2022 Volume 45 Issue 6 Pages 743-750
2022 Volume 45 Issue 6 Pages 743-750
Asthma is a respiratory disease characterized by heterogeneous chronic airway inflammation. Activation of nucleotide-binding oligomerization domain-like receptor pyrin domain containing 3 (NLRP3) inflammasome is involved in the development of many pulmonary inflammatory diseases. The role and regulatory mechanism of carbenoxolone (CBX) in ovalbumin (OVA)-induced asthma models are not fully clear. Therefore, the study investigated whether CBX ameliorates airway inflammation and remodeling, as well as its mechanism in OVA induced-inflammation in mice. Wright–Giemsa staining was used to count inflammatory cells in bronchoalveolar lavage fluid (BALF). The level of inflammatory cells infiltration, mucus cell proliferation, and collagen deposition in lung tissue were separately assessed by hematoxylin and eosin, periodic acid-Schiff, and Masson trichrome staining, respectively. Airway resistance (AR) was measured by non-invasive airway system. Immunohistochemical assay was used to observe NLRP3 expression area. The expression of nuclear factor-kappaB (NF-κB), p-NF-κB, inhibitor of kappaB (IκB)-α, p-IκB-α, NLRP3, pro-caspase-1, caspase-1, and interleukin (IL)-1β in lung tissue were measured using quantitative real-time PCR or Western blotting. Our results showed that CBX can significantly attenuate the leukocyte count and the percentage of eosinophils and neutrophils in the BALF, peribronchial inflammation, airway mucus secretion, collagen deposition area, and AR in OVA-induced airway inflammation. In addition, the expression of p-NF-κB, p-IκB-α, NLRP3 and related factors were dramatically alleviated after CBX treatment. These data suggest that CBX has a significant protective effect on allergic airway inflammation by suppressing the activation of NLRP3 inflammasome through NF-κB pathway in asthmatic mice.
Bronchial asthma is the most common chronic airway inflammatory disease in children, and it has a detrimental effect on the QOL of affected children and their families. Moreover, it is the second leading cause of death among chronic respiratory diseases and is a growing global economic burden. Its prevalence has increased over the last decades, especially among children, affecting about 14% of children worldwide.1,2) Chronic inflammatory responses are the main factors leading to the progression of asthma. This inflammatory process is relevant to high mucus secretion, airway hyper-responsiveness (AHR), and even irreversible airflow restriction, which lead to chronic airway obstruction.3,4) Therefore, research should focus on the mechanisms of chronic airway inflammation in asthma and the measures to control this inflammation.
Nucleotide-binding oligomerization domain-like receptor pyrin domain containing 3 (NLRP3) inflammasome is a multi-protein complex which plays a central role in asthma. It contains an intracellular natural immune receptor NLRP3, apoptosis-related speck-like protein, and the protease caspase-1. These proteins identify pathogen-associated molecular patterns or damage-related molecular patterns to recruit and activate caspase-1 that promotes the release of interleukin (IL)-1β and IL-18. These interleukins initiate or amplify multiple downstream signaling transduction mechanisms that mediate pro-inflammatory responses in the airway.5) A previous study reported that eosinophilic airway inflammation depends on the activation of the NLRP3 inflammasome.6) NLRP3, caspase-1, and IL-1β-related gene expression levels were higher in sputum samples of patients with asthma. In the absence of NLRP3, peribronchial inflammatory cells and mucus secretion were significantly reduced in asthma models than in wild-type mice.6,7) A study found that the NLRP3 inflammasome-mediated inflammatory response is associated with asthma severity and glucocorticoid resistance.8) Therefore, activation of NLRP3 plays a significant role in the development of airway inflammation in asthma. In addition, nuclear factor-kappaB (NF-κB) signaling pathway is a well-known inflammatory signaling pathway. The NF-κB pathway regulates the expression of many cytokines and chemokines and exacerbates and prolongs the inflammatory process, which involve the development of inflammatory diseases.9,10) At present, the incidence of asthma is increasing; however, the control measures against asthma are inadequate and drug resistance is constantly emerging. Thus, new therapies and treatment targets are required to have better control of symptoms and exacerbations in refractory asthma. NLRP3 regulation may be the key factor to control asthmatic inflammation.
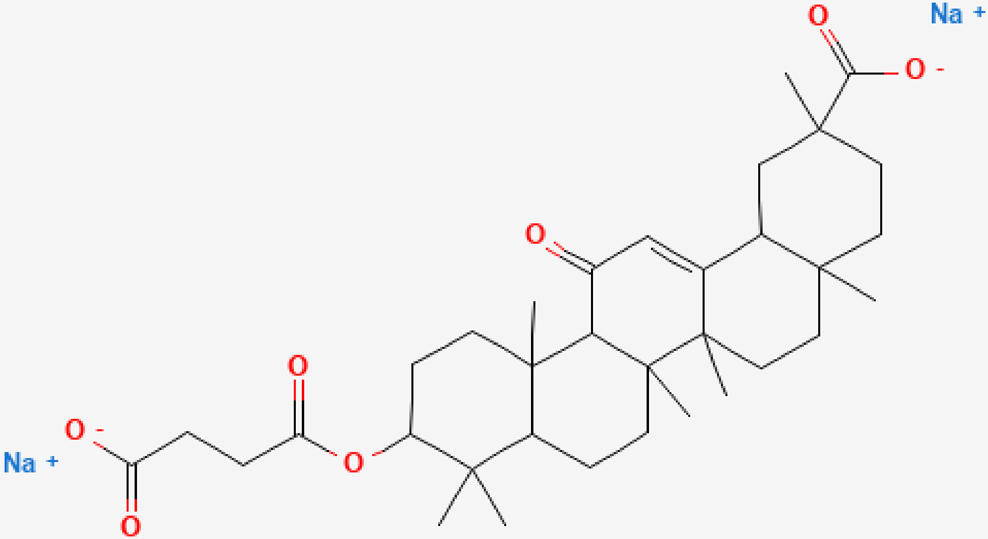
Carbenoxolone (CBX) is a semi-synthetic derivative of glycyrrhetinic acid obtained from the licorice plant (Fig. 1); it has anti-inflammatory, -viral, -tumor, -bacterial, -fibrosis properties and neuroprotective activities amongst other extensive pharmacological activities.11–16) It has been reported that CBX administration decreased lung inflammation in rats with pulmonary hypertension by increasing an anti-inflammatory cytokine, IL-10; decreasing tumor necrosis factor (TNF)-α, IL-1β, IL-6 and monocyte infiltration; and reducing the number of CD3+ and CD4+ T cells in lung tissues.11) Another study indicated that CBX significantly inhibits NLRP3 activation and proinflammatory cytokine production thereby alleviating renal function and pathological damage in sepsis-induced acute kidney injury.17) However, the interaction between CBX and NLRP3 in ovalbumin (OVA)-induced airway inflammation has not been elucidated. This experiment investigated whether CBX can improve airway inflammation via inhibiting NF-κB/NLRP3 pathway in asthmatic mice and provide some new opportunity and theoretical basis for the treatment of asthma.
Four-week-old BALB/c female mice were acquired from China Medical University. All mice were housed under laboratory conditions with libitum food and water access after they were housed for 7 d to acclimate to the standard pathogen-free environment. Mice in this study were grouped as follows: (control; asthma; dexamethasone (DEX); and CBX groups) (n = 8/group). All mice were sensitized with 50 µg OVA (Grade V; Sigma, MO, U.S.A.) mixed with 1 mg aluminum hydroxide (Imject Alum Adjuvant; Thermo, MA, U.S.A.) in sterile saline by intraperitoneal (i.p.) injection on days 1, 8, and 15. On days 22–28, the mice were challenged for 30 min with 2% OVA by atomization inhalation (DeVilbiss, Bornemouth, England). The control group was sensitized and challenged with the same amount of normal saline. DEX [1 mg/kg·d] (Sinopharm, Shanghai, China) was diluted with sterile saline and CBX [5 ; 10 ; 20 ; 40 mg/kg·d] (Sigma; C4790) was dissolved in ultra-pure water, and heated to 45 °C for 30 min. DEX and CBX treatments were both by injecting 0.2 mL of the respective solutions by i.p once daily from 22–28 d for one hour before the challenge (Fig. 2). All mice were sacrificed by an overdose of sodium pentobarbital. This experiment was approved by the Animal Ethics Committee of China Medical University. The whole process of the experiments followed the Chinese National Regulations for Animal Care. (Approval No. 2021PS666K).

Mice were sensitized on days 1, 8, and 15 and challenged from day 22 to day 28 with the same volume of saline (control) or 2% ovalbumin (OVA) solution for 30 min (test groups). Some of the mice were injected with dexamethasone or carbenoxolone before the OVA challenge. Airway responsiveness was measured in all mice before they were sacrificed on day 29.
All left lung specimens were immersed in 4% paraformaldehyde for 48 h. Subsequently; the fixed paraffin-embedded blocks were sliced to 4 µm sections. Hematoxylin-eosin (H&E), periodic acid-Schiff (PAS; Beyotime, Shanghai, China) and Masson trichrome staining (Solarbio, Beijing, China) were performed to assess peribronchial and perivascular inflammation, goblet cell metaplasia, and collagen deposition, respectively. The H&E-stained sections were semi-quantified; a scoring standard was created for stain assessment as previously described.18) Finally, a suitable CBX dose was chosen for follow-up experiments based on the pathological staining results of H&E. The percentage of PAS-positive cells and collagen fibril deposition were analyzed using the ImageJ 1.8.0 software (National Institutes of Health).18,19) Histopathological analysis was done in six mice from each group.
Cell Counts and ClassificationBronchoalveolar lavage fluid (BALF) was collected by repeated (×3) lung washing with 1 mL of phosphate-buffered saline (PBS) in each mouse. The BALF was centrifuged at 1000 rpm for 5 min at 4 °C, and the cell pellets were resuspended in 0.5 mL PBS. The resuspention was prepared for cell count using a hemocytometer and was stained using Wright–Giemsa staining (Solarbio). Approximately 200 cells were counted to analyze the proportion of eosinophils, neutrophils, monocytes, and lymphocytes for each mouse.
Detection of Airway Resistance (AR)Non-invasive airway mechanics (NAM; Data Sciences International Inc., Minneapolis, MN, U.S.A.) was used to measure AR in animals using barometric plethysmography. The animals were placed in a specially designed respiratory plethysmography chamber; specific airway resistance (sRaw) was calculated by detecting the phase difference between the nasal airflow and the chest airflow. The maximum box airflow signal occurring during one breath in a negative direction is defined as the peak inspirations flow rate, whereas the maximum box airflow signal occurring during one breath in a positive direction is defined as the peak expiratory flow rate. The time gap between the airflow from the chest to the nasal cavity is called delay time (DT). The changes in box pressure during expiration (PEP) are more apparent than that during inspiration (PIP) in bronchoconstriction. The formula of enhanced pause (Penh) empirically monitors airway function in this study: Penh = (DT × PEP)/PIP. Enhanced pause is a function of the proportion of the airflow signal from PIP and PEP and the timing of expiration.20) The time constant of the box was 0.02 s; the AR of each mouse was recorded for 5 min.
ImmunohistochemistryLung sections (3 µm) were dewaxed, hydrated, and boiled for antigen modification. Immunohistochemistry kits (MXB, Fuzhou, China) were used, according to the manufacturer’s instructions. Primary antibodies NLRP3 were obtained from Affinity Biosciences (1 : 200; Jiangsu, China). Evaluation of NLRP3 expression regions and the average optical density were analyzed by ImageJ software.
RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)Total mRNA was isolated using Trizol Reagent (Vazyme, Nanjing, China). The cDNA was synthesized using the extracted mRNA and a cDNA Synthesis Kit (Vazyme). It was amplified using an ABI7500 Fast thermocycler (Applied Biosystems, U.S.A.) and SYBR primerScript RT-PCR Kit (Vazyme). The primer sequences used were as follows: NLRP3: 5′-GGAGGAAGAAGAAGAGAGGAGAGGAG-3′ (forward = F), 5′-CTTGAGAAGAGACCACGGCAGAAG-3′ (reverse = R); Caspase-1: 5′-AGAGGATTTCTTAACGGATGCA-3′ (F), 5′-TCACAAGACCAGGCATATTCTT-3′ (R); IL-1β: 5′-CACTACAGGCTCCGAGATGAACAAC-3′ (F), 5′-TGTCGTTGCTTGGTTCTCCTTGTAC-3′ (R); and β-actin: 5′-GTGCTATGTTGCTCTAGACTTCG-3′ (F), 5′-ATGCCACAGGATTCCATACC-3′ (R). The relative mRNA fold changes were assessed using the 2−ΔΔct method with β-actin serving as the reference gene.
Western BlottingThe expression of NF-κB, p-NF-κB, inhibitor of kappaB (IκB)-α, p-IκB-α, NLRP3, pro-caspase-1, caspase-1, and IL-1β in each group were accessed using Western blotting. In each mouse, 20 mg of right lungs was homogenized in radioimmunoprecipitation assay buffer with a protease inhibitor cocktail. The lung samples (20 µg/lane) were separately electrophoresed in a 10% sodium dodecyl sulfate (SDS) polyacrylamide gel and transferred to polyvinylidene difluoride membranes. The membranes were sealed for 2 h in 5% skim milk in Tris-buffered saline containing 0.1% Tween 20 (TBST) at 22 ± 2 °C. The membranes were then incubated with primary rabbit NLRP3 (1 : 1000; CST, MA, U.S.A.), rabbit caspase-1 (1 : 1000; Proteintech, Hubei, China), mouse IL-1β (1 : 1000; CST), NF-κB, p-NF-κB, IκB-α, p-IκB-α (1 : 1000; CST) and rabbit tubulin antibodies overnight at 4 °C and incubated with secondary antibodies [Goat anti-mouse/Goat anti-rabbit (1 : 10000; Proteintech)] for 2 h at 22 ± 2 °C. After rinsing with TBST, the chemiluminescent signals from membranes were transformed into images using an Amersham Imager 600 (GE Healthcare Life Sciences, U.K.) and analyzed using the ImageJ software.
Statistical AnalysisAll data were analyzed using one-way ANOVA for differences between groups using the GraphPad Prism software version 9.0 (GraphPad Inc., U.S.A.) and are expressed as means ± standard deviation. p < 0.05 was considered statistically significant.
The results of H&E-stained lung sections showed that there was significantly higher inflammatory cell lung infiltration in the OVA group than in the normal group. Compared with the asthma group, CBX at 5 and 10 mg/kg doses had no significant effect on airway inflammation, and the difference was not statistically significant. However, DEX and CBX (20 and 40 mg/kg·d) group sharply attenuated histological changes, and there was no significant difference in the treatment group (Figs. 3A, B).

(A) Pictures show representative samples from the different groups (magnification ×200); (B) Inflammation analysis of every group is shown (n = 6); Wright–Giemsa staining results of each group (C) and changes in (D) the total numbers of cells, (E) eosinophils, (F) neutrophils, (G) monocytes, and (H) lymphocytes in BALF (n = 3). * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, and ns represents nonsignificant differences.
Furthermore, the inflammatory cells of morphological analysis in BALF were detected by Wright’s–Giemsa staining. A higher total number of leucocytes and a higher percentage of eosinophils and neutrophils were observed after the induction of OVA. Notably, the 20 mg/kg and 40 mg/kg dose of CBX group distinctly lowered the count of inflammatory cells in BALF compared to that in the asthma group (Figs. 3C–H). Therefore, we chose CBX at a dose of 20 mg/kg over CBX at a dose of 40 mg/kg for subsequent experiments, as the dosage was lower, yet it significantly reduced lung inflammation.
CBX Inhibits AR of Allergic MiceWe used sRaw and Penh to measure the effects of CBX on AR in animals. The OVA-challenged mice had remarkably increased Penh and sRaw values than the normal mice, which were significantly attenuated by CBX (Fig. 4).
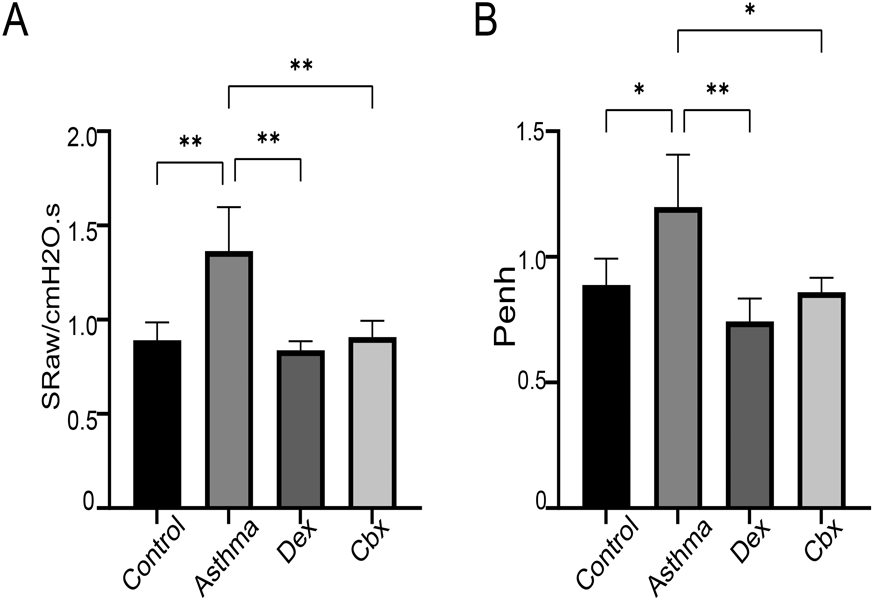
* p < 0.05, ** p < 0.01, and *** p < 0.001.
To estimate the effects of CBX on OVA-induced goblet cell hyperplasia and collagen deposition in the airway epithelium around the bronchi, PAS and Masson stainings were performed, respectively. As shown in Figs. 5A and B, in comparison with that in the control group, the percentage of PAS+ goblet cell in the OVA group was dramatically increased. Similarly, thicker-walled alveoli and an accumulation of collagen around the bronchi were heavier in the asthma model than in the normal group. However, CBX decreased these pathological changes (Figs. 5C, D).

(A) Representative periodic acid-Schiff (PAS)-stained showing airway mucus secretion in each group. (B) The number of PAS+ epithelial cells was quantified as a percentage by ImageJ. (C) Representative Masson-stained lung sections showing collagen deposition in different groups. (D) Semiquantitative of Masson staining was performed using ImageJ software; (magnification ×200). * p < 0.05, ** p < 0.01, and *** p < 0.001.
Immunohistochemistry results showed little or negligible NLRP3 expression in the control mice; however, we found a significant increase of NLRP3 expression after sensitization and challenge with OVA, which was reversed by CBX (Figs. 6A, B).

(B) Semi-quantification of NLRP3 expression in the airway epithelial cells of lungs was analyzed using ImageJ software; (magnification ×200). * p < 0.05, ** p < 0.01, and *** p < 0.001.
NLRP3, caspase-1, and IL-1β mRNA expression levels were largely elevated in the asthma group than in the control group, and CBX had markedly decreased mRNA expression level (Figs. 7A–C). The results revealed that the airway of asthmatic mice was in a state of inflammation, however, it improved significantly after CBX treatment.

(A) NLRP3 mRNA, (B) caspase-1 mRNA, (C) IL-1β mRNA in every group. * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001.
The protein expression of NF-κB, p-NF-κB, IκB-α, p-IκB-α, NLRP3, pro-caspase-1, caspase-1, and IL-1β increased in OVA-induced mice, as compared with that in the control mice, whereas CBX reduced the proteins level of p-NF-κB/NF-κB, p-IκB-α/IκB-α, NLRP3, pro-caspase-1, caspase-1, and IL-1β. These finding showed that the NF-κB pathway up-regulates the expression of NLRP3 and inflammatory factors, thereby promoting airway inflammation in the asthma group. CBX attenuates OVA-induced-inflammation by modulating NF-κB signaling. In addition, Caspase-1 is separated from pro-caspase-1, and our study showed that NLRP3, pro-caspase-1, caspase-1, and IL-1β protein expression was consistent with the changes in the mRNA expression (Figs. 8A–H).
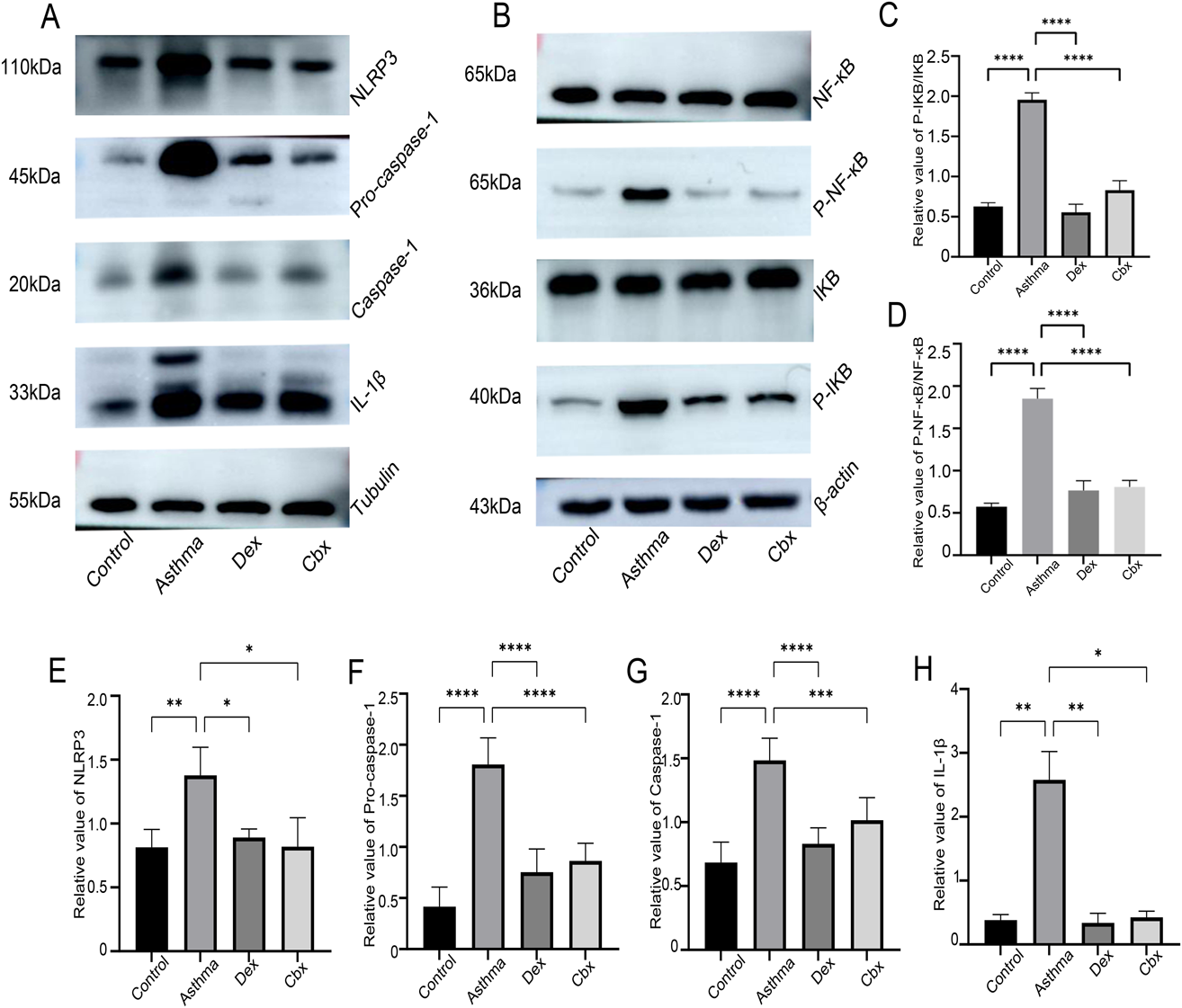
Relative levels of (C) p-NF-κB/NF-κB, (D) p-IκB-α/IκB-α, (E) NLRP3, (F) pro-caspase-1, (G) caspase-1, and (H) IL-1β are shown. * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001.
Asthma is a respiratory disease characterized by heterogeneous chronic airway inflammation. The pathobiology of airway inflammation involves multiple cell types, including airway epithelial cells, dendritic cells, airway smooth muscle, endothelial cells, eosinophils, neutrophils, mast cells, and CD4+ T-cells and is characterized by airway infiltration.21) The airway epithelium plays a vital role in host defense and immune responses. There is considerable evidence that the airway epithelium is dysfunctional in asthma and plays a key role in the progression and exacerbation of the disease.22) In addition, airway inflammation promotes mucus production, airway wall remodeling, and AHR; asthma exacerbation induces an immunoglobulin E response that leads to hypersecretion of mucus from goblet cells.23) Due to airway inflammation, eosinophils and mast cells are regulated to release numerous different mediators, such as major basic proteins, histamine, and leukotrienes. This may lead to AHR, bronchoconstriction and eventually lead to remodeling of the lung.24) Airway remodeling is usually detrimental and can lead to continued airflow limitation, impaired lung function, and AHR.25) The results of our study were consistent with those from previous studies on OVA-stimulated allergic asthma models. The number of leukocytes, particularly eosinophils, in BALF increased significantly. There were many inflammatory cells infiltrating the bronchi and blood vessels, with mucus hypersecretion, goblet cell hyperplasia, peribronchial fibrosis, and increased airway resistance in the lungs. Meanwhile, we found that treatment with CBX significantly attenuated inflammatory infiltration in BALF and lung tissue. Moreover, we confirmed the anti-inflammatory properties of CBX in allergic asthma. Notably, CBX alleviated hyperplasia of airway goblet cells and collagen deposition around bronchi. The decreased AR may be related to these characteristics of CBX. These results show that CBX has protective effects on the airways against allergic asthma.
In recent years, research studies have identified five major inflammasomes: NLRP1, NLR CARD domain-containing protein 4, retinoic acid–inducible gene I, absent in melanoma 2, and NLRP3.2) Extensive evidence suggests that NLRP3 is the most widely known phenotypic inflammasome, and it is involved in the development of asthma.2,7) Inflammasomes are significantly expressed in the airway epithelium during inhalation of allergens and other pathogens, which activates the innate immune responses and has protective effects on the body.26) However, over-activated inflammasomes can prompt excessive inflammation and tissue damage and induce asthma. Kim et al.27) showed that OVA activates NLRP3 and promotes the expression of caspase-1 and IL-1β by inducing mitochondrial reactive oxygen species production. In OVA-stimulated asthmatic mice, NLRP3 was blocked with an OLT1177® inhibitor, which diminished airway inflammation as evidenced by reducing eosinophil and neutrophil numbers; TNF, IL-1β, IL-6, and IL-13 cytokine release in BALF; inflammatory cell infiltration; and significantly reduced AHR.28) Our data indicated that NLRP3 is mainly expressed in bronchial epithelial cells and the expression of NLRP3, caspase-1, and IL-1β mRNA and protein in lungs are significantly elevated after OVA stimulation, further confirming that allergic airway inflammation is related to NLR signal activation. Meanwhile, our results also indicated that CBX significantly inhibited the expression of NLRP3, caspase-1, and IL-1β mRNA and protein in lungs and alleviated pulmonary inflammation in OVA-induced asthma.
Inflammation appears to be a factor in the majority of chronic diseases. NF-κB is a key mediator of inflammatory mechanisms, and its dysregulation has been associated with a variety of chronic diseases, including asthma, cancer, diabetes, rheumatoid arthritis, and neurological disorders.29) The research showed that inflammatory responses mediated by NF-κB signaling pathway have a significant impact on status epilepticus.30) Increased NF-κB activity in the airway has been observed in human and animal asthma studies, and it is critical in the initiation and persistence of allergic inflammation.31) In unstimulated cells, NF-κBs are predominantly located in the cytoplasm and bind to the proprietary inhibitor proteins including IκB family. The NF-κB pathway is activated after proteasome degradation of IκB proteins, which results in NF-κB dimer transfer from the cytoplasm to nucleus, to complete nuclear localization and induce gene expression. The canonical P50/P65 heterodimer is predominantly regulated by IκB-α.32,33) Previous articles have proved that activation of NF-κB leads to increasing the transcription of NLRP3 gene.34) In acute kidney injury, activated NF-κB pathway induces downstream NLRP3 activation to promote inflammatory response.35) In this study, the phosphorylation rates of IκB-α and NF-κB were significantly increased in the lung tissues of asthmatic mice induced by OVA. Our results further support the activation of the NF-κB pathway that mediates airway inflammation in asthma, and we also observed changes in NLRP3 and inflammatory factors following IκB/NF-κB activation in asthmatic mice. In addition, after CBX treatment, the phosphorylation ratio of the IκB/NF-κB was reduced; further reducing NLRP3 and inflammatory factors expression. These results suggest that CBX regulates pulmonary inflammation in asthmatic mice by partially inhibiting the activity of NF-κB/NLRP3 pathway.
CBX is a known 11β-hydroxysteroid dehydrogenase (11β-HSD) inhibitor, and this enzyme is highly expressed in the liver, adipose tissue, lungs, and areas in the central nervous system.36) Asthmatic effects are enabled by impaired pulmonary endogenous glucocorticoid activation that is attributed to the upregulation of 11β-HSD levels.37) Therefore, CBX exerts anti-inflammatory effects by stimulating the adrenal gland or increasing endogenous corticosteroid secretion. Its anti-inflammatory properties are mainly used for dyspepsia, digestive ulcers, and to a lesser extent, skin disease.38) CBX is also a pannexin 1 channel inhibitor. The pannexin 1 channel is a nonselective, large-pore channel that is responsible for the release of nucleotides and ATP, which induces cations in intracellular and extracellular fluid to cross the plasma membrane, resulting in K+ outflow and NA+ inflow. Evidently, K+ outflow triggers NLRP3 activation. Therefore, CBX may play an anti-inflammatory role by inhibiting extracellular ATP release, weakening K+ outflow, and preventing excessive activation of the inflammasome.39,40)
In conclusion, our data demonstrated that CBX significantly alleviates asthmatic features, particularly, airway inflammation, mucus hypersecretion, airway remodeling, and AR most likely by inhibiting the activation of the NF-κB pathway and further preventing the excessive expression of NLRP3 inflammasome. Our results also made theoretical foundation for the mechanism of CBX in treating bronchial asthma. To the best of our knowledge, only a few studies have proposed the relationship between CBX and NLRP3 in allergic asthma. However, the specific regulatory mechanism between CBX and NF-κB pathway has not been studied in this experiment. Hence, further research is required to elucidate the potential of CBX as a drug therapy for inflammatory disease.
This project was supported by the National Natural Science Foundation of China (81800029) and Liaoning Higher Education Innovation Team Support Plan of the Educational Department of Liaoning Province [(2018) 479].
The authors declare no conflict of interest.