2022 Volume 45 Issue 3 Pages 378-381
2022 Volume 45 Issue 3 Pages 378-381
Enterococcus avium, producing 5R-hexahydrocurcumin metabolized tetrahydrocurcumin to octahydrocurcumin in vitro. Based on a detailed analysis of the two secondary alcohols, the metabolite obtained from tetrahydrocurcumin via 5R-hexahydrocurcumin was identified as 3R,5R-octahydrocurcumin. The activities of 5R-hexahydrocurcumin and 3R,5R-octahydrocurcumin were compared to those of the synthetic compounds, using monocyte chemoattractant protein-1 produced via murine adipocytes in vitro. The optically active curcuminoids reduced the cytokine production similar to tetrahydrocurcumin without any difference in their stereochemistry.
Curcumin (1, Fig. 1), a well-known yellowish ingredient of the rhizomatous herbaceous perennial plant turmeric (Curcuma longa) of the ginger family, has received immense attention owing to its use in various aspects,1) especially its therapeutic value in fighting coronavirus disease 2019 (COVID-19), as recently reported.2,3) Unfortunately, the in vivo activity of curcumin is limited by its low bioavailability.4,5) Therefore, the identification of active metabolites in vivo has attracted considerable interest. Recent studies have described some biologically active curcumin metabolites, such as glucuronide6) and an oxidative metabolite.7) However, reduced products, such as tetrahydrocurcumin (THC: 3, Fig. 1), hexahydrocurcumin (HHC), and octahydrocurcumin (OHC), which have been detected in vivo,8–10) have played important functions in the human body. Considers the HHC structure, the metabolite is expected to exist as an enantiomers: however, no reports have described the stereochemistry of this curcumin metabolite produced in vivo. We recently isolated a human intestinal bacterium that produced 5R-HHC (4, Fig. 1) from THC in vitro.11) This bacterium named 2a1-2b, was identified as Enterococcus avium according to its 16S ribosomal DNA (rDNA) sequence.

Originally, 2a1-2b strain was obtained from the human intestinal bacterial fraction 1C, producing kazuranol (2, Fig. 1) from curcumin.12) To study the metabolic pathway between 5R-HHC (4) and kazuranol (2), we aimed to identify the HHC-metabolizing bacterium.
Curcumin was obtained from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). THC was purchased from Sigma (St. Louis, MO, U.S.A.). NaBH4 was obtained from Kishida Chemical Co., Ltd. (Osaka, Japan). HHC was prepared from THC using NaBH4 in ethanol (EtOH) with TLC monitoring,13) whereas OHC was obtained after completion of the reaction.14) Gifu Anaerobic Medium (GAM) was a product of Nissui Pharmaceutical Co., Ltd. (Tokyo, Japan).
HPLC Analysis of HHC and OHCHHC and OHC were analyzed using an HPLC system equipped with a 250 × 4.6 mm i.d. Wakosil-II 5C18HG column (Wako Pure Chemical Corporation, Osaka, Japan) and an UV detector (280 nm) by eluting with a solvent mixture of aq. 0.1% acetic acid (AcOH)/acetonitrile (MeCN) (8 : 2) at a flow rate of 1.0 mL/min at 40 °C.
Separations of OHC Enantiomers by HPLCThe OHC enantiomers were separated using the same method described previously11) with slight modifications. Briefly, samples were subjected to HPLC using a CHIRALPAK IF-3 column (i.d. 4.6 × 250 mm: Daicel Chemical Industries Ltd., Osaka, Japan) eluted with a solvent mixture of aq. 0.1% AcOH/MeCN (75 : 25) at a flow rate 0.8 mL/min at 30 °C with UV monitoring at 280 nm.
Synthesis and Isolation of OHCsBriefly, THC (37 mg) was treated with NaBH4 (6.0 mg).14) Then, the product was purified using preparative HPLC on a 250 × 20 mm i.d. Wakosil-II 5C18HG column eluted with 20% MeCN at a flow rate of 6.0 mL/min at ambient temperature affording raceme OHC (9.5 mg) and meso-OHC (15.3 mg).
Isolation of 2a1-2b-Producing OHCThis bacterial product was isolated using a previously mentioned method,11) except with a longer incubation time. Briefly, the bacterium was incubated in 9.0 L of GAM containing THC (100 μM) for nine days under anaerobic static conditions at 37 °C. Then, the ethyl acetate (EtOAc) extract was applied to two Merck 1 mm-thick TLC plates and eluted with a solvent mixture containing hexane/EtOAc (1 : 2). Finally, the bacterial OHC (17.2 mg) was purified using preparative HPLC as described above.
Monocyte Chemoattractant Protein-1 (MCP-1) Reducing Activity of Curcuminoid in VitroExperiments were conducted in a similar manner as described previously.12) Briefly, 3T3-L1 cells (Japanese Collection of Research Bioresources Cell Bank, Osaka, Japan) were seeded at 105 cells/well in a 24-well plate and differentiated to adipocytes. Fresh media containing the samples were applied to the cells and incubated for 24 or 27 h. The concentration of MCP-1 in the medium was measured using a mouse CCL2 enzyme-linked immunosorbent assay (ELISA) Kit (R&D Systems; Minneapolis, MN, U.S.A.) according to the manufacturer’s instructions, except for using 80 μL of samples. Because of the cell growth, the activities were compared by analyzing the results obtained from a single 24-well plate for each examination.
The following equation was used to determine cell viability:
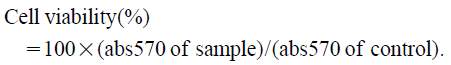 |
Each analysis was performed in triplicate, and results are reported as mean ± standard deviation. Statistical significance was calculated by Dunnett’s tests comparing to vehicle (EtOH) to show their activity. p-Values of <0.05 were considered statistically significant.
Previously, we isolated the 5R-HHC-producing bacterium strain 2a1-2b from human feces and identified it as E. avium based on the 16S rDNA sequence.11) Considering that 5R-HHC (4) might be an intermediate to kazuranol (2),11) we tried to identify an HHC-metabolizing bacterium using HHC as the substrate. The test results indicated that 2a1-2b, which produced 5R-HHC from THC, metabolized HHC to OHC. Moreover, strain 2a1-2b produced OHC from THC. Although 2a1-2b produced 5R-HHC, the stereochemistry of OHC is still unclear. Owing to two secondary alcohols, OHC has three possible structures, i.e., 3R,5R-OHC (5), 3S,5S-OHC, and the meso-OHC (6, Fig. 1). Meso-OHC can be separated from the optically active OHCs using chromatographic methods, as described by Maehara et al.15) We found that the product prepared from THC and NaBH4 exhibited two peaks in the HPLC analysis, an m/z of 375 [M − H]− for each peak in the LC-MS analysis using the 3200 QTRAP (AB Sciex, Framingham, MA, U.S.A.). Using an IF-3 column, this sample exhibited three peaks in the HPLC analysis (Fig. 2a). To identify the signals shown in Fig. 2a, we isolated raceme OHC and meso-OHC using a preparative octadecyl silica (ODS) column and measured the 1H-NMR spectra. The NMR data were comparable with those of a study reported in a study on 199116) rather than those in a more recent report.17) The raceme OHC showed two equivalent signals on the chromatogram, obtained using the IF-3 column (Fig. 2b), confirming the OHC structures. The three OHC isomers were separated via two HPLC separations, and the OHC produced by 2a1-2b was analyzed. Thus, the bacterium was incubated in a 1.0-L GAM containing 100 μM THC for seven days. The crude EtOAc extract of the culture media was first analyzed using an ODS column. The extract contained 10.8-mg HHC and 2.9-mg OHC; however, meso-OHC was present in a small amount (diastereomeric excess, >90%). Then, the biosynthetic OHC was isolated as described and confirmed via 1H-NMR spectroscopy. These results suggested that the bacterial OHC predominantly comprised 3R,5R- or 3S,5S-OHC. However, this OHC might be produced via 5R-HHC as 2a1-2b produced 5R-HHC from THC.11) Therefore, the product has at least one center with R-stereochemistry, highlighting 3R,5R as the absolute stereochemistry of the biosynthetic OHC. Indeed, the isolated bacterial OHC exhibited high optical activity upon chiral HPLC separation using the IF-3 column (Fig. 2c). To confirm the metabolite structure, 5R-HHC (4)11) was treated with NaBH4, and the product was subjected to chiral separation. This synthetic product, containing 3R,5R-OHC (5) and meso-OHC (6), delivered two peaks on the chromatogram (Fig. 2d), one of which agreed well with the bacterial metabolite. As concluded from Fig. 2, 2a1-2b produced 3R,5R-OHC from THC via 5R-HHC. The structure was also supported by the optical rotation ([α]D25=+9.7° (c 0.1, EtOH)) as compared to the data concerning 3S,5S-OHC.15–17)

Samples were subjected to HPLC using a CHIRALPAK IF-3 column (i.d. 4.6 × 250 mm) eluted with a solvent mixture of aq. 0.1% acetic acid/acetonitrile (75 : 25) at a flow rate of 0.8 mL/min at 30 °C.
The stereochemistry of HHC and OHC in vivo remains unclear. However, our results suggested that optically active HHC and OHC are produced by a human intestinal bacterium in vitro. Therefore, identifying the optically active HHC and OHC is important to reveal their biological activities. As we could not identify the 5S-HHC-producing bacterium, 5R-HHC was compared with the racemate via measuring the MCP-1 reducing activity in murine adipocytes in vitro. In our previous study, curcumin showed cytotoxicity at 100 μM12); in this study, we compared the activity with that of THC, which showed no toxicity, and thus, used as a substrate in this study. Similar to THC, HHCs showed no cytotoxicity at 100 μM (Fig. 3a). Thus, the MCP-1 production was tested at 50 and 100 μM. As a result, all three samples reduced MCP-1 production more than 50% comparing to vehicle (EtOH) at 100 μM. However, we could not find a critical difference among the three curcumin metabolites (Fig. 3b). Regarding the stereochemistry, we found that synthetic HHC tended to show more potent activity than the bacterial product.

3T3-L1 cells were differentiated to adipocytes in a 24-well plate as described previously.12) Fresh media containing samples were applied to the cells and incubated for 24 h. (a) Cell viability was evaluated by the MTT assay testing. (b) MCP-1 secretion. Data are represented as the mean ± standard deviation of three experiments. * p < 0.05 vs. vehicle (ethanol; EtOH), ** p < 0.01 vs. vehicle (EtOH).
Several OHC activities have been described, including MCP-1 reduction in RAW264.7 macrophages.18) However, the stereochemistry of the sample used was ambiguous although meso-OHC might be separated by HPLC. In this study, we tackled this issue by evaluating MCP-1 using synthetic and bacterial OHCs. First, 3R,5R-OHC was compared with the two synthetic OHCs. We observed no toxicity for any of the tested OHCs, even at 100 μM, via the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method (Fig. 4a) or microscopic observation (data not presented), as reported.18) Among the three samples, meso-OHC was not sufficient to show statistical significance even at 100 μM (Fig. 4b) comparing to vehicle (EtOH). Although further tests are required, meso-OHC can have unfavorable conformation owing to the 1,3-diol structure. No evident difference was observed between 3R,5R-OHC and racemate (Fig. 4b). However, raceme OHC was slightly more active than the bacterial product, similar to HHC (Fig. 3). Finally, raceme HHC and OHC were compared with curcumin at 50 μM because curcumin exhibited cytotoxicity at 100 μM.12) Under this condition, we could not find any critical difference between curcumin and OHC (Fig. 4c). Based on these results, it can be concluded that curcumin, THC, HHC, and OHC, showed similar activities, and no difference could be attributed to their stereochemistry (Figs. 3, 4). However, this result might depend on the assay.19,20) Therefore, the evaluation of curcumin metabolites must be performed by considering their stereochemistry for a precise conclusion.

Data were collected as described in Fig. 3. (a) Cell viability of octahydrocurcumins (OHC). (b, c) MCP-1 secretion. Data are represented as the mean ± standard deviation of three experiments. * p < 0.05 vs. vehicle (EtOH), ** p < 0.01 vs. vehicle (EtOH).
This research was supported by the Japan Food Chemical Research Foundation and Urakami Foundation for Food and Food Culture Promotion. Support from The Food Science Institute Foundation (Ryoushoku-kenkyukai) is gratefully acknowledged.
The authors declare no conflict of interest.