple model for USV assessments in adult mice.
Mice USV as a Screening Model for AnxiolyticsIn the present study, we found that USVs induced by exposure to cold-restraint stress in mice decreased significantly and dose-dependently after treatment with midazolam, without any effects on mouse locomotor activity. Similar results were obtained with KNT-127.10,26–30) On the other hand, there were no effects on the USV number in mice exposed to cold-restraint stress after treatment with ketamine, which was recently approved by the U.S. Food and Drug Administration (FDA) as a rapid antidepressant, or haloperidol.
The results of these pharmacological studies indicate that the USVs induced by exposure to cold-restraint stress in mice were suppressed by treatments comprising only anxiolytic midazolam or KNT-127. The dose used in either the midazolam or KNT-127 treatment has been reported previously in an anxiety-related behavioral study.10,11) These results suggest no differences in the effects of these drugs, including those of anxiolytics, between cold-restraint stress-induced USVs and other anxiety-related behavioral assessments. Conversely, the monoaminergic antidepressant duloxetine significantly increased the USV number. Although duloxetine as a chronic treatment is effective for anxiety disorders, a single treatment of duloxetine produced anxiety as an adverse event.31) Furthermore, previous animal studies have reported that a single treatment with monoaminergic antidepressants such as fluoxetine and paroxetine induces anxiety-like behaviors in rodents.32–35) The present study indicated no effects of duloxetine on the plasma corticosterone levels after stress exposure compared with the levels noted in mice treated with midazolam or KNT-127. Taken together, duloxetine at the dose used in the present study induced anxiety-like behaviors in mice after stress exposure. Furthermore, these results strongly support our suggestion that USVs induced by exposure to cold-restraint stress reflect anxiety-like behaviors in mice. In the near future, further studies are warranted to elucidate the clinical efficacy of cold-restraint stress-induced USVs.
Several studies have reported on the interaction between opioid and monoaminergic neuronal systems to modulate USVs in rodents. For example, the relationship between the emission of positive,36–38) negative,39) and social40) USVs and the changes in neurochemicals such as dopamine and noradrenalin indicates cross-talk between gamma-aminobutyric acid (GABA)ergic neurotransmission and the opioid receptor subtype (opioid µ or κ receptor) in the limbic forebrain region of rodents. To our knowledge, the present study is the first to reveal the involvement of DOP in rodent ultrasonic vocalization. We hypothesize that GABA or DOP receptors are involved in the mechanism underlying stress-induced USVs in mice. In order to clarify the differences among the midazolam, KNT-127, and duloxetine treatments, further molecular studies are needed to elucidate possible neurons or receptor mechanisms.
In the single stress exposure paradigm, the training session (day 1) was conducted the day before the experiments to stabilize USVs after stress exposure to which the mice adapted on the day of experiments (day 2). Therefore, we performed repeated experiments on the effects of cold-restraint stress on USVs to investigate whether adaptation influences the occurrence of USVs. We found no changes in the USV number after repeated exposure to cold-restraint stress on days 1 and 3 (Fig. 8). Furthermore, similar results were obtained for the suppressive effect of midazolam on days 1 and 3. In rodents, exposure to a new environment induces a biologically adaptive anxiety response. However, with repeated exposure, the anxiety response is reduced as the rodent adapts to the environment.41) According to a previous study, repeated exposure to an elevated apparatus plus a maze environment caused less anxiety-like behaviors in mice over time than those exhibited when less time was spent on the open arms.42) Therefore, a general anxiety assessment could not be repeated on the same animal because an adaptation change in the behavioral response occurred after repeated exposure to environmental stimuli. However, we found no differences between the results of experiments performed on days 1 and 3; thus, no habituation due to repeated exposure was observed. Therefore, we propose the cold-restraint stress-induced USV paradigm as an animal model for the repeated assessments of anxiety. The fact that anxiety-like behaviors in rodents can be assessed repeatedly using the objective measure of USVs is an interesting finding of the present study.
On the basis of these findings, we propose the cold-restraint stress paradigm in mice as a valid screening model for anxiolytics, which may be evaluated repeatedly, but not for antidepressants or antipsychotics. Furthermore, the cold-restraint stress exposure paradigm may be reproducible as a simple tool to quantify USVs in adult mice. This simple test may also constitute a valuable mice model to explore the physiology underlying anxiety.
Effects of Anxiolytics on Mouse USV Spectrogram ClassificationReportedly, the proportions of the different USV types on the spectrogram differ under different stress conditions such as isolation and restraint.8) However, to our knowledge, no previous studies have evaluated the effects of psychotropic drugs on USV spectrogram classification. Thus, the present study is possibly the first to reveal significant differences in the expression of each classified spectrum between the benzodiazepine anxiolytic midazolam and DOP agonist anxiolytic treatments. Notably, KNT-127 increased the probability of the appearance of 22 kHz straight and sweep spectrograms but decreased that of short spectrograms. This effect may be attributable to the differences in the neurotransmitter levels after psychotropic drug administration because neurotransmitter levels are reported to be closely related to USV spectrogram classification.38,40) In our previous study, KNT-127 and midazolam showed similar anxiolytic effects, resulting in similar times spent in the open arm in the elevated plus maze test.27) Therefore, the classification of USV spectrograms may enable a more detailed categorization of different anxiolytic-like effects in rodent models.
ConclusionIn conclusion, we propose cold-restraint stress-induced USVs as a novel tool to measure anxiety in rodents as well as a useful anxiolytic-screening system that allows for repeated assessments. The understanding of the natural vocal behavior of mice and better interpretation of the vocalizations produced by mice across behavioral contexts may facilitate the development of mouse models of psychological disorders.
Conflict of Interest
The study was supported by a Grant from FUJIMIC, Inc., Tokyo, Japan.
REFERENCES
- 1) Zampieri BL, Fernandez F, Pearson JN, Stasko MR, Costa AC. Ultrasonic vocalizations during male-female interaction in the mouse model of Down syndrome Ts65Dn. Physiol. Behav., 128, 119–125 (2014).
- 2) Wöhr M, Roullet FI, Crawley JN. Reduced scent marking and ultrasonic vocalizations in the BTBR T + tf/J mouse model of autism. Genes Brain Behav., 10, 35–43 (2011).
- 3) Hariri AR, Holmes A. Finding translation in stress research. Nat. Neurosci., 18, 1347–1352 (2015).
- 4) Portfors CV, Perkel DJ. The role of ultrasonic vocalizations in mouse communication. Curr. Opin. Neurobiol., 28, 115–120 (2014).
- 5) Lahvis GP, Alleva E, Scattoni ML. Translating mouse vocalizations: prosody and frequency modulation. Genes Brain Behav., 10, 4–16 (2011).
- 6) Bishop SL, Lahvis GP. The autism diagnosis in translation: shared affect in children and mouse models of ASD. Autism Res., 4, 317–335 (2011).
- 7) Panksepp JB, Jochman KA, Kim JU, Koy JJ, Wilson ED, Chen Q, Wilson CR, Lahvis GP. Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS ONE, 2, e351 (2007).
- 8) Grimsley JM, Sheth S, Vallabh N, Grimsley CA, Bhattal J, Latsko M, Jasnow A, Wenstrup JJ. Contextual modulation of vocal behavior in mouse: newly identified 12 kHz “mid-frequency” vocalization emitted during restraint. Front. Behav. Neurosci., 10, 38 (2016).
- 9) Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat. Rev. Drug Discov., 4, 775–790 (2005).
- 10) Yamada D, Yanagisawa S, Yoshizawa K, Yanagita S, Oka JI, Nagase H, Saitoh A. Selective agonists of the δ-opioid receptor, KNT-127 and SNC80, act differentially on extinction learning of contextual fear memory in mice. Neuropharmacology, 160, 107792 (2019).
- 11) Miao YL, Guo WZ, Shi WZ, Fang WW, Liu Y, Liu J, Li BW, Wu W, Li YF. Midazolam ameliorates the behavior deficits of a rat posttraumatic stress disorder model through dual 18 kDa translocator protein and central benzodiazepine receptor and neurosteroidogenesis. PLOS ONE, 9, e101450 (2014).
- 12) Wróbel A, Serefko A, Wlaź P, Poleszak E. The effect of imipramine, ketamine, and zinc in the mouse model of depression. Metab. Brain Dis., 30, 1379–1386 (2015).
- 13) Song JC, Seo MK, Park SW, Lee JG, Kim YH. Differential effects of olanzapine and haloperidol on MK-801-induced memory impairment in mice. Clin. Psychopharmacol. Neurosci., 14, 279–285 (2016).
- 14) Ramezany Yasuj S, Nourhashemi M, Keshavarzi S, Motaghinejad M, Motevalian M. Possible role of cyclic AMP response element binding/brain-derived neurotrophic factor signaling pathway in mediating the pharmacological effects of duloxetine against methamphetamine use-induced cognitive impairment and withdrawal-induced anxiety and depression in rats. Adv. Biomed. Res., 8, 11 (2019).
- 15) Saitoh A, Hirose N, Yamada M, Yamada M, Nozaki C, Oka T, Kamei J. Changes in emotional behavior of mice in the hole-board test after olfactory bulbectomy. J. Pharmacol. Sci., 102, 377–386 (2006).
- 16) Nicolakis D, Marconi MA, Zala SM, Penn DJ. Ultrasonic vocalizations in house mice depend upon genetic relatedness of mating partners and correlate with subsequent reproductive success. Front. Zool., 17, 10 (2020).
- 17) Dinel AL, Guinobert I, Lucas C, Blondeau C, Bardot V, Ripoche I, Berthomier L, Pallet V, Layé S, Joffre C. Reduction of acute mild stress corticosterone response and changes in stress-responsive gene expression in male Balb/c mice after repeated administration of a. Food Sci. Nutr., 7, 3827–3841 (2019).
- 18) Maggio JC, Maggio JH, Whitney G. Experience-based vocalization of male mice to female chemosignals. Physiol. Behav., 31, 269–272 (1983).
- 19) Grimsley JM, Monaghan JJ, Wenstrup JJ. Development of social vocalizations in mice. PLoS ONE, 6, e17460 (2011).
- 20) Maggio JC, Whitney G. Ultrasonic vocalizing by adult female mice (Mus musculus). J. Comp. Psychol., 99, 420–436 (1985).
- 21) Liu RC, Miller KD, Merzenich MM, Schreiner CE. Acoustic variability and distinguishability among mouse ultrasound vocalizations. J. Acoust. Soc. Am., 114, 3412–3422 (2003).
- 22) Scattoni ML, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in adult BTBR T + tf/J mice during three types of social encounters. Genes Brain Behav., 10, 44–56 (2011).
- 23) Chabout J, Serreau P, Ey E, Bellier L, Aubin T, Bourgeron T, Granon S. Adult male mice emit context-specific ultrasonic vocalizations that are modulated by prior isolation or group rearing environment. PLOS ONE, 7, e29401 (2012).
- 24) Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur. J. Pharmacol., 463, 235–272 (2003).
- 25) Lahti RA, Barsuhn C. The effect of various doses of minor tranquilizers on plasma corticosteroids in stressed rats. Res. Commun. Chem. Pathol. Pharmacol., 11, 595–603 (1975).
- 26) Saitoh A, Sugiyama A, Nemoto T, Fujii H, Wada K, Oka J, Nagase H, Yamada M. The novel δ opioid receptor agonist KNT-127 produces antidepressant-like and antinociceptive effects in mice without producing convulsions. Behav. Brain Res., 223, 271–279 (2011).
- 27) Saitoh A, Sugiyama A, Yamada M, Inagaki M, Oka J, Nagase H, Yamada M. The novel δ opioid receptor agonist KNT-127 produces distinct anxiolytic-like effects in rats without producing the adverse effects associated with benzodiazepines. Neuropharmacology, 67, 485–493 (2013).
- 28) Nagase H, Saitoh A. Research and development of κ opioid receptor agonists and δ opioid receptor agonists. Pharmacol. Ther., 205, 107427 (2020).
- 29) Saitoh A, Nagase H. Delta opioid receptor (DOR) ligands and pharmacology: development of indolo- and quinolinomorphinan derivatives based on the message-address concept. Handb. Exp. Pharmacol., 247, 3–19 (2018).
- 30) Saitoh A, Yamada M. Antidepressant-like effects of δ opioid receptor agonists in animal models. Curr. Neuropharmacol., 10, 231–238 (2012).
- 31) Shelton RC. Serotonin and Norepinephrine Reuptake Inhibitors. Handb. Exp. Pharmacol., 250, 145–180 (2019).
- 32) Drapier D, Bentué-Ferrer D, Laviolle B, Millet B, Allain H, Bourin M, Reymann JM. Effects of acute fluoxetine, paroxetine and desipramine on rats tested on the elevated plus-maze. Behav. Brain Res., 176, 202–209 (2007).
- 33) Sánchez C, Meier E. Behavioral profiles of SSRIs in animal models of depression, anxiety and aggression: are they all alike? Psychopharmacology (Berl), 129, 197–205 (1997).
- 34) Ravinder S, Burghardt NS, Brodsky R, Bauer EP, Chattarji S. A role for the extended amygdala in the fear-enhancing effects of acute selective serotonin reuptake inhibitor treatment. Transl. Psychiatry, 3, e209 (2013).
- 35) Marcinkiewcz CA, Mazzone CM, D’Agostino G, Halladay LR, Hardaway JA, DiBerto JF, Navarro M, Burnham N, Cristiano C, Dorrier CE, Tipton GJ, Ramakrishnan C, Kozicz T, Deisseroth K, Thiele TE, McElligott ZA, Holmes A, Heisler LK, Kash TL. Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature, 537, 97–101 (2016).
- 36) Hamed A, Daszczuk P, Kursa MB, Turzyńska D, Sobolewska A, Lehner M, Boguszewski PM, Szyndler J. Non-parametric analysis of neurochemical effects and Arc expression in amphetamine-induced 50-kHz ultrasonic vocalization. Behav. Brain Res., 312, 174–185 (2016).
- 37) Hamed A, Szyndler J, Taracha E, Turzyńska D, Sobolewska A, Lehner M, Krząścik P, Daszczuk P. κ-Opioid receptor as a key mediator in the regulation of appetitive 50-kHz ultrasonic vocalizations. Psychopharmacology (Berl), 232, 1941–1955 (2015).
- 38) Millan MJ, Brocco M, Gobert A, Dorey G, Casara P, Dekeyne A. Anxiolytic properties of the selective, non-peptidergic CRF(1) antagonists, CP154,526 and DMP695: a comparison to other classes of anxiolytic agent. Neuropsychopharmacology, 25, 585–600 (2001).
- 39) Nazarian A, Rodarte-Freeman AL, McDougall SA. Dopaminergic modulation of kappa opioid-mediated ultrasonic vocalization, antinociception, and locomotor activity in the preweanling rat. Behav. Neurosci., 113, 816–825 (1999).
- 40) Grant LM, Barth KJ, Muslu C, Kelm-Nelson CA, Bakshi VP, Ciucci MR. Noradrenergic receptor modulation influences the acoustic parameters of pro-social rat ultrasonic vocalizations. Behav. Neurosci., 132, 269–283 (2018).
- 41) van der Goot MH, Keijsper M, Baars A, Drost L, Hendriks J, Kirchhoff S, Lozeman-van T Klooster JG, van Lith HA, Arndt SS. Inter-individual variability in habituation of anxiety-related responses within three mouse inbred strains. Physiol. Behav., 239, 113503 (2021).
- 42) Tucker LB, McCabe JT. Behavior of male and female C57BL/6J mice is more consistent with repeated trials in the elevated zero maze than in the elevated plus maze. Front. Behav. Neurosci., 11, 13 (2017).