2022 Volume 45 Issue 9 Pages 1225-1231
2022 Volume 45 Issue 9 Pages 1225-1231
In veterinary medicine, various drugs are used on a daily basis. Using inappropriate medications poses health hazards to companion animals and humans; thus, assessing adverse events in veterinary medicine has great social significance but remains an untapped area of research. In this study, to promote the appropriate use of veterinary drugs and clarify common pharmaceutical issues in Japanese veterinary medicine, we analyzed information in the Veterinary Drug Side Effects Database (National Veterinary Assay Laboratory of the Ministry of Agriculture, Forestry and Fisheries, Japan). We found that the number of reports has been increasing annually, including those on high-risk drugs, molecular-targeted drugs, and antibody-based drugs. The details of the reports were similar to those from the United States, including the misadministration of veterinary drugs to humans, improper drug management, and re-administering drugs with a history of side effects. Furthermore, 46.50% of all reports mentioned the administration of one or more drugs, with the highest number of concomitant drugs being 10. In addition, 37.78% of all reports described the use of drugs in manners deviating from the intended use indicated in the package insert. Therefore, to avoid adverse events, pharmacists may have to be involved in dispensing and aseptically preparing veterinary medicines and providing drug information and medication guidance. To optimize pharmacotherapy for ill companion animals, “veterinary pharmacy” and “veterinary medicine pharmacy” must be developed in line with clinical situations in Japan, while considering knowledge from countries that are advanced in terms of veterinary medicine.
In 2020, approximately 18133000 companion animals were being raised in Japan,1) exceeding the population of Japanese children younger than 15 years of age.2) This situation reflects the increased longevity of pets owing to advancements in veterinary medicine and the increasing incorporation of pets into families. Similar to humans, pets develop various diseases such as cancer and diabetes with increasing life span; thus, an increasing social need exists for more advanced veterinary medicine.3)
The global market for animal health is expected to expand further; domestic sales of veterinary drugs in 2019 totaled approximately 130 billion yen.4) Various medicines are used for animals on a daily basis, including those that humans have little experience with, such as molecular-targeted drugs. Indeed, improper use of medicines worsens not only the treatment outcome of the ill animals, but it may also adversely affect human health. Therefore, it is of great social significance to determine drug-related adverse events in veterinary medicine.
Pharmacists already play an important part in veterinary medicine in other countries.5) For example, the U.S. Food and Drug Administration (FDA) collects information on adverse events and medical malpractice pertaining to drugs used in veterinary medicine, and the utilization of such information is progressing.6) In Japan, a publicly available database hosting information regarding reported adverse effects of veterinary drugs has been established.7) However, there are only a few reports on the involvement of pharmacists in veterinary medicine or pharmaceutical research in Japan. Therefore, in this study, we analyzed the side effects of veterinary drugs in Japan and compared them with those reported in the United States.
Of 3874 reports, spanning from February 2002 to December 2020, available in the Veterinary Drug Side Effects Database of the Japanese Ministry of Agriculture, Forestry and Fisheries,7) 1438 reports on drugs (excluding vaccines and those related to industrial animals) were analyzed (aggregation period: February 12, 2021 to May 17, 2021). The database was created to store anonymized data of side effects related to veterinary drugs, which were reported according to the Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices (Act No. 145 of August 10, 1960).
Of 66525 reports submitted to the U.S. Animal & Veterinary Adverse Events database8) (made publicly available by openFDA in 2020), we analyzed 15860 reports with clear details related to off-label use of veterinary drugs to determine any deviations in use indicated in the package inserts.
Items InvestigatedWe retrieved information from the Japanese database regarding the drug name, compliance with the package insert, storage conditions, number of concomitant drugs, presence or absence of side effects history, and opinions of veterinarians and manufacturers.
Therapeutic Categories of the DrugsVeterinary drugs with the same active ingredients as human drugs were classified into the corresponding therapeutic categories. In addition, those with a different intended use from the corresponding human drugs, and those that could not be properly classified, were assigned the therapeutic category name(s) described in the drug package insert.
According to the therapeutic category, the highest number of reports (577, 55.46%) was associated with antiparasitic drugs, and the top three categories of drugs were mentioned in 81.25% of all reports (Table 1). As shown in Table 2, 12 reports (0.83%) were related to humans: 5 reports of needlestick accidents (Nos. 6–10), 4 reports of drug exposure (Nos. 2, 3, 5, 11), 1 report of accidental ingestion of oral medicine by an owner (No. 4), 1 report of injury when opening a drug vial (No. 1), and 1 report of adverse effects of unknown origin (No. 12).
| Therapeutic category | Number of reported cases | Proportion (%) |
|---|---|---|
| Antiparasitic drugs | 577 | 55.46 |
| Antimicrobial drugs | 207 | 14.39 |
| Non-steroidal anti-inflammatory drugs (NSAIDs) | 164 | 11.40 |
| General anesthesia | 46 | 3.20 |
| Osteoarthritis symptom-improving agent*1 | 43 | 2.99 |
| JAK inhibitor*1 | 41 | 2.85 |
| Corticosteroids | 35 | 2.43 |
| Antiemetic drugs (neurokinin 1 receptor antagonists) | 35 | 2.43 |
| Interferon formulations*2 | 33 | 2.29 |
| Ophthalmological drugs (NSAIDs) | 32 | 2.23 |
| Antineoplastic drugs | 31 | 2.16 |
| Immunosuppressants | 29 | 2.02 |
| ACE inhibitors | 18 | 1.25 |
| Injectable anesthesia for dogs and cats*1 | 15 | 1.04 |
| Antiepileptic drugs | 15 | 1.04 |
| Corticosteroid synthesis inhibitor | 14 | 0.97 |
| Sedative hypnotic | 13 | 0.90 |
| Analgesics and painkillers | 12 | 0.83 |
| Otitis externa medicines | 9 | 0.63 |
| Canine anti-dog interleukin-31 monoclonal antibody preparation*1 | 9 | 0.63 |
| Gastrointestinal disease medicines | 8 | 0.56 |
| Prostaglandin I2-derived preparation*2 | 7 | 0.49 |
| Heart failure treatment | 6 | 0.42 |
| Analgesics/sedative antagonist*1 | 5 | 0.35 |
| Biological preparations (vaccines) | 3 | 0.21 |
| Drugs for non-communicable canine osteoarthritis *1 | 3 | 0.21 |
| Diabetes medicine | 3 | 0.21 |
| Thyroid disease medicine | 3 | 0.21 |
| Anti-inflammatory agent for the acute phase of canine Pancreatitis medicine*1 | 3 | 0.21 |
| Angiotensin receptor blocker | 3 | 0.21 |
| Canine benign prostatic hyperplasia treatment*1 | 3 | 0.21 |
| Other | 13 | 0.90 |
| Total | 1438 | 100 |
*1 Indicates the drug name as written in its package insert, and *2 indicates a drug with a different use purpose as a human drug or veterinary drug.
| No. | Report date | Product name | Therapeutic category | Non-proprietary name | Dosage form | Sex | Age | Administered by | Prefecture | Situation | Time to onset | Main Symptom(s) | Measures taken | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10/16/2020 | Draxxin | Antimicrobial drug (macrolide) | Turraslomycin | Injection | — | — | Veterinarian | Hokkaido | A finger was injured by the metal piece at the mouth of the vial after removing the vial cap. | — | Fingertip injury | — | — |
| 2 | 9/23/2020 | Ivomec topical | Antiparasitic drug | Ivermectin | Injection | Male | — | Breeder | Gifu | The breeder was administering this drug to cattle when about 200–300 mL of the drug splashed onto both of his thighs over his pants. After about 5 min, the breeder took his pants off and washed the area with soap. Despite the absence of skin issues, asthma-like symptoms were noted. | 50 min | Asthma-like symptoms | Washing with soap | Recovered |
| 3 | 3/30/2020 | Andres ointment | Non-steroidal anti-inflammatory drug | Methyl salicylate, l-menthol, dl-camphor | Topical drug | Male | 31 | Breeder | Aichi | After applying this drug to cattle with bare hands several times, the breeder experienced recurrence of alopecia areata. | — | Recurrence of alopecia areata | Treatment | Under treatment |
| 4 | 3/6/2020 | Remadile tablet 25 | Non-steroidal anti-inflammatory drug | Carprofen | Tablet | Female | Owner | Chiba | The owner mistakenly ingested this drug and developed gastric discomfort. | — | Gastric discomfort | — | — | |
| 5 | 10/3/2019 | Revolution plus | Antiparasitic drug | Selamectin, saloralenol | Topical drug | — | — | Owner | Shizuoka | The drug adhered to human fingers and caused skin redness | <1 h | Skin redness | Washing with running water | Recovered |
| 6 | 7/5/2018 | Ingelvac flex combo mix | Vaccine | Inactivating antigen of porcine circovirus type 2 | Injection | Male | 40 | Breeder | Aichi | While vaccinating pigs, the person performing the injections had a needlestick accident, which later led to injection site swelling. It is unclear whether the agent was injected into the affected person. | <12 h | Injection site swelling | Treatment with antibiotics | Recovered |
| 7 | 5/24/2018 | Ingelvac flex combo mix | Vaccine | Inactivating antigen of porcine circovirus type 2 | Injection | Male | — | Breeder | Yamanashi | While vaccinating pigs, the person performing the injections had a needlestick accident, which later led to injection-site swelling. It is unclear whether the agent was injected into the affected person. | <1 h | Injection site swelling, pain | Treatment with antibiotics | Recovered |
| 8 | 5/9/2018 | Micotil 300 injectable fluid | Antimicrobial agent (macrolide) | Tilmicosin | Injection | Male | 58 | Veterinarian | Oita | A used needle held by a veterinarian at the time of treating cattle stuck in the thigh of a breeder who was holding the animal in place. Pain and swelling of the affected area were observed later. | <10 min | Injection site swelling, pain | Treatment (cleaning and cooling affected site, drip infusion) | Recovered |
| 9 | 4/12/2018 | Micoral oral solution | Antimicrobial agent (macrolide) | Tilmicosin phosphate | Injection | Female | — | Breeder | Hokkaido | During treatment of cattle, misadministration of the drug to the breeder caused symptoms such as poor physical condition, difficulty in standing up, loss of appetite, abdominal pain, and loose stool. | 2 d | Difficulty standing, loss of appetite, abdominal pain, soft stool | Treatment | Recovered |
| 10 | 7/31/2015 | Respisure one | Vaccine | Mycoplasma hyopneumoniae NL1042 strain Inactivated bacteria | Injection | Male | 36 | — | Kagoshima | While vaccinating pigs, a man accidentally stabbed himself in the finger with a needle when replacing a used needle, which caused pain, numbness, and swelling. | — | Pain, swelling, numbness | Treatment (disinfection at the hospital, taking antibiotics and herbal medicine) | Under treatment |
| 11 | 11/8/2006 | Pices | Other (antifungal agent) | Bronopol | Liquid for medicated bath | Male | 65 | — | Shizuoka | A man used this drug without wearing personal protective equipment (such as gloves, glasses, and a mask), which led to allergic reactions, eczema, and conjunctivitis in areas not covered with clothing. . | 10 d | Allergic reaction, eczema, conjunctivitis | Treatment (vitamin C injection, ointment prescription at dermatology clinic) | Recovered |
| 12 | 4/25/2003 | Tilmicosin | Antimicrobial agent (macrolide) | Tilmicosin | Injection | Male | 38 | — | Tokyo | — | — | Chest and back pain, cyanosis, death | — | Death |
Analysis of the storage conditions revealed that proper storage was recorded in 771 reports (53.62%), whereas in 647 (44.99%) reports the storage conditions were unknown, and in 20 (1.39%) reports, improper storage/management was evident (Fig. 1). Among the latter 20 reports, 18 cases of improper storage reflected room temperature storage of medicines that should have been refrigerated, and the remaining 2 cases involved the administration of drugs that were stored at proper temperatures but kept open for an unknown number of days before use (Table 3).

Storage according to the conditions described in the package insert was classified as “appropriate storage.” Storage deviating from the recommended conditions or instances of inappropriate use were considered “inappropriate storage.” Instances where the storage conditions were unknown or not described were classified as “unknown storage.”
| No. | Product name | Therapeutic category | Non-proprietary name | Dosage form | Storage conditions indicated in the package insert | Actual storage conditions and notes | Number of reports |
|---|---|---|---|---|---|---|---|
| 1 | Cytopoint® 10 | Canine anti-dog interleukin-31 monoclonal antibody preparation | Lokivetmab | Injection | 2–8 °C | Room temperature | 1 |
| 2 | Convenia® Injection | Antimicrobial drug | Cefovecin | Injection | 2–8 °C | Room temperature | 2 |
| 3 | Onsior® Injection | Non-steroidal anti-inflammatory drug | Robenacoxib | Injection | 2–8 °C | Room temperature | 14 |
| 4 | Antisedan® | Analgesic/sedative antagonist | Medetomidine hydrochloride | Injection | Room temperature | Room temperature, use of drug for which the date of opening was unknown | 2 |
| 5 | Allermmune® HDM0.1 | Canine atopic dermatitis-desensitization therapy | Recombinant Der f 2 - pullulan conjugate | Injection | 2–8 °C | Room temperature | 1 |
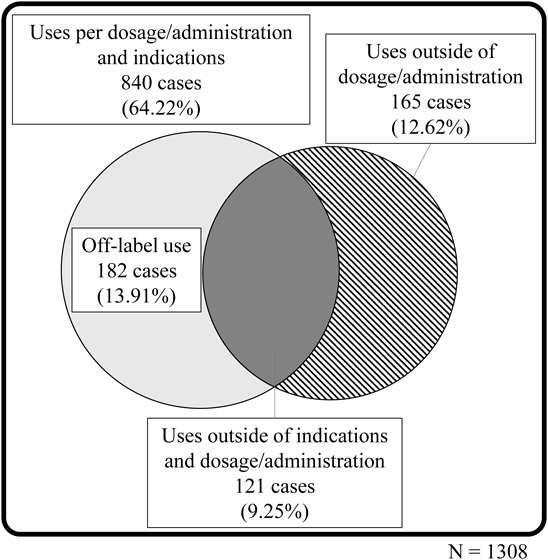
Of the 1308 reports containing information regarding clear compliance with the drug package insert, 840 reports (64.22%) mentioned that the medicine was used according to the intended dosing, administration, and indications described in the package insert (Fig. 2). Nevertheless, 468 reports (35.78%) mentioned uses that deviated from the package-insert instructions, of which 5 cases involved the use of a drug with the understanding that it would deviate from the package-insert instructions, and 20 cases involved the evaluation of an adverse event based on human drug information. Table 4 provides a detailed description of the 467 reports in which deviated drug use was apparent. The most common deviation was deviation from the indication (259 reports, 55.46%). The number of deviations reported to the FDA was different than that reported in Japan, although similarities in the types of deviations were observed (Table 5).
| Deviation from the method of use described in the package insert | Number of reports | Proportion (%) |
|---|---|---|
| Indication | 259 | 55.46 |
| Dosage method (other than the intended route of administration) | 83 | 17.77 |
| Animal species | 43 | 9.21 |
| Route of administration | 30 | 6.42 |
| Exceeding the maximum number of treatment days | 13 | 2.78 |
| Administration to an animal below the dosing standard age | 12 | 2.57 |
| Contraindication | 11 | 2.36 |
| Overdosing | 11 | 2.36 |
| Underdosing | 3 | 0.64 |
| Exceeding the maximum number of doses | 2 | 0.43 |
| Total | 467 | 100 |
Note: Based on the results of compliance with the instructions in the package insert (Fig. 4), the deviations and corresponding numbers of reports are indicated for 467 out of 468 instances where the details of deviation were clear.
| Deviation from the method of use described in the package insert | Number of reports | Proportion (%) |
|---|---|---|
| Off-label treatment regimen | 5080 | 32.03 |
| Off-label indication | 2915 | 18.38 |
| Overdosed | 2600 | 16.39 |
| Underdosed | 2016 | 12.71 |
| Other off-label issues | 1494 | 9.42 |
| Off-label species | 1243 | 7.84 |
| Off-label route | 352 | 2.41 |
| Product expired | 108 | 0.68 |
| Off-label storage condition | 22 | 0.14 |
| Total | 15860 | 100 |
Note: The information presented is based on data for animal-related adverse events submitted to the U.S. openFDA Animal & Veterinary Adverse Events during 2020.
Regarding the use of concomitant drugs, 727 (50.98%) of 1426 reports indicated the use of only the suspect drug, whereas 663 reports (46.50%) indicated concomitant drug administration (Fig. 3A). Among the concomitant drug cases, most were a combination of two drugs (327 reports) and two reports indicated the involvement of 10 concomitant drugs (Fig. 3B).

(A) Presence or absence of concomitant drugs use. (B) Number of concomitantly administrated drugs identified in 663 reports.
Regarding the presence or absence of side effect history, 682 (47.83%), 655 (45.93%), and 89 reports (6.24%) described unknown, absence, and presence of side effect history, respectively (Fig. 4A). Of the 89 “presence” reports, 35 indicated the occurrence of side effects to the same drug and same active ingredient that were administered previously, and 6 reports indicated side effects to a similar ingredient that was administered previously. These findings show that nearly half of all reports of adverse reactions after administering drugs with a history of side effects involved the same drug, a drug with the same active ingredient, or a similar active ingredient to a drug that had been administered previously (Fig. 4B).

(A) Characterization of drug-related side effects. (B) Suspected drugs identified in 89 reports as having a history of side effects.
To the best of our knowledge, this study is the first to elucidate pharmaceutical issues related to drugs used in veterinary medicine in Japan based on reports of their side effects. Notably, some drugs with reported adverse reactions were found to be managed and/or administered in manners that deviated from the conditions described in the package insert. This indicated the fact that veterinary professionals may be struggling to treat companion animals owing to lack of drugs for appropriate treatment. The identified package-insert deviations (Tables 4, 5) were presumed to include carelessness-associated medical malpractice. On the other hand, from another perspective, the Japan Veterinary Medical Association allows the use of medicines deviating from the package insert instructions in the absence of other available medical treatment.9) Hence, off-label drug use may be the result of the large unmet medical needs of approved veterinary medicines. The background factors that lead to adverse events are considered to be rooted in the underdevelopment of evidence-based veterinary medicine,3) in addition to other problems related to veterinary medicine, such as the species differences,10) lack of pharmacist job positions,11) and medical economic aspects, such as considering the balance between medical expenses and cost price under treatment not covered by health insurance.12)
Although the risk of adverse events in polypharmacy has been shown to be high in humans,13) taking several drugs concomitantly, as demonstrated in this study, is also considered a risk factor in the field of veterinary medicine. Furthermore, the side effect that often occurred upon re-administering a drug to an ill animal with a previous adverse effect history, is also a risk factor. Thus, proper pharmaceutical management based on information regarding the affected animal may contribute to reducing the risk of adverse events. In general, students have limited opportunities to learn about animals during their pharmacy education, but pharmaceutical researchers and pharmacists are in a position to contribute to veterinary medicine. The provision of more detailed drug information and appropriate drug management could not only assist animal health care providers, but could also contribute to the promotion of the proper use of drugs and the enhancement of human health. However, studies based on pharmaceutical perspective in Japanese veterinary medicine have been limited to a few areas, such as investigations on adverse reactions to vaccines14) and the handling of anticancer drugs.15)
Taken together, these findings suggest that there is room for improving the collection and utilization of drug information, research, and pharmaceutical care to avoid adverse events in veterinary medicine in Japan. Development of veterinary medicine pharmacology in response to the actual clinical situation in Japan, and increased opportunities for specialized education, are still warranted to optimize pharmacotherapy for ill companion animals.
We would like to express our sincere gratitude to Dr. Kenji Hoshi of the Division of Medical and Pharmaceutical Information Science, Tohoku Medical and Pharmaceutical University for all valuable insights. We would also like to express our sincere gratitude to Shiho Miyauchi of our group for collecting the data.
The authors declare no conflict of interest.
This article contains supplementary materials.