Abstract
Cancer immunotherapies are powerful therapeutic options for cancer patients. To enhance the therapeutic effects of cancer immunotherapies, we plan to develop novel immunostimulatory drugs for use in combination with cancer immunotherapy. In the present study, we focused on tetracyclines, the effects of which are controversial for immunotherapy. We examined the effects of tetracyclines on human T cells in the peripheral blood of healthy donors and the tumor tissues of non-small cell lung cancer (NSCLC) patients. By using bispecific T-cell engager technology to assess the cytotoxicity of peripheral T cells against tumor cells, we showed that tetracyclines (minocycline, tetracycline, doxycycline, meclocycline, chlortetracycline, and demeclocycline) enhanced T-cell cytotoxicity through granzyme B expression and CD4+ and CD8+ T-cell proliferation. In analyses of the peripheral blood mononuclear cells (PBMCs) and lung tumor-infiltrated cells of NSCLC patients, we found that demeclocycline enhanced T-cell cytotoxicity not only in PBMCs, but also in lung tumor tissues. These results support the further application of tetracyclines to combination cancer immunotherapy.
INTRODUCTION
Tetracyclines, widely utilized as bacteriostatic antibiotics, have been reported to exert immunomodulatory effects.1) For example, tetracyclines were previously shown to inhibit T-cell activation and proliferation.2–6) However, the concentrations of tetracyclines used in these studies were markedly higher, in many cases more than 10-fold higher, than those in clinical use.7) Furthermore, tetracyclines may induce immune disorders as adverse events.8) These findings imply the immunostimulatory properties of tetracyclines.
To assess the ability of tetracyclines to enhance anti-tumor T-cell responses, we herein utilized ex vivo models for human T cells. We previously developed an ex vivo assay system using a bispecific T-cell engager (BiTE), which redirects human T cells to tumor cells.9–11) The pattern of T-cell cytotoxicity induced by BiTE showed some similarities to tumor cell killing by endogenous tumor antigen-specific T cells.12) In contrast to immune checkpoint inhibitors (ICIs), BiTE directly enhances T-cell cytotoxicity in tumor cells by engaging CD3 on T cells and the specific antigen on tumor cells.13) Blinatumomab, an anti-CD19/CD3 BiTE, is clinically used to treat CD19-positive hematological malignancies.14) It has several limitations, including a short half-life and missing co-stimulation, such as CD28. Therefore, combination therapies with blinatumomab to enhance anti-tumor T-cell activities are desired for better clinical outcomes.
In the present study, we examined the abilities of tetracyclines to enhance anti-tumor T-cell responses induced by BiTE. We also focused on demeclocycline, which is used as an antibiotic less frequently than other tetracyclines, such as minocycline. T-cell cytotoxicity in the lung tumor tissues of non-small cell lung cancer (NSCLC) patients was evaluated. By using BiTE in these analyses, we aimed to investigate the abilities of tetracyclines to enhance human T-cell responses resembling endogenous cytotoxic T-cell activities and increase the therapeutic effects of BiTE as combination cancer immunotherapy.
MATERIALS AND METHODS
Sample PreparationPeripheral blood mononuclear cells (PBMCs) were isolated from peripheral blood by gradient density centrifugation using Lymphoprep (Axis Shield, Dundee, U.K.), and this was followed by a T-cell cytotoxicity analysis.
The surgically resected fresh tumors of NSCLC patients were minced in a 6-cm dish and digested to a single cell suspension using a Tumor Dissociation Kit for humans (Miltenyi Biotec, Bergisch Gladbach, Germany) and gentleMACS Dissociator (Miltenyi Biotec) according to the manufacturer’s instructions. The cell suspension was applied to a 70-µm nylon cell strainer (BD Biosciences, Franklin Lakes, NJ, U.S.A.) with the lysis of red blood cells by BD Pharm lyse. Dead cells and debris were removed by centrifugation in isodensity Percoll solution (Pharmacia Biotech, Uppsala, Sweden), followed by FACS and T-cell cytotoxicity analyses. These methods were performed as described in our previous study.9,10,15)
Construction of the EphA2-Specific T-Cell EngagerThe construction of the EphA2-specific engager containing EphA2-specific scFv 4H5, a short serine (S)-glycine (G) linker, and CD3-specific scFv derived from OKT3 is described elsewhere.16) Specifically, the EphA2-specific engager consists of the 4H5 heavy-chain, a G-S linker [(G4S)3], the 4H5 light-chain, a short G4S linker, the OKT3 heavy-chain, a (G4S)3 linker, and the OKT3 light-chain. A 6 × His-Myc tag was inserted at the C terminus before the stop codon. This recombinant protein was custom-made by Thermo Fisher Scientific (Waltham, MA, U.S.A.). Briefly, EphA2-specific engager DNA was synthesized and subcloned into the pcDNA3.3 vector. This plasmid vector was transfected into Expi293™ cells. After the culturing of cells, the protein was purified from the culture supernatant using His-tag affinity chromatography. These methods were performed as described in our previous study.9,10)
Chemical ReagentsDemeclocycline hydrochloride was purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). Chlortetracycline hydrochloride, doxycycline hydrochloride, meclocycline sulfosalicylate, minocycline hydrochloride, and tetracycline hydrochloride were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). Entinostat and olaparib were purchased from Chem Scene (Monmouth Junction, NJ, U.S.A.).
T-Cell Cytotoxicity AnalysisThe U251 cell line was kindly provided by Dr. Yasuko Mori (Kobe University, Japan). Cell line authentication by short tandem repeat profiling and mycoplasma testing were performed by the JCRB Cell Bank. U251 cells were plated on 96-well flat-bottomed cell culture plates (Corning, Corning, NY, U.S.A.) at a density of 1 × 104 cells per well with RPMI medium 1640 (Nacalai Tesque, Kyoto, Japan) containing 10% fetal bovine serum (FBS; HyClone, Thermo Scientific). After 24 h, 5 × 104 PBMCs or freshly isolated cells from normal lung or tumor tissues were added to plates with 100 ng/mL of EphA2/CD3 BiTE ± tetracyclines. After a co-culture, culture supernatants were cryopreserved for interferon γ (IFNγ) and interleukin (IL)-2 enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN, U.S.A.), non-adherent cells were removed by gentle washing four times with RPMI medium 1640 containing 10% FBS, and the remaining adherent viable cells were detected using the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay (CellTiter 96 aqueous one solution cell proliferation assay; Promega, Madison, WI, U.S.A.). The assay was performed in triplicate. The calculation of EphA2/CD3 BiTE-mediated killing was based on the degree of the reduction in viable target cells using the following formula:
Each treated well consisted of 1 × 104 U251 cells and 5 × 104 PBMCs, isolated CD4+ or CD8+ T cells from PBMCs, or freshly isolated cells from normal lung or tumor tissues with 100 ng/mL of EphA2/CD3 BiTE ± tetracyclines. CD4+ or CD8+ T cells were isolated from PBMCs using the CD4+ or CD8+ T Cell Isolation Kit (Miltenyi Biotec) according to the manufacturer’s instructions. Each non-treated well consisted of 1 × 104 U251 cells and 5 × 104 PBMCs, isolated CD4+ or CD8+ T-cells from PBMCs, or freshly isolated cells from normal lung or tumor tissues without EphA2/CD3 BiTE. These methods were performed as described in our previous study.9,10)
Collected non-adherent cells were analyzed by granzyme B staining and the CellTrace assay.
Flow Cytometric AnalysisSurface marker staining was performed after the FcR block using Human TruStain FcX Fc Receptor blocking solution (BioLegend, San Diego, CA, U.S.A.). Cells were incubated with the Zombie NIR Fixable Viability Kit (BioLegend). Surface marker-stained cells were analyzed on BD LSRFortessa with FACSDiva software (BD Biosciences). The following antibodies were used for FACS staining: anti-CD45RA-FITC (clone HI100), anti-CD25-PE (BC96), anti-4-1BB-BV421 (4B4-1), anti-CD8-BV786 (RPA-T8), anti-CD8-BV510 (RPA-T8), anti-CD103-BV605 (Ber-ACT8), anti-CD4-BV711 (OKT4), anti-Tim-3-APC (F38-2E2), anti-CD3-Alexa Fluor 700 (UCHT1), anti-CD45-BV786 (HI30), and immunoglobulin G1 (IgG1) isotype control (MOPC-21) purchased from BioLegend. Anti-ICOS-PerCP eFluor 710 (ISA-3) and IgG1 isotype control (P3.6.2.8.1) were purchased from eBioscience (San Diego, CA, U.S.A.). Anti-OX-40-PE-CF594 (ACT35) and anti-PD-1-PE Cy7 (EH12.1) were purchased from BD Bioscience.
Regarding granzyme B staining, cells were washed, fixed, and permeabilized with Cytofix/Cytoperm solution (BD Bioscience) at 4 °C for 30 min, followed by FACS staining using an anti-granzyme B antibody (clone GB11, BioLegend) (Supplementary Fig. 1).
CellTrace AssayU251 cells were plated on 96-well flat-bottomed cell culture plates (Corning) at a density of 1 × 104 cells per well with RPMI medium 1640 (Nacalai Tesque) containing 10% FBS (HyClone, Thermo Scientific). After 24 h, 5 × 104 PBMCs labeled with CellTrace Violet (Thermo Fisher Scientific) were added to plates with 100 ng/mL of EphA2/CD3 BiTE ± tetracyclines. After a co-culture, cell proliferation was evaluated by flow cytometry (Supplementary Fig. 2).
Statistical AnalysisA paired two-tailed Student’s t-test was used to examine the significance of differences between samples. A one-way ANOVA with Dunnett’s post hoc test was employed for multiple comparisons to compare differences with respective values for the control, with a p value <0.05 indicating a significant difference.
Study ApprovalThe present study was conducted according to the principles of the Declaration of Helsinki. The study protocol was approved by the Osaka University Hospital Ethics Committee, and written informed consent was obtained from participants prior to their inclusion in the study.
RESULTS
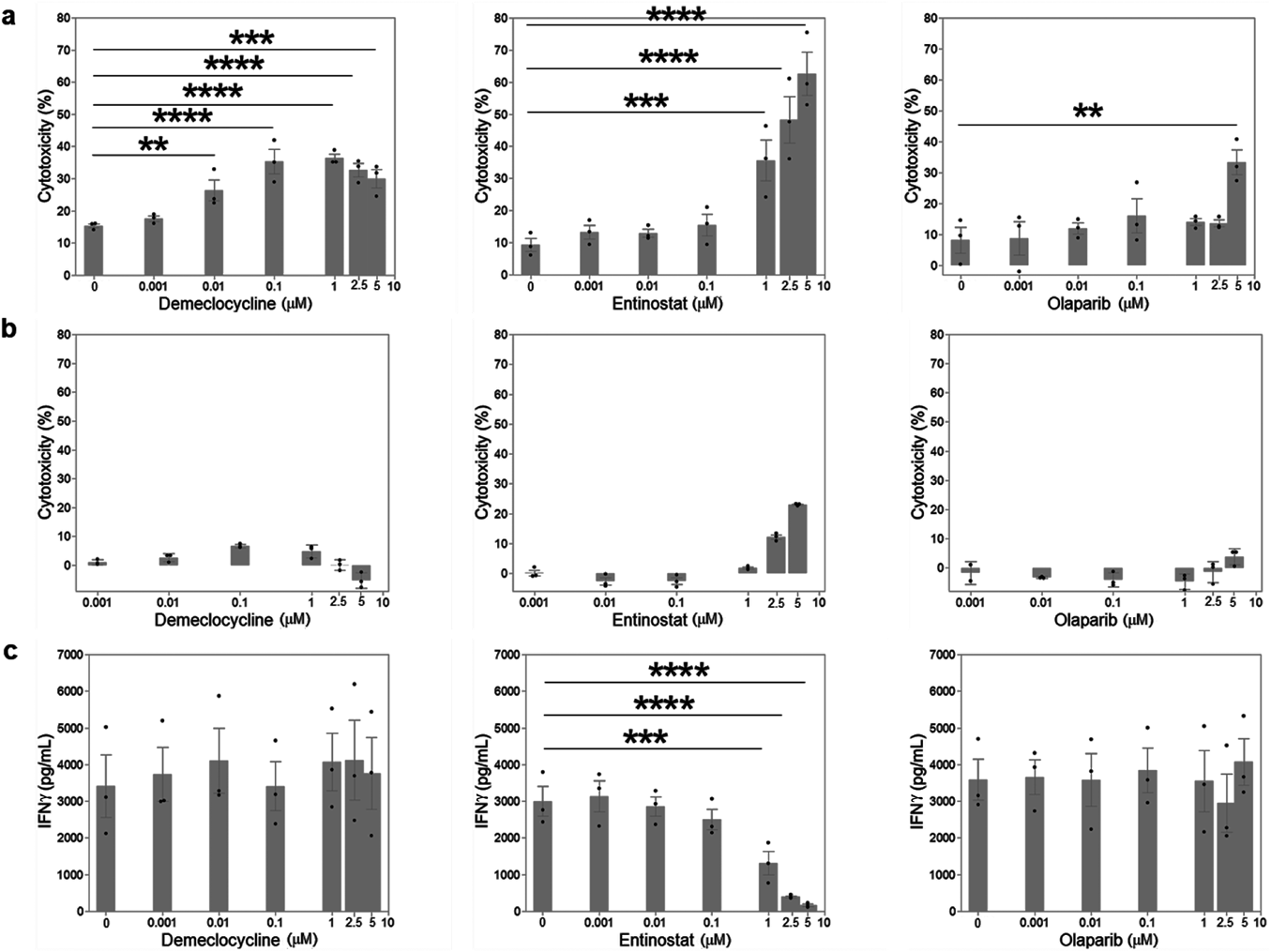
Differences between Demeclocycline and HDAC and PARP InhibitorsTo evaluate the ability of tetracyclines to enhance anti-tumor T-cell responses, we developed an assay system using BiTE for human T cells (Fig. 1). The pattern of T-cell cytotoxicity induced by BiTE shows some similarities to tumor cell killing by endogenous tumor antigen-specific T cells. In this assay, we used BiTE specific for the tumor antigen EphA2 on U251 cells and CD3 on human T cells. Based on our previous findings, we selected co-culture conditions for the effector-to-target ratio (5 : 1) and BiTE concentration (100 ng/mL).9) By adding tetracyclines to this co-culture, we evaluated the ability of tetracyclines to enhance T-cell cytotoxicity. Among the tetracyclines tested, we focused on demeclocycline, which is used as an antibiotic less frequently than other tetracyclines, such as minocycline. The repurposing of minor antibiotics for other usages is advantageous due to the reduced risk of antimicrobial resistance.
To establish the potential of demeclocycline as a candidate agent for combination cancer immunotherapy, we compared demeclocycline with the histone deacetylase (HDAC) inhibitor entinostat and poly(ADP-ribose) polymerase (PARP) inhibitor olaparib, which are used in combination with ICIs in clinical trials.
Demeclocycline significantly increased T-cell cytotoxicity at concentrations between 0.01 and 5 µM (Fig. 2a). This activity was elevated at concentrations between 0.1 and 2.5 µM. On the other hand, demeclocycline did not exhibit cytotoxicity of more than 10% against U251 cells at concentrations between 0.001 and 5 µM (Fig. 2b). These results indicated that demeclocycline enhanced T-cell cytotoxicity induced by BiTE in a dose-independent manner without direct cytotoxicity against U251 cells. We also examined the secretion of IFNγ in these co-culture supernatants and found that IFNγ concentrations were slightly high in the presence of demeclocycline (Fig. 2c). These results suggest that demeclocycline exhibited cytotoxicity against U251 cells by enhancing T-cell cytotoxicity.
In contrast to demeclocycline, entinostat significantly increased T-cell cytotoxicity in a dose-dependent manner (Fig. 2a). It also directly exhibited cytotoxicity against U251 cells in a dose-dependent manner (Fig. 2b). On the other hand, the secretion of IFNγ into the co-culture supernatant decreased at a higher concentration of entinostat (Fig. 2c). Olaparib did not exhibit a significant increase in T-cell cytotoxicity from 0 µM, except at 5 µM (Fig. 2a). It also did not exhibit cytotoxicity of more than 10% against U251 cells at concentrations between 0.001 and 5 µM (Fig. 2b). IFNγ concentrations did not change in an olaparib concentration-dependent manner (Fig. 2c).
These results suggest that among the three drugs examined, demeclocycline enhanced T-cell cytotoxicity in U251 cells without direct cytotoxicity.
Time Dependency of Effects of DemeclocyclineTo elucidate the mechanisms by which demeclocycline enhances T-cell responses, we examined the time courses of cytotoxicity, cytokine production, granzyme B expression, and T-cell proliferation induced by demeclocycline. Co-cultures with demeclocycline were performed at a concentration of 2.5 µM, at which demeclocycline increased T-cell cytotoxicity, but did not exhibit direct cytotoxicity against U251 cells (Figs. 2a, b). A demeclocycline concentration of 2.5 µM is nearly equivalent to the maximum serum concentration after a 150-mg oral dose of demeclocycline.7)
T-cell cytotoxicity was enhanced within one day by demeclocycline (Fig. 3a). To assess the effects of demeclocycline on each T-cell subset, CD4+ or CD8+ T cells isolated by negative selection were co-cultured. Although CD4+ T cells did not exhibit cytotoxicity, CD8+ T-cell cytotoxicity was enhanced by demeclocycline (Figs. 3b, c). The production of IFNγ and IL-2 in co-cultures with demeclocycline was slightly high within one day (Figs. 3d, e). Granzyme B expression and the proliferation of CD8+ T cells were enhanced within two days by demeclocycline (Figs. 4a, c). In contrast to CD8+ T cells, granzyme expression and the proliferation of CD4+ T cells were slightly high with demeclocycline (Figs. 4b, d).
Therefore, CD8+ rather than CD4+ T-cell responses were enhanced by demeclocycline within one to two days via cytotoxicity, granzyme B expression, and cell proliferation.
Sensitization of Tumor Cells by Demeclocycline for T-Cell CytotoxicityAlthough demeclocycline did not exhibit direct cytotoxicity against tumor cells, it may sensitize tumor cells for T-cell cytotoxicity. To establish whether demeclocycline is effective for tumor cells or T cells, we pre-treated tumor cells or PBMCs with demeclocycline before a co-culture with BiTE in the absence of demeclocycline. The results obtained showed that T-cell cytotoxicity was stronger against tumor cells pre-treated with demeclocycline than non-treated tumor cells (Fig. 5a). EphA2 expression on tumor cells was not up-regulated by demeclocycline (Supplementary Fig. 3). On the other hand, T cells pre-treated with demeclocycline did not exhibit stronger cytotoxicity against tumor cells than non-treated T cells (Fig. 5a). Furthermore, the activation of T cells by an anti-CD3 antibody stimulation was not enhanced by demeclocycline through the production of IFNγ and increases in intracellular calcium concentrations (Fig. 5b, Supplementary Fig. 4). These results indicated that demeclocycline sensitized tumor cells for T-cell cytotoxicity.
Anti-tumor T-Cell Responses Induced by Six TetracyclinesWe investigated the ability of six tetracyclines, minocycline, tetracycline, doxycycline, meclocycline, chlortetracycline, and demeclocycline, to enhance T-cell responses induced by BiTE. We evaluated cytotoxicity, cytokine production, granzyme B expression, and T-cell proliferation induced by the six tetracyclines. Based on the results obtained on anti-tumor T-cell responses induced by demeclocycline, we analyzed six tetracyclines at a concentration of 2.5 µM, at which demeclocycline increased T-cell cytotoxicity, but did not exhibit direct cytotoxicity against tumor cells (Figs. 2a, b). The co-culture period was set to two to four days, during which T-cell responses were enhanced by demeclocycline via cytotoxicity, granzyme B expression, and cell proliferation (Figs. 3, 4).
All six tetracyclines enhanced T-cell cytotoxicity induced by BiTE after a two-day co-culture (Fig. 6a). IFNγ and IL-2 production in the co-culture with tetracyclines was slightly high (Fig. 6b). In contrast, none of the six tetracyclines enhanced IFNγ production from T cells without BiTE (Supplementary Fig. 5). Granzyme B expression in CD8+ T cells was enhanced after three days of the co-culture with all six tetracyclines. Regarding CD4+ T cells, granzyme B expression was enhanced by chlortetracycline, doxycycline, and meclocycline (Fig. 6c). The proliferation of CD8+ T cells was enhanced after four days of the co-culture with all six tetracyclines. Regarding CD4+ T cells, proliferation was enhanced by chlortetracycline, demeclocycline, doxycycline, and tetracycline (Fig. 6d).
These results indicated that tetracyclines enhanced T-cell responses, particularly CD8+ T cells, induced by BiTE.
Effects of Demeclocycline on T Cells in Tumor Tissues of NSCLC PatientsWe assessed the ability of demeclocycline to enhance T-cell cytotoxicity in the tumor tissues of NSCLC patients. A flow cytometric analysis of PBMCs and lung tumor-infiltrated cells from NSCLC patients revealed that the rates of not only Tim-3+ in CD8+ T cells and CD4 + CD25++, namely, regulatory T cells in CD4+ T cells, but also CD103+ in CD8+ T cells and OX40+ in CD4+ T cells were significantly higher in lung tumor tissues than in peripheral blood (Fig. 7a, Supplementary Fig. 6). Isolated PBMCs or lung tumor-infiltrated cells from NSCLC patients were co-cultured with U251 cells and BiTE. Following the addition of demeclocycline to these co-cultures, we noted that demeclocycline enhanced T-cell cytotoxicity not only in PBMCs, but also in lung tumor tissues (Fig. 7b).
DISCUSSION
The present results indicate that tetracyclines enhanced T-cell responses induced by BiTE through T-cell cytotoxicity, cytokine production, granzyme B expression, and the proliferation of CD4+ and CD8+ T cells. Since all six tetracyclines tested, namely, minocycline, tetracycline, doxycycline, meclocycline, chlortetracycline, and demeclocycline, enhanced T-cell responses, the basic chemical structure of tetracyclines is critical for their ability to enhance T-cell responses.
In our analysis, demeclocycline did not directly exhibit cytotoxicity against U251 cells at the concentration that enhanced T-cell cytotoxicity in U251 cells. On the other hand, the HDAC inhibitor entinostat enhanced T-cell cytotoxicity at the concentration that exhibited direct cytotoxicity against U251 cells. In contrast, demeclocycline sensitized tumor cells for T-cell cytotoxicity. Since demeclocycline did not exhibit direct cytotoxicity against tumor cells, the mechanism by which demeclocycline enhances T-cell cytotoxicity does not appear to be based on immunogenic cell death, which is triggered by dying tumor cells.17) Demeclocycline may increase T-cell cytotoxicity by regulating the interaction between T cells and tumor cells. Due to this regulation, T-cell responses will be enhanced through granzyme B expression for cytotoxicity against tumor cells.
Demeclocycline enhanced T-cell cytotoxicity at a concentration as low as 0.01 µM. On the other hand, the maximum serum concentration after a 150-mg oral dose of demeclocycline is approximately 2.5 µM, which is an effective concentration for antibiotic therapy. Among tetracyclines, minocycline was previously reported to exert inhibitory effects on T-cell activity.2–6) However, the concentration of minocycline for T-cell suppression in these in vitro studies was approximately 25 µM, which was 10-fold higher than that in clinical use for antibiotic therapy.7) Therefore, in contrast to previous findings, the present results indicate that tetracyclines increased anti-tumor T-cell responses at the same or lower concentrations for antibiotic therapy, which have been confirmed for clinical safety.
Our analysis of lung tumor tissues revealed that demeclocycline increased T-cell cytotoxicity not only in peripheral blood, but also in lung tumor tissues. Based on our flow cytometric analysis, a higher frequency of regulatory T cells (CD4 + CD25++) in lung tumor tissues implied an immunosuppressive tumor microenvironment. On the other hand, we found a high frequency of tissue-resident memory CD103 + CD8+ T cells in lung tumor tissues, which has been linked to the magnitude of cytotoxic T-cell responses in human lung cancer.18) Therefore, demeclocycline may enhance the activity of cytotoxic T cells in a suppressive tumor microenvironment including regulatory T cells.
There are several limitations that need to be addressed. Although the results obtained suggest that tetracyclines enhanced anti-tumor T-cell responses, the mechanism of activation for T-cell cytotoxicity has not yet been elucidated in detail. Furthermore, we only examined the effects of demeclocycline on T cells in the lung tumor tissues of NSCLC patients. Animal models were not investigated because of the difficulties associated with producing BiTE specific for murine antigens.
In conclusion, we herein demonstrated that tetracyclines enhanced anti-tumor T-cell responses induced by BiTE. Therefore, tetracyclines are potential candidates for combination cancer immunotherapy.
Acknowledgments
We thank all the patients and healthy donors who participated in the present study. This work was supported by AMED under Grant No. JP18lm0203007 (KI) and JSPS KAKENHI Grant No. 21K08153 (KI).
Conflict of Interest
The Department of Clinical Research in Tumor Immunology is a collaborating laboratory of Osaka University and Shionogi Co., Ltd.
Supplementary Materials
This article contains supplementary materials.
REFERENCES
- 1) Garrido-Mesa N, Zarzuelo A, Gálvez J. What is behind the non-antibiotic properties of minocycline? Pharmacol. Res., 67, 18–30 (2013).
- 2) Kloppenburg M, Verweij CL, Miltenburg AM, Verhoeven AJ, Daha MR, Dijkmans BA, Breedveld FC. The influence of tetracyclines on T cell activation. Clin. Exp. Immunol., 102, 635–641 (1995).
- 3) Kloppenburg M, Brinkman BM, de Rooij-Dijk HH, Miltenburg AM, Daha MR, Breedveld FC, Dijkmans BA, Verweij C. The tetracycline derivative minocycline differentially affects cytokine production by monocytes and T lymphocytes. Antimicrob. Agents Chemother., 40, 934–940 (1996).
- 4) Giuliani F, Hader W, Yong VW. Minocycline attenuates T cell and microglia activity to impair cytokine production in T cell-microglia interaction. J. Leukoc. Biol., 78, 135–143 (2005).
- 5) Szeto GL, Brice AK, Yang HC, Barber SA, Siliciano RF, Clements JE. Minocycline attenuates HIV infection and reactivation by suppressing cellular activation in human CD4+ T cells. J. Infect. Dis., 201, 1132–1140 (2010).
- 6) Szeto GL, Pomerantz JL, Graham DR, Clements JE. Minocycline suppresses activation of nuclear factor of activated T cells 1 (NFAT1) in human CD4+ T cells. J. Biol. Chem., 286, 11275–11282 (2011).
- 7) Agwuh KN, MacGowan A. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J. Antimicrob. Chemother., 58, 256–265 (2006).
- 8) Elkayam O, Yaron M, Caspi D. Minocycline-induced autoimmune syndromes: an overview. Semin. Arthritis Rheum., 28, 392–397 (1999).
- 9) Iwahori K, Shintani Y, Funaki S, Yamamoto Y, Matsumoto M, Yoshida T, Morimoto-Okazawa A, Kawashima A, Sato E, Gottschalk S, Okumura M, Kumanogoh A, Wada H. Peripheral T cell cytotoxicity predicts T cell function in the tumor microenvironment. Sci. Rep., 9, 2636 (2019).
- 10) Yamamoto Y, Iwahori K, Funaki S, Matsumoto M, Hirata M, Yoshida T, Kanzaki R, Kanou T, Ose N, Minami M, Sato E, Kumanogoh A, Shintani Y, Okumura M, Wada H. Immunotherapeutic potential of CD4 and CD8 single-positive T cells in thymic epithelial tumors. Sci. Rep., 10, 4064 (2020).
- 11) Iwahori K. Cytotoxic CD8. Adv. Exp. Med. Biol., 1224, 53–62 (2020).
- 12) Schreiner J, Thommen DS, Herzig P, Bacac M, Klein C, Roller A, Belousov A, Levitsky V, Savic S, Moersig W, Uhlenbrock F, Heinzelmann-Schwarz VA, Umana P, Pisa P, von Bergwelt-Baildon M, Lardinois D, Müller P, Karanikas V, Zippelius A. Expression of inhibitory receptors on intratumoral T cells modulates the activity of a T cell-bispecific antibody targeting folate receptor. OncoImmunology, 5, e1062969 (2015).
- 13) Baeuerle PA, Reinhardt C. Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res., 69, 4941–4944 (2009).
- 14) Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N. Engl. J. Med., 376, 836–847 (2017).
- 15) Haruna M, Hirata M, Iwahori K, Kanazawa T, Yamamoto Y, Goto K, Kawashima A, Morimoto-Okazawa A, Funaki S, Shintani Y, Kumanogoh A, Wada H. Docetaxel upregulates HMGB1 levels in non-small cell lung cancer. Biol. Pharm. Bull., 43, 399–403 (2020).
- 16) Iwahori K, Kakarla S, Velasquez MP, Yu F, Yi Z, Gerken C, Song XT, Gottschalk S. Engager T cells: a new class of antigen-specific T cells that redirect bystander T cells. Mol. Ther., 23, 171–178 (2015).
- 17) Yatim N, Cullen S, Albert ML. Dying cells actively regulate adaptive immune responses. Nat. Rev. Immunol., 17, 262–275 (2017).
- 18) Ganesan AP, Clarke J, Wood O, Garrido-Martin EM, Chee SJ, Mellows T, Samaniego-Castruita D, Singh D, Seumois G, Alzetani A, Woo E, Friedmann PS, King EV, Thomas GJ, Sanchez-Elsner T, Vijayanand P, Ottensmeier CH. Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat. Immunol., 18, 940–950 (2017).