2022 Volume 45 Issue 3 Pages 301-308
2022 Volume 45 Issue 3 Pages 301-308
Neuroinflammation induced by activated microglia is a key feature of neurodegenerative diseases such as Alzheimer’s disease. The natural flavonoid 3′,4′,7-trihydroxyflavone protects nerve cells from oxidative stress-mediated apoptosis and inhibits the aggregation of amyloid β protein in vitro. However, little is known about its effects on microglial activation. In this study, we investigated the effects of 3′,4′,7-trihydroxyflavone on lipopolysaccharide (LPS)- or interferon-γ (IFN-γ)-induced neuroinflammatory responses in MG6 microglial cells. 3′,4′,7-Trihydroxyflavone inhibited LPS- or IFN-γ-mediated nitric oxide (NO) generation and the upregulation of inducible NO synthase (iNOS) in MG6 cells. 3′,4′,7-Trihydroxyflavone also suppressed LPS- or IFN-γ-mediated phosphorylation of signal transducer and activator of transcription 1 (STAT1), which is crucial for iNOS expression. LPS stimulation induced rapid phosphorylation of c-Jun N-terminal kinase (JNK), p38 mitogen-activated protein kinase (MAPK), and extracellular signal-regulated kinase (ERK) in MG6 cells. 3′,4′,7-Trihydroxyflavone significantly inhibited the LPS-mediated phosphorylation of JNK, but not that of ERK and p38 MAPK. The inhibitory effect of 3′,4′,7-trihydroxyflavone on NO generation was mimicked by pharmacological inhibition of the JNK signaling pathway with SP600125. Furthermore, SP600125 significantly inhibited LPS- or IFN-γ-mediated phosphorylation of STAT1 in MG6 cells. These results suggest that 3′,4′,7-trihydroxyflavone exerts anti-neuroinflammatory effects via inhibition of the JNK-STAT1 pathway in microglia.
Microglia, the tissue resident macrophages of the central nervous system, regulate immune responses and maintain brain homeostasis by clearing various pathogens. However, overactivated microglia produces large amounts of proinflammatory factors including nitric oxide (NO), which induces neuroinflammation.1,2) NO is endogenously produced from L-arginine by inducible NO synthase (iNOS). Recent studies have demonstrated that excessive NO generation in brain microglia is responsible for the pathogenesis of several neurodegenerative disorders such as Alzheimer’s disease (AD), multiple sclerosis, and Parkinson’s disease.2,3) Thus, suppression of proinflammatory factors including NO and iNOS in microglia may be an attractive therapeutic strategy for these disorders.
Overactivated microglia induces activation of multiple signaling pathways such as the signal transducer and activator of transcription 1 (STAT1) and mitogen-activated protein kinase (MAPK) pathways. Recent studies have indicated that STAT1 activation drives microglia-induced neuroinflammatory responses by upregulating the expression of the downstream target, iNOS.2,4–6) Indeed, STAT1 silencing counteracted the upregulation of iNOS expression in microglial cells, demonstrating the key role of STAT1 signaling in microglial inflammatory states.5)
The bacterial endotoxin lipopolysaccharide (LPS) and the cytokine interferon-γ (IFN-γ) are among the most potent microglia-activating factors. These stimulants lead to STAT1 activation, which mediates the expression of iNOS in microglial cells. Therefore, LPS and IFN-γ are widely used to study inflammation in vitro and in vivo.2,7–9) Additionally, previous studies have indicated that the mouse microglial cell line MG6 strongly responds to LPS and/or IFN-γ and retains various primary microglial characteristics.10–13) Thus, MG6 microglial cells have been widely used in a variety of neurotoxicological and immunological studies.
3′,4′,7-Trihydroxyflavone is a bioactive polyphenolic flavonoid isolated from Albizia julibrissin, Butea monosperma, Cissus sicyoides, and Spatholobus suberectus.14–17) These plants have been used in folk medicines to treat a wide range of diseases such as acute or chronic inflammation.14,18–20) Previously, we found that 3′,4′,7-trihydroxyflavone inhibited the formation of toxic aggregates of amyloid β protein (Aβ), which plays an important role in neuronal degeneration in AD.21,22) 3′,4′,7-Trihydroxyflavone also exhibited an anti-apoptotic effect on neuronal cells induced by oxidative stress.23) However, the anti-neuroinflammatory effects of 3′,4′,7-trihydroxyflavone have not been investigated yet. Therefore, in the present study, we explored the anti-neuroinflammatory activities of 3′,4′,7-trihydroxyflavone in LPS- or IFN-γ-activated MG6 microglial cells. Furthermore, we examined the potential underlying mechanisms of 3′,4′,7-trihydroxyflavone including the MAPK and STAT1 signaling pathways.
3′,4′,7-Trihydroxyflavone (T-414) was obtained from Indofine Chemical (NJ, U.S.A.). IFN-γ (I3275) and insulin (I0516) were purchased from Sigma-Aldrich (MO, U.S.A.). AZD1480 (S2162) was obtained from ChemScene (NJ, U.S.A.). Rabbit monoclonal anti-phospho-STAT1 (Tyr701) antibody (7649) and rabbit monoclonal anti-STAT1 antibody (14995) were from Cell Signaling Technology (MA, U.S.A.). The details of the other chemicals and antibodies used in this study have been described in our previous reports.7,24)
Cell CultureMG6 cells (Cell No. RBC2403) were obtained from RIKEN BRC (Tsukuba, Japan). As previously described,25,26) MG6 cells were grown and maintained in Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS), 10 µg/mL insulin, and 100 µM 2-mercaptoethanol (198-15781, Wako, Osaka, Japan) in a CO2 incubator. MG6 cells cultured for 5 d were reseeded in 96-well or 48-well plates at densities of 5 × 104 cells/well or 12.5 × 104 cells/well, respectively. After 1–3 h, the medium was changed to FBS-free DMEM, and 24 h later, the indicated assays were performed.
Griess AssayThe nitrite concentration, an oxidation product of NO, in the culture medium was measured using the Griess reaction. The detailed procedures have been described in our previous reports.7,24)
Western BlottingAs previously described,7,24) polyvinylidene fluoride membranes were blocked with TBS-T (Tris-buffered saline containing 1% Tween 20 and 2% bovine serum albumin) for 1.5 h and then incubated with primary antibodies. The next day, the membranes were rinsed and further incubated with secondary antibodies. The proteins were visualized using an enhanced chemiluminescence prime Western blot detection reagent (12316992, Thermo Fisher Scientific, MA, U.S.A.) and quantitatively analyzed using a LAS-4000 imaging system (FUJIFILM, Tokyo, Japan).
Cell Counting Kit-8 (CCK8) AssayMG6 cells were cultured in FBS-free DMEM with 3′,4′,7-trihydroxyflavone (0–100 µM) for 24 h. Cell toxicity was measured using a Cell Counting Kit-8 (CCK8, CK04, Dojindo, Kumamoto, Japan).
Statistical AnalysisThe data are presented as the mean ± standard error of the mean (S.E.M.) of n independent observations. To compare multiple groups, statistical analyses were performed using one-way ANOVA or Kruskal–Wallis ANOVA on Ranks followed by Tukey’s test, Dunnett’s test, or Steel–Dwass’s test.
To examine whether 3′,4′,7-trihydroxyflavone is cytotoxic, the CCK8 assay was conducted in MG6 cells. At concentrations of 0.1–30 µM, 3′,4′,7-trihydroxyflavone had no effect, but at 100 µM, it remarkably decreased cell viability (Fig. 1B). Therefore, 0.1–10 µM 3′,4′,7-trihydroxyflavone was used in subsequent experiments.
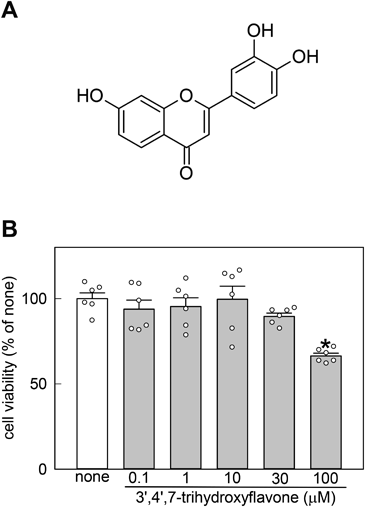
(A) Chemical structure of 3′,4′,7-trihydroxyflavone. (B) MG6 cells were treated with 3′,4′,7-trihydroxyflavone (0.1–100 µM) for 24 h, and then a CCK8 assay was performed (n = 6). Kruskal–Wallis ANOVA on Ranks with Dunnett's test (H = 16.822, p = 0.005). * p < 0.05 vs. untreated cells (none).
To investigate the anti-neuroinflammatory effects of 3′,4′,7-trihydroxyflavone, the nitrite concentration, an NO oxidation product, in the culture medium was measured using the Griess reagent. Stimulation with 100 ng/mL LPS for 48 h induced a significant increase in NO release from MG6 cells (Fig. 2A). The increased NO level was inhibited by 3′,4′,7-trihydroxyflavone in a concertation-dependent manner. 3′,4′,7-Trihydroxyflavone alone did not induce NO generation in MG6 cells (none, 0.53 ± 0.06 µM; 0.1 µM 3′,4′,7-trihydroxyflavone, 0.47 ± 0.13 µM; 1 µM 3′,4′,7-trihydroxyflavone, 0.30 ± 0.15 µM; 10 µM 3′,4′,7-trihydroxyflavone, 0.36 ± 0.08 µM, n = 6; F3, 20 = 0.952, p = 0.434, one-way ANOVA). We also checked the effects of 3′,4′,7-trihydroxyflavone on tumor necrosis factor-α (TNF-α) production in MG6 cells. Stimulation with 100 ng/mL LPS for 24 h induced a significant increase in TNF-α generation, but the increase in TNF-α was significantly inhibited by 3′,4′,7-trihydroxyflavone (none, 0.02 ± 0.05 ng/mL; LPS, 17.44 ± 0.80 ng/mL; 0.1 µM, 14.92 ± 0.66 ng/mL; 1 µM, 15.57 ± 0.67 ng/mL; 10 µM, 9.24 ± 0.76 ng/mL, n = 6; F4, 25 = 119.69, p < 0.001, one-way ANOVA with Tukey’s test). Because LPS stimulation upregulates iNOS expression in microglia, resulting in excessive NO generation, we investigated the effect of 3′,4′,7-trihydroxyflavone on iNOS expression in LPS-activated MG6 cells. Stimulation with 100 ng/mL LPS for 24 h induced a significant increase in iNOS expression, but the increase in iNOS was inhibited by 3′,4′,7-trihydroxyflavone (Fig. 2B).
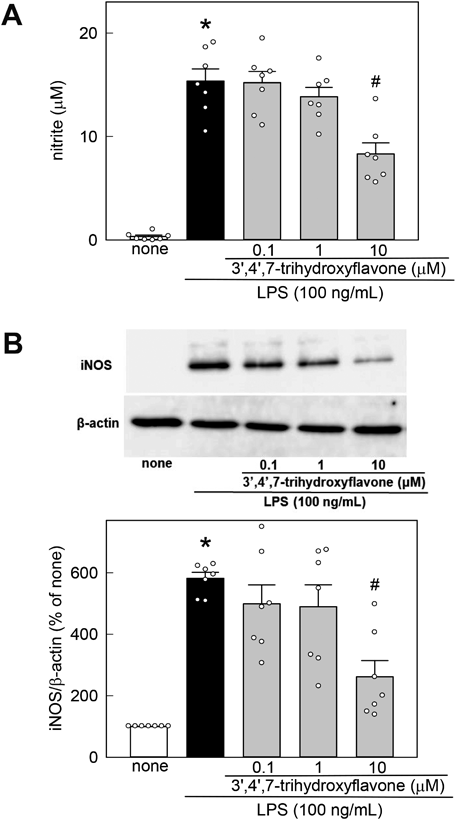
(A) MG6 cells were cotreated with 3′,4′,7-trihydroxyflavone (0.1–10 µM) and LPS (100 ng/mL) for 48 h, and then the Griess assay was performed (n = 7). One-way ANOVA with Tukey’s test (F4, 30 = 45.463, p < 0.001) (B) MG6 cells were cotreated with 3′,4′,7-trihydroxyflavone (0.1–10 µM) and LPS (100 ng/mL) for 24 h, and then Western blotting was performed (n = 7). Kruskal–Wallis ANOVA on Ranks with Steel–Dwass’s test (H = 23.551, p < 0.001). * p < 0.05 vs. untreated cells (none), # p < 0.05 vs. LPS.
Because STAT1 activation plays a key role in the upregulation of iNOS protein in microglia, we investigated the effect of 3′,4′,7-trihydroxyflavone on LPS-mediated STAT1 phosphorylation and total STAT1 upregulation in MG6 cells. We first examined the time course of LPS-mediated changes in phosphorylated STAT1, total STAT1, and β-actin in MG6 cells. The STAT1 phosphorylation level was low 3 h after LPS stimulation, peaked at 6 h, and then decreased (Fig. 3A). The total STAT1 level began to increase 3 h after LPS stimulation and continued to increase until 24 h. In contrast, LPS stimulation did not affect the β-actin level in MG6 cells. Thus, we chose 6 h as the time point to determine whether 3′,4′,7-trihydroxyflavone can inhibit LPS-induced STAT1 activation.

(A) Time course of the changes in the levels of phosphorylated STAT1 (P-STAT1), total STAT1 (T-STAT1), and β-actin after LPS stimulation. (B) MG6 cells were cotreated with 3′,4′,7-trihydroxyflavone (0.1–10 µM) and LPS (100 ng/mL) for 6 h. The expression levels of P-STAT1 and T-STAT1 were detected by Western blotting (n = 6). Kruskal–Wallis ANOVA on Ranks with Steel–Dwass’s test (H = 22.493, p < 0.001). (C) Effects of AZD1480 on LPS-mediated NO generation in MG6 cells. MG6 cells were cotreated with AZD1480 (10 µM) and LPS (100 ng/mL) for 48 h (n = 5). One-way ANOVA with Tukey’s test (F2, 12 = 326.818, p < 0.001). * p < 0.05 vs. untreated cells (none), # p < 0.05 vs. LPS.
Stimulation with 100 ng/mL LPS for 6 h induced a remarkable increase in STAT1 phosphorylation (Fig. 3B). However, 3′,4′,7-trihydroxyflavone inhibited LPS-mediated STAT1 phosphorylation. Additionally, stimulation with LPS for 6 h induced a remarkable increase in the total STAT1 level. The LPS-mediated increase in total STAT1 expression was also inhibited by 3′,4′,7-trihydroxyflavone (none, 100.0 ± 0.00%; LPS, 325.1 ± 9.3%; 0.1 µM, 311.6 ± 18.6%; 1 µM, 318.2 ± 19.3%; 10 µM, 231.4 ± 11.6%, n = 6; H = 21.900, p < 0.001, Kruskal–Wallis ANOVA on Ranks with Steel–Dwass’s test). To examine whether STAT1 activation is required for NO generation, we investigated the effects of AZD1480, a selective inhibitor of the Janus kinase (JAK)/STAT signaling pathway. Because previous studies reported that 10 µM AZD1480 can effectively inhibit STAT1 activation,27,28) we chose this concentration to examine whether AZD1480 can inhibit LPS- or IFN-γ-mediated STAT1 activation in MG6 cells. When MG6 cells were cotreated with 10 µM AZD1480 and 100 ng/mL LPS for 48 h, the LPS-induced increase in NO generation was remarkably suppressed in the presence of AZD1480 (Fig. 3C). AZD1480 alone did not induce NO generation in MG6 cells (none, 0.34 ± 0.09 µM; 10 µM AZD1480, 0.41 ± 0.13 µM, n = 5; t9=−0.447).
Effects of 3′,4′,7-Trihydroxyflavone on LPS-Mediated Activation of MAPKs in MG6 CellsBecause the MAPK signaling pathways are involved in LPS-induced microglial neuroinflammatory responses, we next examined the effects of 3′,4′,7-trihydroxyflavone on LPS-mediated phosphorylation of MAPKs (ERK, JNK, and p38) in MG6 cells. Stimulation with 100 ng/mL LPS for 0.5 h induced phosphorylation of JNK, p38, and ERK (Fig. 4). 3′,4′,7-Trihydroxyflavone significantly inhibited LPS-mediated phosphorylation of JNK (Fig. 4A), but not that of p38 or ERK (Figs. 4B, C). The total protein levels of JNK, p38, and ERK were not affected by any of the treatments tested here.

MG6 cells were cotreated with 3′,4′,7-trihydroxyflavone (0.1–10 µM) and LPS (100 ng/mL) for 0.5 h, and then Western blotting was performed (A: JNK; n = 6, B: p38 MAPK; n = 4, C: ERK; n = 4). Kruskal–Wallis ANOVA on Ranks with Steel–Dwass’s test; (A: H = 20.577, p < 0.001; B: H = 10.522, p = 0.032; C: H = 9.255, p = 0.055). * p < 0.05 vs. untreated cells (none), # p < 0.05 vs. LPS.
To examine whether JNK activation is required for NO generation, the effects of the selective JNK inhibitor SP600125 were determined. As shown in Fig. 5A, LPS-mediated NO generation was significantly suppressed by SP600125. In this study, 30 µM SP600125 showed a robust and significant effect in inhibiting NO generation, and previous studies have reported that 25 or 50 µM SP600125 can effectively inhibit STAT1 activation.29,30) Therefore, we chose 30 µM SP600125 to examine whether it can inhibit LPS-mediated STAT1 activation. Because JNK has been demonstrated to be involved in the activation of STAT1 signaling,29) we examined the effects of pharmacological blockage of JNK signaling on LPS-mediated STAT1 activation in MG6 cells. SP600125 (30 µM) significantly suppressed LPS-mediated STAT1 phosphorylation (Fig. 5B) and upregulation of the total STAT level (none, 100.0 ± 0.00%; LPS, 192.6 ± 12.3%; SP600125, 101.7 ± 11.6%, n = 5; H = 9.852, p = 0.007, Kruskal–Wallis ANOVA on Ranks with Steel–Dwass’s test).

(A) Effects of the JNK inhibitor SP600125 on LPS-mediated NO generation. MG6 cells were cotreated with SP600125 (1–30 µM) and LPS (100 ng/mL) for 48 h (n = 6). One-way ANOVA with Tukey’s test (F4, 25 = 64.648, p < 0.001). (B) Effects of SP600125 on LPS-mediated STAT1 activation. MG6 cells were cotreated with SP600125 (30 µM) and LPS (100 ng/mL) for 6 h. The expression levels of P-STAT1 and T-STAT1 were detected by Western blotting (n = 6). Kruskal–Wallis ANOVA on Ranks with Steel–Dwass’s test (H = 13.542, p = 0.001). * p < 0.05 vs. untreated cells (none), # p < 0.05 vs. LPS.
To examine whether the anti-inflammatory effects of 3′,4′,7-trihydroxyflavone are specific for LPS stimulation, we investigated the effects of 3′,4′,7-trihydroxyflavone on microglial NO production induced by IFN-γ. Stimulation with 100 ng/mL IFN-γ for 48 or 24 h induced a significant increase in NO generation and iNOS expression in MG6 cells, respectively (Fig. 6). The increased NO release and iNOS expression were inhibited by 3′,4′,7-trihydroxyflavone.

(A) MG6 cells were cotreated with 3′,4′,7-trihydroxyflavone (0.1–10 µM) and IFN-γ (100 ng/mL) for 48 h, and then the Griess assay was performed (n = 10). One-way ANOVA with Tukey's test (F4, 45 = 43.062, p < 0.001). (B) MG6 cells were cotreated with 3′,4′,7-trihydroxyflavone (0.1–10 µM) and IFN-γ (100 ng/mL) for 24 h, and then Western blotting was performed (n = 6). Kruskal–Wallis ANOVA on Ranks with Steel–Dwass’s test (H = 22.736, p < 0.001). * p < 0.05 vs. untreated cells (none), # p < 0.05 vs. IFN-γ.
We next examined the effects of 3′,4′,7-trihydroxyflavone on IFN-γ-induced STAT1 activation in MG6 cells. As shown in Fig. 7A, the time course of the IFN-γ-induced changes in phosphorylated STAT1 and total STAT1 levels was similar to that observed in the LPS experiments. Thus, we chose 6 h to examine whether 3′,4′,7-trihydroxyflavone can inhibit the IFN-γ-induced STAT1 activation. Stimulation with IFN-γ for 6 h induced a remarkable increase in phosphorylated STAT1, whereas 3′,4′,7-trihydroxyflavone suppressed IFN-γ-induced STAT1 phosphorylation in a concertation-dependent manner (Fig. 7B). In contrast, 3′,4′,7-trihydroxyflavone did not suppress the IFN-γ-induced increase in the total STAT1 level (none, 100.0 ± 0.00%; IFN-γ, 240.7 ± 45.2%; 0.1 µM, 250.5 ± 45.1%; 1 µM, 244.6 ± 43.3%; 10 µM, 231.3 ± 45.5%, n = 4; H = 9.860, p = 0.043, Kruskal–Wallis ANOVA on Ranks with Steel–Dwass’s test). Finally, we examined the effect of the selective inhibitor of the JAK/STAT pathway, AZD1480, on IFN-γ-induced NO release. As shown in Fig. 7C, AZD1480 (10 µM) significantly inhibited the IFN-γ-induced NO release in MG6 cells.
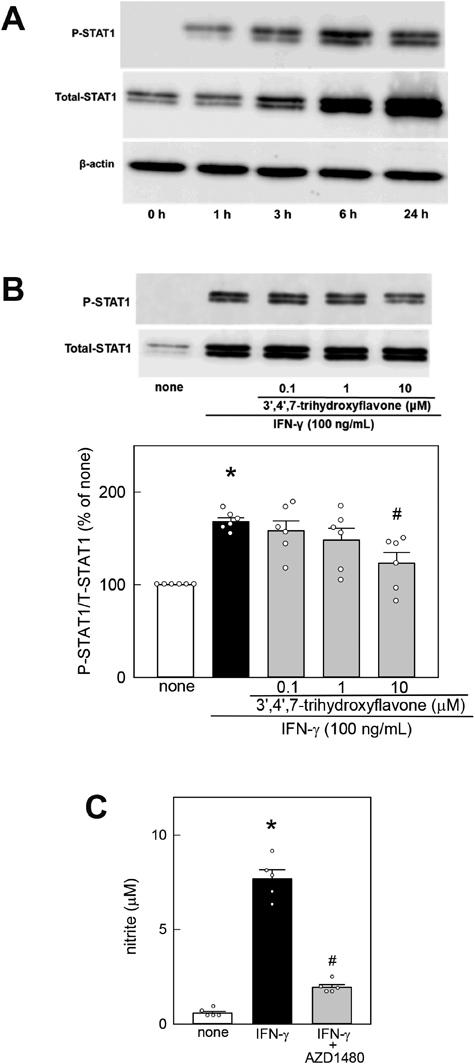
(A) Time course of the changes in the levels of P-STAT1, T-STAT1, and β-actin after IFN-γ stimulation. (B) MG6 cells were cotreated with 3′,4′,7-trihydroxyflavone (0.1–10 µM) and IFN-γ (100 ng/mL) for 6 h. The expression levels of P-STAT1 and T-STAT1 were detected by Western blotting (n = 6). Kruskal–Wallis ANOVA on Ranks with Steel-Dwass’s test (H = 17.482, p = 0.002). (C) Effects of AZD1480 on the IFN-γ-mediated NO generation in MG6 cells. MG6 cells were cotreated with AZD1480 (10 µM) and IFN-γ (100 ng/mL) for 48 h (n = 5). One-way ANOVA with Tukey’s test (F2, 12 = 162.643, p < 0.001). * p < 0.05 vs. untreated cells (none), # p < 0.05 vs. IFN-γ.
Because JNK has been demonstrated to be involved in the activation of STAT1 signaling,29) we also examined the effects of pharmacological blockage of JNK signaling on IFN-γ-mediated NO generation and STAT1 activation in MG6 cells. As shown in Fig. 8A, IFN-γ-mediated NO generation was significantly suppressed by SP600125. SP600125 (30 µM) significantly suppressed IFN-γ-mediated STAT1 phosphorylation without affecting the total STAT level (Fig. 8B).

(A) Effects of the JNK inhibitor SP600125 on IFN-γ-mediated NO generation. MG6 cells were cotreated with SP600125 (1–30 µM) and IFN-γ (100 ng/mL) for 48 h (n = 6). One-way ANOVA with Tukey's test (F4, 25 = 161.801, p < 0.001). (B) Effects of SP600125 on IFN-γ-mediated STAT1 activation. MG6 cells were cotreated with SP600125 (30 µM) and IFN-γ (100 ng/mL) for 6 h. The expression levels of P-STAT1 and T-STAT1 were detected by Western blotting (n = 5). Kruskal–Wallis ANOVA on Ranks with Steel–Dwass’s test (H = 12.963, p = 0.002). * p < 0.05 vs. untreated cells (none), # p < 0.05 vs. IFN-γ.
The current study demonstrated that 3′,4′,7-trihydroxyflavone, a natural flavonoid, attenuated LPS- and IFN-γ-mediated NO generation and iNOS expression in MG6 microglial cells (Figs. 2, 6). Microglial overactivation can cause neuroinflammatory responses. NO is synthesized by iNOS from L-arginine and plays a key role in normal immune functions, but excessive NO generation causes brain damage, which eventually leads to AD, multiple sclerosis, and Parkinson’s disease.1,31–33) Therefore, the inhibitory effect of 3′,4′,7-trihydroxyflavone on microglial NO generation may be useful for therapy for these neurodegenerative diseases. Additionally, a previous study reported that 3′,4′,7-trihydroxyflavone inhibited H2O2-induced neuronal apoptosis by attenuating oxidative stress.23) We have also found that 3′,4′,7-trihydroxyflavone inhibits the aggregation of Aβ, which plays a key role in neuronal degeneration in AD.21,22) In this study, we found that 3′,4′,7-trihydroxyflavone attenuated LPS-mediated TNF-α generation in MG6 cells. Thus, the biological activities of this flavonoid may be therapeutic resources for the prevention and/or treatment of AD.
STAT1 and MAPKs (JNK, p38, ERK) have been implicated in iNOS and proinflammatory cytokine expression in microglia.3–7,24) Phosphorylated STAT1 forms homodimers that translocate to the nucleus. STAT1 and concomitantly induced molecules such as IFN regulatory factor-1 regulate gene expression at the transcriptional level. In the present study, 3′,4′,7-trihydroxyflavone suppressed LPS-induced STAT1 phosphorylation and total STAT1 upregulation (Fig. 3) and selectively suppressed the LPS-mediated activation of JNK, but not that of p38 and ERK (Fig. 4). Furthermore, pharmacological inhibition of JNK signaling attenuated LPS-induced NO release, STAT1 phosphorylation, and total STAT1 upregulation (Fig. 5). Involvement of JNK signaling in the regulation of STAT1 activation has been reported in microglia, RAW 264.7 cells, and fibroblasts.29,30,34) These studies have shown that LPS activates the JNK pathway, which is linked to the STAT1 pathway, resulting in iNOS expression. Therefore, we concluded that 3′,4′,7-trihydroxyflavone suppresses LPS-mediated iNOS expression via inhibition of the JNK-STAT1 pathway in activated microglia.
Recently, Kim et al.35) reported that the structurally similar isoflavonoid 3′,4′,7-trihydroxyisoflavone, found in soybean foods, suppressed LPS-mediated inflammatory responses by inhibiting the ERK and JNK pathways in BV2 microglial cells. This isoflavonoid partly attenuated JNK activation at 50 µM but showed no significant effects at 10–25 µM. 3′,4′,7-Trihydroxyflavone is likely to exhibit better anti-neuroinflammatory activities than the isoflavonoid.
IFN-γ is a cytokine primarily produced by cytotoxic T lymphocytes, natural killer cells, and T helper type 1 cells and plays an essential role in normal immune functions.8,36) IFN-γ is also expressed in the brain and activates microglia. Elevated IFN-γ levels have been reported in several neurological disorders including AD.37,38) It has been shown that iNOS expression mediated by IFN-γ is regulated by the STAT1 pathway.39,40) The current study also demonstrated that 3′,4′,7-trihydroxyflavone attenuated IFN-γ-mediated NO generation and iNOS expression in MG6 cells (Fig. 6). 3′,4′,7-Trihydroxyflavone suppressed IFN-γ-induced STAT1 phosphorylation but not total STAT1 upregulation (Fig. 7B). The selective JAK/STAT signaling pathway inhibitor AZD1480 attenuated IFN-γ-induced NO release (Fig. 7C). Furthermore, pharmacological inhibition of JNK signaling attenuated IFN-γ-induced NO release and STAT1 phosphorylation without affecting the total STAT level (Fig. 8). Taken together, 3′,4′,7-trihydroxyflavone attenuated IFN-γ-induced iNOS expression via inhibition of the JNK-STAT1 pathway in activated microglia.
The exact mechanisms by which 3′,4′,7-trihydroxyflavone attenuated LPS- and IFN-γ-induced STAT1 phosphorylation remain to be elucidated. Previous docking studies reported by Baek et al.41) and Jnawali et al.42) revealed that the plant flavonoids rhamnetin and quercetagetin formed hydrogen bonds with Lys55 and Asp169 at the ATP-binding site of JNK1 via the 3′-and 4′-hydroxy groups of the B-ring. Thus, 3′,4′,7-trihydroxyflavone might directly bind to JNK1 through its hydroxyl arms. In addition, previous studies have shown that several pathways other than JNK-STAT1 are also involved in LPS- and IFN-γ-mediated neuroinflammatory responses. For example, the transcription factor nuclear factor-κB (NF-κB) is considered a major molecule associated with the inflammatory process in microglia.3,7) Signal transduction crosstalk among NF-κB, STAT1, and MAPKs has also been identified in homeostatic and chronic inflammatory conditions.43–45) Therefore, 3′,4′,7-trihydroxyflavone might attenuate LPS- and IFN-γ-induced NF-κB activation. Further investigation is required to identify the target molecule(s) of 3′,4′,7-trihydroxyflavone that are involved in its anti-neuroinflammatory effect.
The current findings demonstrated that the natural flavonoid 3′,4′,7-trihydroxyflavone exerts anti-inflammatory effects through inhibition of the STAT1 pathway in microglial cells. Considering 3′,4′,7-trihydroxyflavone has been found to protect nerve cells from oxidative stress-mediated apoptosis and to inhibit the aggregation of Aβ, this compound may be an attractive agent for preventing AD.
This work was supported by a Grant-in-Aid for Young Scientists from the Japan Society for the Promotion of Science (26860358) and the Takeda Science Foundation awarded to T.A.
We thank Michal Bell, Ph. D., and Lisa Kreiner, Ph. D., for editing a draft of this manuscript.
The authors declare no conflict of interest.