2022 Volume 45 Issue 6 Pages 738-742
2022 Volume 45 Issue 6 Pages 738-742
Nutmeg, a dried seed kernel of a tall evergreen Myristicaceae tree, is widely used as a spice and herbal medicine and is known to have antidepressant-like effects. This study evaluates the mechanisms underlying this antidepressant-like effect and safety of nutmeg n-hexane extract (NNE) in mice. Tail suspension and open field tests showed that NNE (10 mg/kg, per OS (p.o.)) significantly decreased the immobility time of mice without effecting their spontaneous locomotor activity. The reduction of immobility time of mice elicited by NNE was significantly inhibited by ketanserin (5-hydroxytryptamine (5-HT)2A/2C receptor antagonist), ondansetron (5-HT3 receptor antagonist), and yohimbine (α2 receptor antagonist). WAY100635 (5-HT1A receptor antagonist) tended to inhibit the effect of NNE but without significance. Testing according to the Organisation for Economic Co-operation and Development Guidelines, no mice died due to administrated NNE (2000 mg/kg, p.o.), and behavioral and weight changes were not seen in the acute toxicity test. In the Ames test, no increase in the number of revertant colonies for each bacterial strain test strains TA98 and TA100 by nutmeg powder was observed either with or without metabolic activity by S9 mix. These results suggest that NNE shows an antidepressant-like effect involving various serotonergic and noradrenergic nervous systems and maybe a highly safe natural preparation.
Depression is a mental disorder characterized by depressed mood, loss of interest, and decreased energy. An estimated 280 million people are affected by depression worldwide.1) Also, depression significantly influences society because of high suicide rate and problems in education and employment of patients. Therefore, prevention of depression is required in addition to effective treatment, and establishment of countermeasures is critical, including self-medication for people in the primary stage.
Natural products are commonly used as self-medicated health supplements. For example, St. John’s wort (SJW) has been known as a supplement for depressive symptoms,2) and its antidepressant-like effect and safety have been studied.3,4) However, because SJW induces CYP, a human drug-metabolizing enzyme, a decrease in blood drug concentration is induced in some cases.3)
Nutmeg (Myristica fragrans Houttuyn), a dried seed kernel of a tall evergreen tree of Myristicaceae in the East Indies, is widely used as a spice and herbal medicine, such as a carminative agent or an aromatic stomachic. It has been reported that nutmeg has psychotropic, antithrombotic, antiplatelet, antifungal, and anti-inflammatory pharmacological effects.5,6) Moreover, the antidepressant-like effect of nutmeg was also reported.7)
The monoamine hypothesis that serotonergic and/or noradrenergic disorders cause depression has been known. It is suggested that the 5-hydroxytryptamine (5-HT)1A receptors are involved in the hippocampal neurogenesis improving the effect of selective serotonin reuptake inhibitors, the first-line drug against depression. Several clinical studies have reported that combining antidepressants with 5-HT1A receptor agonist treatment is a better therapeutic strategy for depression than antidepressant monotherapy.8) Therefore, among serotonin receptors, the 5-HT1A receptor is considered as an important target for inducing antidepressant-like effect in current depression treatment. Dhingra and Sharma7) reported that the antidepressant-like effect of nutmeg has been involved in the serotonergic nervous system and adrenergic α1 receptors. However, it is unknown which subtypes of serotonin receptors are involved in anti-depressant-like effects. The involvement of the adrenergic α2 receptor is also not clear. In this study, we evaluated the effect of each receptor antagonist in mice by tail suspension test (TST) to clarify the involvement of serotonergic and adrenergic receptors in the antidepressant-like effect of nutmeg. Moreover, we observed the change in spontaneous locomotor activity after nutmeg administration.
Nutmeg has been used as food and herbal medicine. Less than 10 g intake of nutmeg does not cause toxicity effects, such as convulsions, vomiting, and palpitations in humans,9) but consciousness decreases with approximately 18 g intake.10) In addition, weak mutagenicity has been reported.11) In this study, we also assessed nutmeg’s acute oral toxicity and mutagenicity.
Six-week-old male ICR mice were used for this study (Charles River Japan, Yokohama, Japan). Animals were housed under controlled conditions (room temperature 23 ± 2 °C; relative humidity 50 ± 3%; 12 h/12 h light/dark cycle) and had free access to food and water for 7 d before tests were carried out. All experimental procedures were approved by the Animal Care and Use Committee of Kyushu University. The study was conducted following the Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions under the Ministry of Education, Culture, Sports, Science and Technology and ARRIVE guidelines.
Drugs and ChemicalsNutmeg was purchased from P.T. East Indian Agency Products in Indonesia. The following chemicals were used: desipramine hydrochloride (tricyclic antidepressant, Sigma, St. Louis, MO, U.S.A.), ketanserin (5-HT2A/2C receptor antagonist), ondansetron hydrochloride dihydrate (5-HT3 receptor antagonist), and polyethylene glycol 400 (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan), WAY100635 (5-HT1A receptor antagonist, Chemscene, Monmouth Junction, NJ, U.S.A.), yohimbine hydrochloride (α2 receptor antagonist), Tween 80, dimethyl sulfoxide (DMSO), NaCl, 2-aminoanthracene (2AA), 2-nitrofluorene (2NF), 4-nitroquinoline 1-oxide (4NQO), MgSO4·7H2O, citric acid monohydrate, K2HPO4, NH4H2PO4, NaOH and glucose (Wako, Osaka, Japan), nutrient broth (Kanto Kagaku, Tokyo, Japan), agar BA-30 (Ina Food Industry Co., Ltd., Nagano, Japan), and S9 mix (Ieda Trading Corporation, Tokyo, Japan).
Extraction ProcedureThe extraction procedure described by El-Alfy et al.12) was followed. In brief, 120 g of nutmeg was put into an aluminum bag and roughly crushed with a hammer to obtain nutmeg powder. The crushed nutmeg was put through a 4 mm sieve, and 100 g of nutmeg powder was collected. This powder was soaked, with stirring, in 1000 mL of n-hexane for 24 h. The suspension was filtered and washed, and the solution was evaporated on a rotary evaporator to obtain 27.4 g of nutmeg n-hexane extract (NNE).
Preparation of Drug FormulationsThe preparation followed the procedures of Dhingra and Sharma.7) NNE was dissolved in 1 mL of Tween 80 with constant shaking in a 50 °C warm bath, followed by the addition of 4 mL of polyethylene glycol 400 and then dilution to 10 mL by adding 5 mL of physiological saline. Desipramine, WAY 100635, ondansetron, and yohimbine were dissolved in physiological saline. Ketanserin was dissolved in DMSO and diluted 100-times with physiological saline. The volume load of DMSO was 10 µL/g body weight.
Tail Suspension TestThe TST was conducted according to the method described by Steru et al.13) Mice were suspended on the edge of a device made up of expanded polystyrene (33 cm) above a tabletop using adhesive tape placed approximately 2 cm from the tip of the tail. The behavior of mice was recorded on a video camera for 6 min. The immobility time was measured visually using a stopwatch. When mice give up resisting escapable circumstances and stop moving during the experiment for 6 min, it is considered as an immobility state and is regarded as a depression-like symptom. Reduction of the immobility time is considered as an antidepressant-like effect.
NNE (5, 10 mg/kg, per OS (p.o.)), desipramine (15 mg/kg, p.o.) and vehicle (p.o.) were administered for 3 successive days. Mice were subjected to the TST after 60 min of drug administration on day 3. WAY100635 (0.1 mg/kg, subcuteneous injection (s.c.)), ketanserin (1 mg/kg, intraperitoneal injection (i.p.)), ondansetron (1 mg/kg, i.p.), and yohimbine (1 mg/kg, i.p.) were administered 30 min after treatment with NNE (10 mg/kg, p.o.).
Open Field TestEach mouse was placed on the central circle of the open field apparatus (consisting of 19 compartments; diameter: lower 60 cm and upper 88 cm; height: 50 cm). The spontaneous locomotor activity of mice was investigated by counting ambulation (number of line crossings) for a minute, their behavior was recorded on a video camera, and the ambulation was counted visually. Mice were subjected to an open field test (OFT) before treatment with test drugs. Based on the results, mice were classified into four groups so that the locomotor activity within each group was at the same level. After grouping, mice were administered with NNE (5, 10 mg/kg, p.o.), desipramine (15 mg/kg, p.o.), and vehicle (p.o.) for 3 successive days, and were subjected to the OFT after 30, 60, and 120 min of drug administration on day 3.
Acute Toxicity TestThe acute oral toxicity test was based on the Organisation for Economic Co-operation and Development (OECD) Guidelines for Testing Chemicals 423.14) The study was performed at graded doses of 2000 mg/kg p.o. using three mice. Three male ICR mice received a single oral treatment with NNE at the dose of 2000 mg/kg or vehicle (control). Mice were observed whether or not they showed abnormalities, such as death and behavioral change during 14 d and their body weight was measured at four times: before administration, 1, 7, and 14 d after administration. The absence or presence of compound-related mortality of the animals dosed at one step determined the next step, i.e., without further testing, dosing three additional mice with the same dose, or dosing three additional mice at the next higher or the next lower dose level. The method enabled classifying the test substance to a series of toxicity classes defined by a fixed LD50 cut-off value.
Reverse Mutation Test Using Bacteria (Ames Test)The mutagenicity test was carried out according to the modified Ames test’s pre-incubation method.15,16) The Ames test of nutmeg powder was performed in two experimental systems: S9 mix addition (+S9 mix) and non-addition (−S9 mix) by the pre-incubation method. Tester strains were Salmonetla typhimurium TA98 and TA100. Strains containing 8 mL of bacterial suspension were added 0.7 mL of DMSO, dispensed in 0.2 mL portions into screw vials, and stored at −80 °C. Vials were thawed, 20 µL of the strain was added to Nutrient Broth medium, cultured with shaking (60 reciprocations/min) for 9 h at 37 °C, and used as a bacterial suspension. DMSO was used as a negative control; 2-aminoanthracene (2AA), 2-nitrofluorene (2NF), and 4-nitroquinoline 1-oxide (4NQO) dissolved in DMSO at prescribed concentrations were used as positive controls. The maximum concentration of nutmeg powder was set at 3000 µg/plate, and it was carried out at a concentration of public ratio of about 3.
In a sterilized culture tube, 0.1 mL of a sample, 0.5 mL of S9 mix (or phosphate-buffered saline as −S9 mix), and 0.1 mL of a bacterial suspension were sequentially added and mixed well, and then pre-incubated at 37 °C for 20 min. Soft agar (2 mL) was added to each tube and mixed, poured onto an agar medium plate, and spread evenly. When solidified, the plates were incubated at 37 °C for 48 h.
The number of induced revertant colonies was counted. In the dose-setting test to check growth inhibition, the average value was obtained using two plates, and in the main test, the average value and the standard deviation were obtained using three plates. The presence or absence of precipitation of the nutmeg sample was watched with the naked eye. Growth inhibition and antibacterial activity were judged from the pellicle’s state. When the number of revertant colonies increased more than twice that of negative controls and dose dependence was observed, it was determined to be positive, indicating genetic toxicity.
Statistical AnalysisAll experimental results are expressed as the mean ± standard error (S.E.). Statistical analysis was performed by Student’s t-test and one-way ANOVA followed by Dunnett’s test using JMP 11 software package (SAS Institute Inc., NC, U.S.A.). A value of p < 0.05 was considered statistically significant.
Figure 1 shows effects of NNE (5 and 10 mg/kg, p.o.) and desipramine (15 mg/kg, p.o.) administered for three successive days in the TST. The NNE showed a dose-dependent effect on the decrease in immobility time. The effect was significant at a dose of 10 mg/kg (p < 0.05), reducing the immobility time from 90.4 s to 46.0 s when compared with the vehicle-treated group (control). Desipramine used as a positive control also significantly decreased the immobility time from 90.4 s to 43.0 s (p < 0.05).
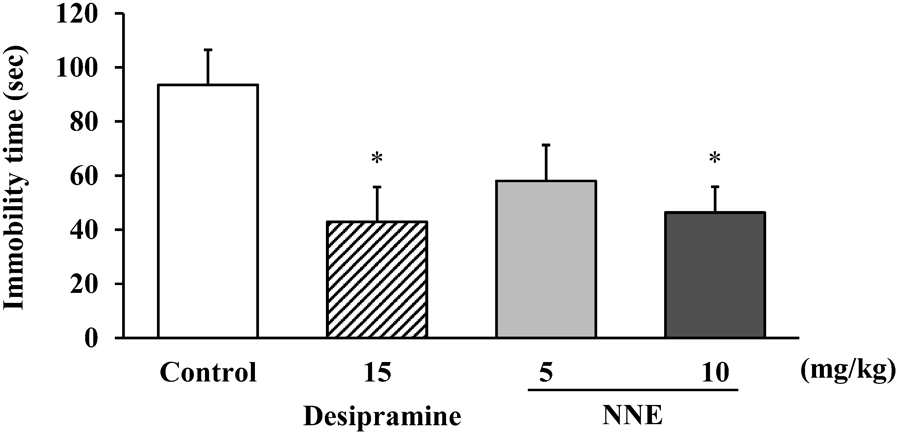
NNE and desipramine were administered for 3 successive days. The immobility period was recorded at 60 min after administration on day 3. Data represent means ± S.E. (n = 10). * p < 0.05; compared with the vehicle-treated group (control). Dunnett’s test.
NNE (10 mg/kg) decreased the immobility time in the mouse TST. Several receptor antagonists were co-administrated to investigate the mechanism of the antidepressant-like effect of NNE. The NNE at the dose of 10 mg/kg significantly decreased the immobility time from 62.0 to 18.9 s (p < 0.05) compared with the control (Fig. 2). In the presence of WAY100635 (0.1 mg/kg, s.c.; a 5-HT1A receptor antagonist), ketanserin (1 mg/kg, i.p.; a 5-HT2A/2C receptor antagonist), ondansetron (1 mg/kg, i.p.; a 5-HT3 receptor antagonist), and yohimbine (1 mg/kg, i.p.; an α2 receptor antagonist), there was no significant reduction in the immobility time of NNE compared to the control group. (Fig. 2). The immobility time at WAY100635, ketanserin, ondansetron, and yohimbine in non-NNE were 62.4, 60.2, 57.5, and 71.5 s, respectively.
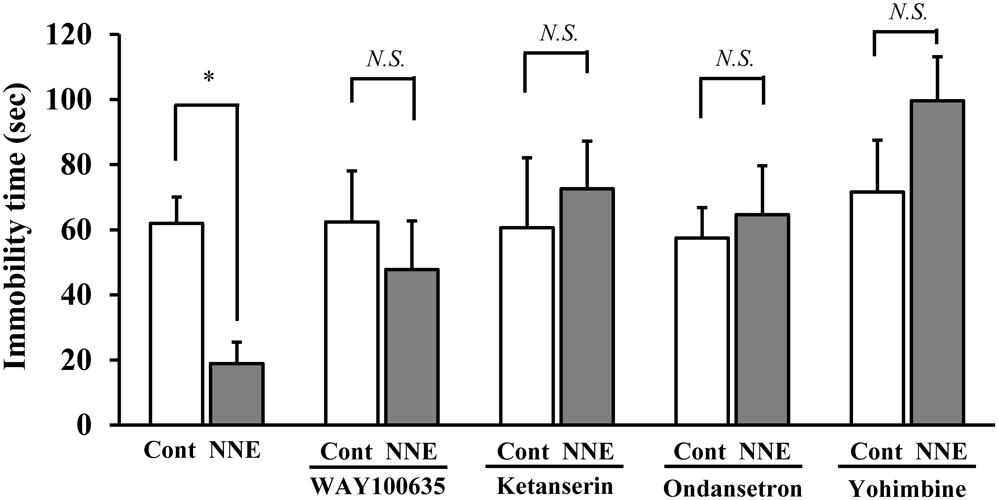
NNE was administered for 3 successive days. WAY100635, ketanserin, ondansetron, and yohimbine were administered 30 min after administration of NNE for 3 successive days. The immobility period was recorded 60 min after administration of NNE on day 3. Data represent means ± S.E. (n = 8). * p < 0.05; compared with the vehicle treated group (control). Student’s t-test.
The administration of NNE at doses of 5 and 10 mg/kg and desipramine at 15 mg/kg for three successive days did not significantly alter the number of line crossings as compared with that of the control group (Fig. 3). There was no effect of NNE on spontaneous locomotor activity in mice.
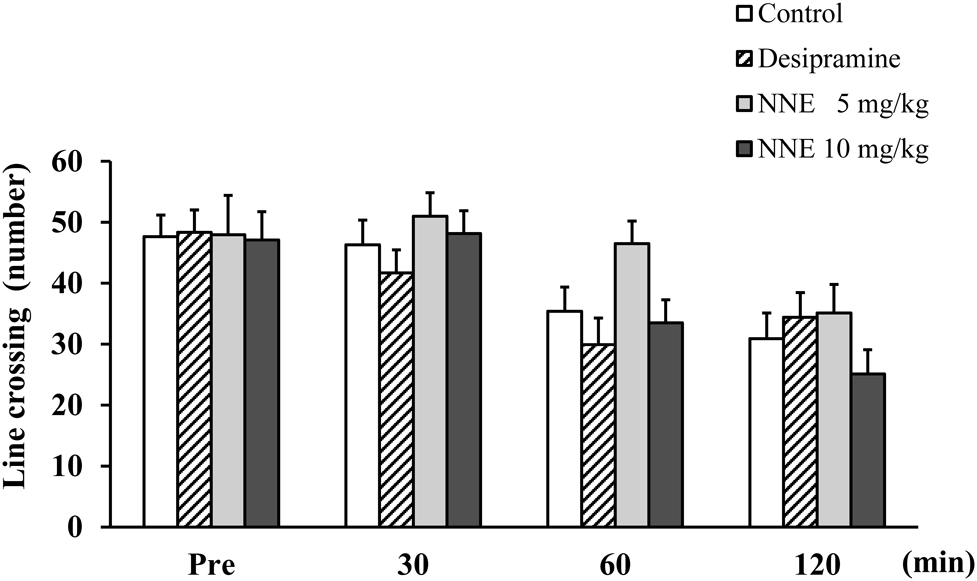
NNE and desipramine were administered for 3 successive days. The line crossing was recorded 30, 60, and 120 min after administration on day 3. Data represent means ± S.E. (n = 10).
Three mice were administrated 2000 mg/kg of NNE and observed for 14 d after treatment; mice did not die. NNE at the dose of 2000 mg/kg was administered to three mice again and observed for 14 d following the OECD Guidelines for the Testing of Chemicals 423. No mice died, and behavioral and weight changes were not shown compared with the control group (Fig. 4). These results indicate that the LD50 of NNE in mice is greater than 2000 mg/kg.
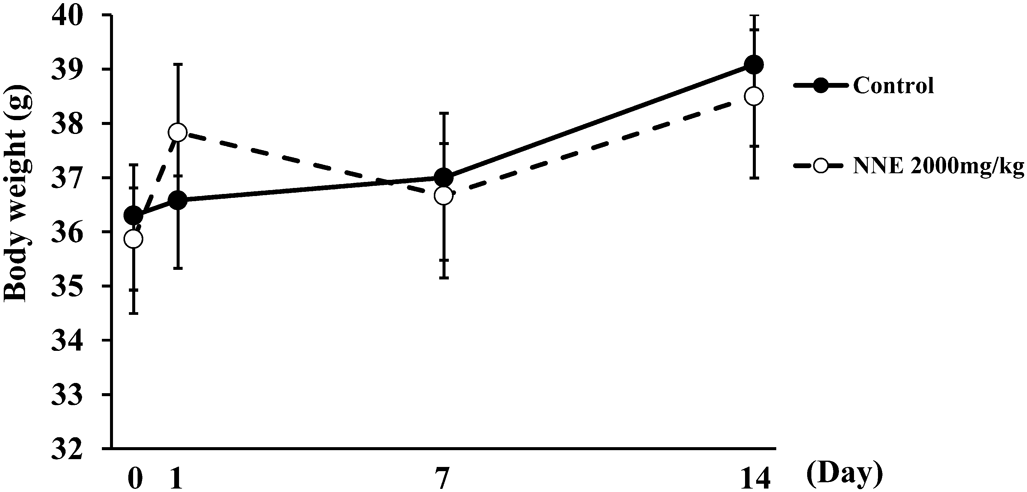
NNE was administered at 2000 mg/kg orally. Data represent means ± S.E. (n = 6).
No increase in revertant colonies in the bacterial strain test of TA98 and TA100 by nutmeg powder was observed either with or without metabolic activity induced by the S9 mix (Table 1). However, the positive controls (2AA, 2NF, and 4NQO) increased 2-fold or more the number of revertant colonies than the negative control (DMSO). These results suggested that nutmeg powder has no mutagenic activity.
| Concentration (µg/plate) | Number of revertant colonies per plate | ||||
|---|---|---|---|---|---|
| TA98 | TA100 | ||||
| +S9 mix | −S9 mix | +S9 mix | −S9 mix | ||
| Negative control | 24 ± 3.2 | 21 ± 3.4 | 100 ± 3.5 | 109 ± 5.2 | |
| Nutmeg powder | 1 | 25 ± 1.7 | 18 ± 2.1 | 106 ± 5.0 | 104 ± 4.5 |
| 3 | 27 ± 3.9 | 19 ± 0.9 | 107 ± 5.0 | 105 ± 5.2 | |
| 10 | 26 ± 0.3 | 21 ± 2.3 | 105 ± 5.7 | 116 ± 7.5 | |
| 30 | 24 ± 4.6 | 18 ± 3.4 | 104 ± 6.2 | 101 ± 3.2 | |
| 100 | 29 ± 1.5 | 26 ± 0.6 | 116 ± 2.9 | 107 ± 9.2 | |
| 300 | 24 ± 3.8 | 19 ± 3.5 | 115 ± 5.0 | 86 ± 7.8 | |
| 1000 | 23 ± 0.9 | 16 ± 3.8 | 113 ± 6.9 | 27 ± 7.8a) | |
| 3000 | 24 ± 2.8 | 7 ± 2.7a) | 21 ± 4.1a) | 0a) | |
| Positive control | |||||
| 2AA | 1 | 486 ± 23.3 | 564 ± 42.6 | ||
| 2NF | 1 | 292 ± 18.4 | |||
| 4NQO | 0.025 | 248 ± 20.3 | |||
Positive controls: TA98/TA100 in the presence of S9 mix, 2AA (1 µ/plate); TA98 in the absence of S9, 2NF (1 µ/plate); TA100 in the absence of S9, 4NQO (0.025 µ/plate). Data represent means ± S.E. (n = 3). a) Growth inhibition.
Nutmeg is widely used as a spice and is known to effect the central nervous system activity,5) for example, as an antidepressant.7) However, it is not clear which subtype of the serotonin receptors are involved and whether noradrenalin α2 receptors are associated with the antidepressant-like effect of nutmeg. Moreover, its safety as a health supplement has not been evaluated. Thus, we evaluated the safety and the mechanism underlying the antidepressant activity a of nutmeg.
We investigated the antidepressant effect and mechanism of NNE. In TST, a mouse suspended to the device by the tail tries to escape; it then alternates running movements forward or backward, undergoes body torsion with attempts to catch the suspending bond, and experiences immobility periods.13) The immobility time is observed after escape behavior of the mouse in an escapable environment. This immobility time is generally interpreted as behavioral despair and is a depressive state. TST is commonly used to determine antidepressant-like effects because the test is very easy to use, reliable, and specific to identify an antidepressant.17,18) Any central nervous system stimulant may affect the overall activity levels, resulting in altered immobility time in the TST. Therefore, it is necessary to distinguish antidepressant-like action from locomotor activity. Therefore, we conducted OFT to clarify the action.
In TST, NNE at a dose of 10 mg/kg significantly decreased the immobility time of mice. In OFT, NNE did not affect spontaneous locomotor activity. Therefore, the result suggests that NNE’s reduction of immobility time is not due to an increase in mice locomotor activity. Because NNE was extracted with n-hexane, a fat-soluble component, it may induce the antidepressant-like action of NNE. Components present in the essential oil of nutmeg are α-pinene, sabinene, myristicin, etc.19,20) Among them, myristicin is an essential and fat-soluble component that inhibits monoamine oxidase.21) Thus, the reduction of immobility time elicited by NNE may be due to the effect of myristicin.
TST was conducted using each antagonist to clarify the mechanisms on antidepressant-like effect of nutmeg in mice. We had conducted TST 60 min after nutmeg administration, and we administered each antagonist 30 min before the behavioral experiment (30 min after NNE) in order to ensure the effects. Since each antagonist is a competitive inhibitor, we think there is no problem in experiments even if it is administered 30 min after the administration of NNE. In the presence of WAY100635, ketanserin, ondansetron, and yohimbine, the significant reduction in immobility time of NNE was abolished. Among the serotonin receptor subtypes, 5-HT1A and 5-HT2A receptors are closely involved in depression.22,23) It has been reported that different classes of antidepressants act as functional 5-HT3 receptor antagonists.24) Moreover, a study by Kondo et al.25) indicated that the 5-HT3 receptor is essential for exercise-induced hippocampal neurogenesis and antidepressant effects. The adrenergic α2 receptor is mainly an autoreceptor in the presynaptic membrane. It induces negative feedback in the neuronal synapse, inhibiting noradrenaline release. However, it was suggested that postsynaptic α2 adrenergic receptors play a major role in antidepressant effects.26) Our study suggests that the antidepressant-like effect of NNE may involve 5-HT2A/2C receptor, 5-HT3 receptor, and α2 receptor besides 5-HT1A receptor, considered to play an important function in antidepressive effects. Further, it has been reported that the antidepressant-like effect of nutmeg involves adrenergic α1 and D2 receptors.7) Thus, it can be inferred that multiple receptors are involved in the antidepressive effect of nutmeg. This may be partly because monoamine oxidase inhibition by nutmeg myristicin increases the activity of various biogenic amines. In this study, we examined the antidepressant-like effect of using naïve mice according to the method described by Steru et al.13) We would like to give mice with depression-like behaviors (e.g. chronic unpredictable stress, repeated social defeat stress, learned helplessness, LPS model) more consideration moving forward.
Moreover, we conducted the acute toxicity test and Ames test to assess the safety of NNE. The results showed that the LD50 of NNE is greater than 2000 mg/kg. In addition, no behavioral and body weight changes were observed during the observation period, and the tolerability at the administered dose was confirmed. Furthermore, the results of the Ames test suggest that nutmeg powder and its metabolites are not mutagenic because the number of revertant colonies did not increase by the action of nutmeg powder extract, regardless of the metabolic activity by S9 mix.
These results indicate that NNE possesses antidepressant-like effect through various serotonergic and noradrenergic nervous systems and is a very safe natural preparation.
We would like to thank Mr. Masakazu Shiga of P.T. East Indian Agency Products for providing a sample of nutmeg, the Department of Clinical Pharmacy and Pharmaceutical Care laboratory members for their comments.
The authors declare no conflict of interest.