2022 年 45 巻 7 号 p. 863-871
2022 年 45 巻 7 号 p. 863-871
Saikosaponin A (SSA)—a natural compound extracted from Radix bupleuri—possesses antitumor properties in several types of carcinomas. However, the role of SSA on bladder cancer and the mechanisms remain unclear. In this study, we have described the effect of SSA on human bladder cancer cell lines T24 and 5637 in the context of the regulation of mitochondrial pathways of apoptosis. In vitro, the Cell Counting Kit-8 (CCK-8) assay and cell wound healing assays were used to determine the proliferative effect of SSA treatment. Flow cytometry and Western blotting were performed to evaluate the apoptosis and related mechanisms. To further confirm that apoptosis is mediated through Caspase activation, Hoechst 33258 fluorescence staining assay was done after cells were treated with SSA and caspase inhibitor-Z-VAD-FMK. In vivo, an orthotopic xenograft mice model was adopted to evaluate the effect of SSA. The tumors were analyzed by hematoxylin-eosin (H&E) staining, immunohistochemical analysis, and Western blotting. In vitro, the results with CCK-8 assay showed obvious SSA-induced suppression in cell growth in a dose- and time-dependent manner. Flow cytometry analysis, Hoechst 33258 fluorescence staining assay and the assessment of the changes in the B-cell lymphoma 2 (Bcl-2) family protein expression level revealed that SSA could significantly induce cell apoptosis, which was associated with apoptosis via the mitochondrial pathways. In vivo, the results revealed a reduction in cell proliferation. In conclusion, our data suggest that SSA inhibits the growth of bladder cancer cells by activating the mitochondrial apoptosis pathway and inducing cell apoptosis.
As the tenth-most common cancer type, bladder cancer (BC) is a urinary system malignant tumor.1) According to the statistical analysis, in 2020, approximately 570000 people across the world were diagnosed with BC, of which 210000 died2); in this affected population, men accounted for the majority (4/5th of all).3,4) Meanwhile, the average age of the patients diagnosed with BC is 70 years, of which the majority of patients were aged >55 years.5,6) Despite the advancement in modern medicine, the annual prevalence of BC has been gradually increasing, while simultaneously showing a trend of affecting a greater number of the younger population.7)
BC is of two types: non-muscle-invasive BC (NMIBC) and muscle-invasive BC (MIBC),8) accounting for approximately 70–80 and 20–30% of all cases, respectively.9) In terms of clinical treatment, the most commonly applied treatment for NMIBC is transurethral resection of the bladder tumor (TURBT).10) After this surgery, Bacillus Calmette–Guerin Vaccine (BCG) infusion is supplemented to the patient.11,12) The common clinical treatment strategy for MIBC is radical cystectomy with urinary diversion.13–15) Nevertheless, the 5-year survival rate with this intervention for BC is relatively low, often accompanying serious adverse reactions and a high recurrence rate.16) Therefore, it is of immense importance to establish a new, effective, and safe therapy urgently.
Radix bupleuri is a traditional Chinese medicine (TCM) that offers the properties of clearing heat and protecting the liver (Fig. 1).17) Recent reports have suggested the anti-inflammatory and anti-tumor roles of the active extract Saikosaponin A (SSA).18,19) However, SSA has not been applied in the treatment of bladder tumors yet. Its effect on BC cells and the mechanism behind its efficacy remain to be established. Thus, in the present study, we explored the anti-tumor efficacy of SSA on human BC cells through in vitro and in vivo experiments.

Human BC cell lines T24 (Item No. CX0309) and 5637 (Item No. CX0002) were acquired from the BOSTER Biological Technology Co., Ltd. (Wuhan, China) and cultured in RPMI-1640 medium supplemented with 10%(v/v) fetal bovine serum (FBS) and 1% penicillin–streptomycin in an atmosphere of 5% CO2 at 37 °C.
Chemicals and ReagentsSSA (CAS Number: 20736-09-8) was provided by Alfa Biotechnology Co., Ltd. (Chengdu, China) with a purity of ≥98%, and it was dissolved in dimethyl sulfoxide (DMSO) before use. The final concentration of the solution was adjusted to 20 mM and the solution was stored at −20 °C. RPMI-1640, phosphate-buffered saline (PBS), and FBS were purchased from Gibco (Grand Island, NY, U.S.A.). Rabbit monoclonal antibodies against bad (#9292), bak (#12105), bax (#2772), B-cell lymphoma 2 (bcl-2) (#3498), caspase-3 (#9662), and caspase-9 (#9508) were obtained from Cell Signaling Technology (MA, U.S.A.). β-Actin (AC026) and β-tubulin (AC021) were acquired from ABclonal Technology (Wuhan, China). Horseradish peroxidase (HRP)-conjugated immunoglobulin G (IgG) (BL003A) was purchased from Biosharp Technology (Hefei, China).
Cell Viability AssayThe efficacy of SSA on cell viability was determined by the cell counting kit-8 (CCK-8 assay) (RUI JIE Biotech Co., Ltd., Hefei, China). Briefly, T24 and 5637 cells were seeded in a 96-well plate at the density of 1 × 104 cells/well and cultured overnight. After the treatment with different concentrations (0, 3.75, 7.5, 15, and 30 µM) and for different periods (12, 24, and 48 h), 10 µL of the CCK-8 reagent was added to each well, followed by incubation at 37 °C for 2 h and the measurement of cell viability and IC50 values via relative optical density reading at 450 nm. The data obtained were averaged for six repetitions.
Wound-Healing AssayA wound-healing assay was performed to detect the different migration rates of cells between the control and experimental groups. After the confluence of T24 and 5637 cells in a 6-well plate reached 80–90%, the confluent cell monolayer was scratched with sterile 10-µL pipette tips, followed by rinsing gently with PBS to remove the detached cells. Then, the cells were treated with 7.5 µM of SSA in an FBS-free medium at 37 °C for 48 h. Cell migration was recorded at 0 h and 48 h and photographed with an inverted microscope (DMI3000B) (Leica Optical Company, Ltd., Germany). The wound healing rate was evaluated using the following formula: [1 − (empty area 48 h/empty area 0 h)] ×100%.
Flow Cytometry of Apoptosis AnalysisThe confluent cultures of T24 and 5637 cells were treated with either DMSO alone or different doses of SSA (0, 3.75, 7.5, and 15 µM) for 24 h. Subsequently, the cells were collected through trypsinization and centrifugation. The cell pellets were washed twice with 4 °C pre-cold PBS and resuspended in 500 µL of the binding buffer. Before testing on the machine, the cells were incubated with Annexin V/propidium iodide (PI) dye solution for 10 min at room temperature in the dark. The percentages of apoptosis were evaluated by the FACS Calibur flow cytometry (BD Biosciences, San Jose, CA, U.S.A.) using the Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection Kit (KeyGEN Biotech Co., Ltd., Jiangsu, China) according to the manufacturer’s protocol.
Hoechst 33258 Fluorescence Staining AssayHoechst staining was performed following the methods of Li et al. to evaluate the apoptosis of 5637 cells.20) Cells were plated into 6-well plates (2 × 105 cells per well) and exposed to Z-VAD-FMK (50 µM) and SSA (7.5 and 15 µM) for 24 h. After that, the cells were rinsed twice with PBS. Then, the cells were incubated with Hoechst 33258 (Beyotime Institute of Biotechnology, China) at 25 °C for 10 min. After washed with PBS, the samples were placed on a slide for fluorescence microscopy. The Hoechst-stained nuclei were visualized by fluorescence microscope with emission 350–460 nm (×200).
Western BlottingWestern blotting was performed using cell lysates treated with the desired concentrations of SSA (0, 3.75, 7.5, 15 µM) for 24 h or with xenograft tumor tissue homogenates. The protein was extracted by incubating the reactants in radio immunoprecipitation assay (RIPA) Lysis Buffer (Dingguo Biotechnology, Beijing, China) on an ice bath for 30 min, followed by centrifuging the lysates at 12000 × g for 10 min at 4 °C. A total of 30 µg of the protein mixture was assessed by the bicinchoninic acid (BCA) Protein Assay Kit separated on 15% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) gel electrophoresis per lane and then transferred onto the polyvinylidene difluoride (PVDF) membranes (Merck Millipore, Burlington, MA, U.S.A.). Subsequently, the membranes were blocked with 5% nonfat milk in tris buffered saline-tween 20 (TBST) at room temperature for 1 h, followed by immunoblotting with specific primary antibodies (dilution at 1 : 1000) at 4 °C overnight. After the incubation period, the membranes were washed with TBST thrice (for 10 min each time) before incubating with peroxidase-conjugated secondary antibodies at room temperature for 1 h. Finally, the membranes were washed thrice with TBST over 30 min and visualized with the enhanced chemiluminescence (ECL) Detection Kit (Merck Millipore) in the ChemiDoc XRS+ (BIORAD, Hercules, CA, U.S.A.). The optical density quantification of protein blot was performed using the Image J software.
The 5637 Tumor Xenograft StudyA total of 30 male BALB/c mice (weight: 18–20 g; age: 4–6 weeks) were purchased from the Charles River Laboratories (Beijing, China). A total of 5 × 106 of the 5637 cells were subcutaneously injected into the right armpit of the experimental mice. On detecting palpable lumps, the mice were randomly categorized into 5 groups (n = 6/group) and respectively treated intraperitoneally (i.p.) with different doses of SSA (2, 5, and 10 mg/kg) or gemcitabine (200 mg/kg) or with vehicle alone once every 2 d. The mice were monitored every alternate day, and the tumor volumes were calculated using the following formula: V (mm3) = length × width2 × 0.5. These mice were then sacrificed by cervical dislocation after a 3-week treatment, and the tumor samples were washed with normal saline and subjected to Western blotting, hematoxylin and eosin (H&E) staining, and immunohistochemical assays. All animal studies were performed as approved by the Laboratory Animal Center at the North Sichuan Medical College.
H&E StainingThe tissue pre-fixed in formalin was placed into a 15% ethylenediaminetetraacetic acid (EDTA) decalcification solution until completing the decalcification process. After decalcification, the tissues were dehydrated and embedded, followed by deparaffinization and hematoxylin staining for 10–20 min. We then treated the tissues with hydrochloric acid and alcohol for 5–10 s and placed them into ammonia solution until blue appears. Finally, the tissues were stained with eosin for 3–5 min and sealed in neutral gum.
Immunohistochemical AnalysisAntigen retrieval was performed by placing the tissues in citrate buffer under microwave for 15 min. After the peroxidase activity was blocked with 3% H2O2 for 25 min, the sections were incubated with the normal goat serum for an additional 30 min to block all nonspecific antibody bindings. The sections were then immunoblotted with primary antibodies against bax (AF0120), bcl-2 (AF6139), and caspase-3 (AF6311) (1 : 100) (Affinity Biosciences, U.S.A.) at 4 °C overnight. Then, the slices were rinsed for 5 min with PBS thrice, followed by the incubation of the sections with biotinylated IgG for 50 min at room temperature. After rinsing, the results were observable with diaminobenzidine (DAB) and counterstained with hematoxylin. The images were acquired under a microscope at 200× magnification in three random high-power fields. The Image Pro-Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, U.S.A.) was used to analyze the images. The brown color developed in the immunohistochemistry test was set as the unified standard for judging the positive reaction of all photographs, and each photograph was analyzed to obtain the integral optical density (IOD) and the pixel area of the tissues (AREA) for each photograph. Then, the average optical density values (AOD) were calculated using the formula AOD = IOD/AREA.
Statistical AnalysisImage J software was employed to analyze the results of the scratch experiment and the Western blot results. The SPSS 24.0 software was employed for data statistical analysis, and the Graphpad Prism 8.0 was used to draw the charts. The data were expressed as ̄x ± s. p < 0.05 was considered to indicate a statistically significant difference. Each experiment was repeated 3 times independently.
In the study, the antiproliferative effect of SSA on the BC cells was calculated by incubating the T24 and 5637 cells were incubated with varying concentrations of SSA (0, 3.75, 7.5, 15, 30 µM) for 12, 24, and 48 h. Then, the cell viabilities were assessed by using the CCK-8 Assay Kit. As is shown in Fig. 2, SSA could inhibit the cell viabilities of T24 and 5637 cells in a dose- and time-dependent manner. After 12, 24, and 48 h, the IC50 values of SSA on T24 and 5637 cells are listed in Table 1.

The cells were seeded in a 96-well plate at the density of 1 × 104 cells/well and treated with different concentrations of SSA (3.75–30 µM) for 12, 24, and 48 h (A) T24 cell line. (B) 5637 cell line. The data are presented as the mean ± standard deviation (S.D.) of three independent experiments; * p < 0.05, ** p < 0.01, *** p < 0.001 statistically significance when compared with control group (one-way ANOVA).
| T24 | 5637 | |
|---|---|---|
| 12 h | 12.11 ± 0.37 | 15.20 ± 0.63 |
| 24 h | 11.06 ± 0.28 | 7.99 ± 0.33 |
| 48 h | 9.67 ± 0.34 | 6.22 ± 0.24 |
The ability of SSA inhibiting T24 and 5637 cell migration was examined by employing a wound-healing assay. In this study, we used a serum-free conditioned medium to eliminate the interference of cell proliferation. After 48 h of SSA treatment (7.5 µM), the percentages of migrated cells markedly decreased in the treatment group relative to that in the control group (Figs. 3A, B). Finally, the cell migration rate of the control group (0 µM) was found to be 39.53 ±3.87%, whereas that of the experimental group (7.5 µM) was found to be 23.51 ±1.20% in T24 cell (p < 0.01; Fig. 3C). The cell migration rate of the control group (0 µM) was 59.30 ±3.62%, while that of the experimental group (7.5 µM) was 11.57 ±1.52% in 5637 cells (p < 0.001; Fig. 3D). The differences were found to be statistically significant.

The cells were seeded in a 6-well plate and treated with SSA (0 and 7.5 µM) for 48 h. At 0 and 48 h, the photomicrographs of cleft formed and the migration rates were obtained. (A, B) Photomicrographs of the scratch in the T24 and 5637 cell lines, respectively. (C, D) Migration percentages in T24 and 5637 cell lines, respectively. Scale bar = 50 µm. The data are presented as the mean ± S.D. of three independent experiments; * p < 0.05, ** p < 0.01, *** p < 0.001 indicate statistical significance when compared with the control group (Student’s t-test).
To examine whether the decline in cell viability was attributable to SSA-induced cell apoptosis, we treated the cells with different concentrations of SSA (0, 3.75, 7.5, 15 µM) for 24 h, and then detected the cell apoptosis rate via the Annexin V-FITC/PI assay. We observed that the percentage of apoptosis increased in a dose-dependent manner (Figs. 4A, B). Specifically, SSA treatment increased the apoptosis rates from 6.29 ±1.25% at 0 µM to 26.44 ±3.71% at 15 µM concentration in T24 cells (p < 0.001; Fig. 4C). Similarly, the apoptosis rates increased from 5.41 ±0.63% at 0 µM to 21.33 ±2.46% at 15 µM in 5637 cells (p < 0.001; Fig. 4D). The differences were found to be statistically significant.

The cells were treated with SSA (3.75–15 µM) for 24 h, and the apoptotic cells were analyzed by FACS to determine the relative percentage of apoptotic Annexin V/PI cells. (A, B) Flow cytometry graphs of T24 and 5637 cells, respectively. (C, D) Quantitative analyses of the percentage of apoptotic T24 and 5637 cells, respectively. The data are presented as the mean ± S.D. of three independent experiments; * p < 0.05, ** p < 0.01, *** p < 0.001 indicate statistical significance when compared with the control group (one-way ANOVA).
We utilized the caspase inhibitor Z-VAD-FMK to find the relationship between SSA promoting apoptosis and inhibiting cell growth. After SSA treatment, cell apoptosis was checked by Hoechst 33258 nuclear staining. As shown in Fig. 5, we observed that compared with the combination of SSA and Z-VAD-FMK, in the SSA alone group, in SSA-treated cell groups, more cells showed bright blue fluorescence and nuclear pyknosis, indicating cell apoptosis. This result suggests that Z-VAD-FMK attenuated SSA-induced apoptosis and SSA may promote apoptosis through the Caspase pathway, which we will further verify in subsequent experiments.

To elucidate the potential mechanisms of SSA on T24 and 5637 cells apoptosis, the protein expression of the Bcl-2 family, caspase-3, caspase-9, and cleaved caspase-9 were detected after treatment with SSA for 24 h. As shown in Fig. 6, the relative expression of pro-apoptotic protein bad, bak, and bax was upregulated, accompanied by a decrease in the anti-apoptotic protein bcl-2 level in a dose-dependent manner. Furthermore, the relative expression levels of cleaved caspase-9 were found to have increased. However, the cleaved caspase-3 protein (17 kDa) was the smallest protein among all caspase-cleaved proteins and hence easily degradable, possibly making it undetectable by Western blotting. Therefore, we conducted a quantitative analysis of the value of the caspase-3/β-actin and found that its ratio decreased with increasing concentration. Therefore, we can conclude that SSA-induced apoptosis was achieved by promoting the expression of pro-apoptotic proteins and inhibiting the expression of anti-apoptotic proteins in the mitochondrial-mediated apoptosis pathway.

(A) Representative Western blotting images of T24 and 5637 cell lines. (B) Quantification of the relative expression levels of apoptosis-associated proteins bad, bak, bax, bcl-2, caspase-3, caspase-9, and cleaved caspase-9. β-Actin served as the loading control. The data were expressed as the mean ± S.D. of three independent experiments; * p < 0.05, ** p < 0.01, and *** p < 0.001 indicates statistical significance when compared with the control group (one-way ANOVA).
Since SSA inhibited the proliferation of BC cells in vitro, we further detected the in vivo effect of SSA on tumor xenografts formed through the hypodermic injection of 5637 cells. Within 4 weeks of cell inoculation, the weight of nude mice in all groups increased (Fig. 7A); however, at the end of the experiment, no significant difference in the body weight was noted between different groups (Fig. 7C). Moreover, the tumors of the SSA treatment group were significantly smaller than those of the control group (Figs. 7B, E). The tumor volumes in the groups treated with SSA at the concentration of 2, 5, and 10 mg/kg were 91.41, 72.33, and 46.98% of the control group, respectively (Fig. 7D). These results revealed that SSA could inhibit the growth of bladder tumors in vivo.
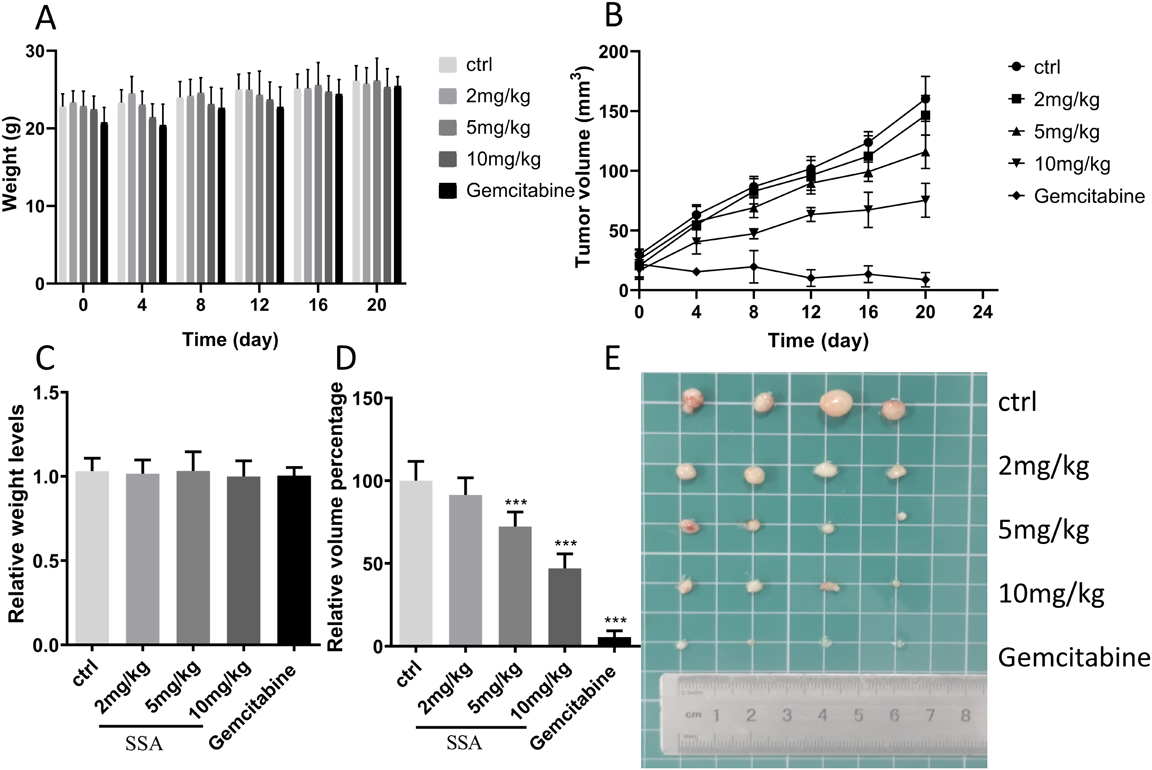
A total of 5 × 106 of the 5637 cells was inoculated into 4-week-old male BALB/c mice subcutaneously in the right flank. Mice were i.p. treated with SSA (2, 5, and 10 mg/kg), vehicle (normal saline), or gemcitabine (200 mg/kg) every alternate day for 3 weeks (n = 6/group). (A) Bodyweight changes in each group. (B) Tumor growth curves of xenografts in nude mice. (C) One-way ANOVA analysis of weight. (D) One-way ANOVA analysis of tumor volumes. (E) Isolated tumors 1-month after inoculation of the 5637 cells. The data were expressed as the mean ± S.D. of three repeated measurements; * p < 0.05, ** p < 0.01, *** p < 0.001 indicate statistical significance compared with the control group (one-way ANOVA).
As is shown in the histological analyses of tumor tissues, the cross-sectional area of the tumor, the proliferation ability of tumor cells, and the pathological mitosis decreased gradually after treatment with SSA when compared with that in the negative control group. The numbers of necrotic cells and swollen cells were increased, and different numbers of nuclear fragments were scattered (Fig. 8A).

The protein expression levels were analyzed based on the average optical density (IOD/area) of three random high-power staining areas by using the Image-pro Plus 6.0 software. (A) Histological analyses of the tumor tissues. Scale bars = 200 µm. (B–D) Photomicrographs of intense immunohistochemical staining of caspase-3, bcl-2, and bax in xenograft tumor tissues. Scale bars = 200 µm. (E) Average optical density analyses. Data are presented as the mean ± S.D. of three independent experiments; * p < 0.05, ** p < 0.01, *** p < 0.001 indicate statistical significance relative to the control group (Student’s t-test).
Then, immunohistochemistry analyses of bax, bcl-2, and caspase-3 were performed on tumor tissues treated with SSA. Collectively, our results revealed that the positive expression rates of caspase-3 and bcl-2 gradually decreased with increasing concentration of SSA (Figs. 8B, C), on the contrary, the expression rate of bax increased (Fig. 8D), with the differences of AOD indicating statistical significance at p < 0.05 (Fig. 8E). These results suggest that SSA inhibited the proliferation and induced apoptosis in BC cell xenograft; this result is consistent with those of our in vitro experiments.
SSA Modulated the Proteins Levels of Bad, Bak, Bax, Bcl-2, Caspase-3, and Caspase-9 in 5637 BC Xenograft TumorsWe had observed that SSA treatment could regulate the expression levels of apoptosis-related proteins under in vitro conditions. In addition, we found that the expression of bad, bak, and bax in the tumor tissues from 5637 xenograft mice treated with SSA showed a significant increase (Figs. 9A–D). In contrast, we found that SSA treatment downregulated the expression of bcl-2 and activated the expressions of caspase-3 and caspase-9 in the intracranial xenograft mouse model (Figs. 9E–H). These results further confirmed the results of our in vitro study, indicating that SSA plays an important role in the process of inducing cell death through the mitochondrial apoptosis pathway.

(A) Representative Western blotting images of the tumor tissues. (B–H) Quantification of the relative expression levels of apoptosis-related proteins. β-Tubulin served as the loading control. The results are consistent with those from in vitro experiments. Data are presented as the mean ± S.D. of three independent experiments; * p < 0.05, ** p < 0.01, *** p < 0.001 indicate statistical significance relative to the control group (one-way ANOVA).
Cancer is a major public health concern all across the world, with an estimated 19.3 million new cases and 10.0 million cancer deaths occurring in 2020 alone.2) Although the TCM has long played a vital role in human healthcare, it has not received much attention. Surprisingly, in recent years, researches on the pharmacological activity and the action mechanism of TCM has gradually stepped onto the right track, with increasing evidence suggesting that TCM offers beneficial anti-inflammatory and anti-tumor properties.21–23)
R. bupleuri has been widely applied in TCM for >2000 years for its liver protection and heat-clearing effects.24) SSA is the main biologically active compound in R. bupleuri owing to its anti-inflammatory,25) antioxidant,26) and anti-tumor properties.27) The anti-tumor effects of SSA have been reported in several other cancer types, such as triple-negative breast cancer28) and colon cancer.29) However, only a few studies so far have focused on the effect of SSA on BC to further promote its anti-tumor effect in vivo.
Apoptosis, which is also referred to as programmed cell death, plays an important role in the physiological process of cell suicide.30,31) As a significant part of the health of an organism, apoptosis can protect organisms from the harmful effects of damaged cells, thereby allowing the healthy cells to perform their normal functions.32) When apoptosis occurs, the cell receives a specific signal that in turn cascades into a series of effects. As soon as this process occurs, the caspase family gets activated, leading to the development of biochemical and morphological changes in the cell.33)
Apoptosis signals received by cells can be mainly divided into two types: extrinsic and intrinsic. The extrinsic signals are mainly composed of ligands that mediate death, including the cell surface molecules of T-lymphocytes and soluble factors.34)
The intrinsic signal is mainly regulated by mitochondria. In this process, the protein family called the Bcl-2 family plays an important role.35) There are two types of proteins in the Bcl-2 family that exert opposite effects, one of which is anti-apoptotic proteins headed by bcl-2. They are distributed in the outer mitochondrial membrane and can inhibit the release of cytochrome c. The other type is pro-apoptotic proteins represented by bad, mainly including bak and bax.36) They are distributed in the cytoplasm and can promote the release of cytochrome c. Following apoptotic stimuli, these pro-apoptotic proteins get translocated to the outer mitochondria membrane and promote the release of cytochrome c.37) Subsequently, cytochrome c released from mitochondria can interact with the apoptosis protease-activating factor-1 (APAF-1) and caspase-9. The apoptotic body can then activate caspase-3 and initiate the caspase cascade that eventually achieves the goal of apoptosis.38,39)
In this study, we first tested the cell viability and migration after being treated with SSA, and the results indicated that SSA could inhibit cells’ growth and cause death (Figs. 2, 3). On the other hand, the flow cytometry results suggested that SSA could promote cell apoptosis in a dose-dependent manner (Figs. 4, 5). To comprehensively illuminate the mechanism of SSA on apoptosis, we detected the relative expression levels of apoptosis-related proteins by Western blotting. When compared with the control group (0 µM), bad, bak, and bax protein expressions were remarkably upregulated, whereas that of bcl-2 protein expression was downregulated (Fig. 6). Moreover, we could observe the changes in the expression of caspase-3 and caspase-9, which further confirmed the activation of the mitochondria-mediated apoptosis pathway.
As we had verified the effective anticancer efficacy of SSA against BC cells T24 and 5637 in vitro, the inhibition of SSA-treated nude mice by 5637-xenograft was further investigated in vivo.
Our results confirmed that SSA could effectively inhibit tumor growth induced by subcutaneous injection of BC cells and demonstrated no toxicity to nude mice (Fig. 7). The HE staining outcomes signified that the proliferation of tumor cells in the SSA treatment group was inhibited and that apoptotic cell death was induced (Fig. 8A). In addition, consistent with the in vitro findings, increased expression of pro-apoptotic proteins and decreased expression of anti-apoptotic proteins were also recorded in vivo (Fig. 9).
Our study findings highlight that a natural product, SSA, can exert an effect against BC cells. This compound could inhibit the proliferation of tumor cells and induce apoptosis. For the first time in the literature, we have revealed that SSA-induced apoptosis in BC cells regulated the mitochondrial apoptosis pathway that eventually induced cellular impairment in BC cells.
In summary, it is of great significance that SSA inhibited cell growth and induced apoptosis in human BC cells T24 and 5637 in this study by modulating the mitochondria-mediated apoptosis pathway with a strong effect. In the future, we expect that the application of SSA in cancer chemoprevention may be extensive.
The present study was supported by a Municipal-school cooperative scientific research project [Grant No. 19SXHZ0242], the Applied Basic research Program of Science and Technology Department of Sichuan Province [Grant No. 2016JY0032], and the Innovation and Entrepreneurship Training Program for College Students in Sichuan Province [Grant Nos. 201810634043, S201910634006, S201910634080, S201910634081, S202010634048, S202010634158].
The authors declare no conflict of interest.