2022 Volume 45 Issue 9 Pages 1254-1258
2022 Volume 45 Issue 9 Pages 1254-1258
Cytotoxic agents are classified according to the severity of skin injury after extravasation. However, injuries caused by extravasation of noncytotoxic agents have not been sufficiently investigated, although the risk of extravasation is mentioned in medical safety information published by the Japan Council for Quality Health Care. Therefore, in this study, we focused on noncytotoxic electrolyte solutions and infusions and evaluated skin injuries during leakage using extravasation model rats. Rats were anesthetized and intradermally injected with 100 µL of an electrolyte solution or infusion. Injection lesions were macroscopically and histopathologically evaluated for extravasation injuries. Each electrolyte solution and infusion were classified into three categories (vesicants, irritants, and non-tissue-damaging agents) depending on the degree of skin injury. Similar to saline, 0.3% potassium chloride and 0.6% magnesium sulfate showed almost no injury, and 3% sodium chloride and BFLUID® caused erythema and induration. Erythema, induration, and ulceration were observed with the following: 10% sodium chloride, 2% calcium chloride, 8.5% calcium gluconate, 12.3% magnesium sulfate, MAGSENT®, FESIN®, and Intralipos®. The duration of damage with these agents was markedly prolonged. Electrolyte solutions and infusions can be classified into vesicants (10% sodium chloride, 2% calcium chloride, 8.5% calcium gluconate, 12.3% magnesium sulfate, MAGSENT®, FESIN®, and Intralipos®), irritants (3% sodium chloride and BFLUID®), and non-tissue-damaging agents (0.3% potassium chloride and 0.6% magnesium sulfate) according to their composition. The characteristic symptoms and severity of each drug extravasation revealed in this study will provide basic information for preparation of guidelines for treatment of extravasation.
Skin injury or refractory ulcers sometimes occur when cytotoxic agents inadvertently leak out of the blood vessels. Several guidelines have been issued on the prevention and treatment of extravasation injuries caused by cytotoxic agents. In these guidelines, cytotoxic agents are classified into three categories according to the severity of skin injury after extravasation: vesicants, irritants, and non-tissue-damaging agents.1–3) As a result, careful monitoring according to severity and initial treatment has been widely undertaken, and the frequency of extravasation of cytotoxic agents has decreased.3)
In contrast, the injuries caused by noncytotoxic agents’ extravasation has not been sufficiently investigated, and very little research for the classification of noncytotoxic agents according to the severity of skin injury after extravasation has so far been done.4) The risk of extravasation by noncytotoxic agents is often underestimated in clinical settings, and attention from medical staff is likely to be insufficient. There are several reports about serious injuries caused by extravasation of noncytotoxic agents, and the risk of extravasation of noncytotoxic agents is mentioned in medical safety information published by the Japan Council for Quality Health Care. However, there are few reports that classify the severity of skin injuries associated with extravasation, and many serious cases are still reported. Therefore, it is important to classify the severity of the extravasation of noncytotoxic agents.
We have clarified the risk of injury induced by extravasation of thiopental and propofol, noncytotoxic agents frequently reported to leak. The therapeutic effects of local cooling/warming were also evaluated.5) In a previous study, we investigated the relationship between extravasation injury and osmotic pressure using glucose and mannitol, and clarified that the osmotic pressure ratio is an important factor in the prediction of skin injury induced by extravasation.6)
In addition to highly osmotic agents, such as acids, bases, and vasoconstrictives, electrolyte injections, and infusions are reported to induce severe skin injury with extravasation.7,8)
Electrolyte solutions and infusions are frequently used clinically, and some package inserts state that caution is required due to extravasation. Many cases of skin damage after leakage have been reported. In this study, we evaluated skin injuries induced by leakage of electrolyte injections and infusions using macroscopic and histological changes in extravasation model rats as indicators.
Each drug was examined in undiluted form or at concentrations used in clinical settings. The clinical concentrations of the electrolytes were as follows: 0.3% potassium chloride (KCl, Terumo Corp., Tokyo, Japan), 10 and 3% sodium chloride (NaCl, Otsuka Pharmaceutical Factory Inc., Tokushima, Japan), 12.3 and 0.6% magnesium sulfate (MgSO4, Otsuka Pharmaceutical Factory Inc.), BFLUID® (amino acid, glucose, electrolytes, vitamin B1 infusion; Otsuka Pharmaceutical Factory Inc.), Intralipos® (20% soybean-oil emulsion, Otsuka Pharmaceutical Factory Inc.), 2% calcium chloride (CaCl2, NIPRO Corp., Osaka, Japan), 8.5% calcium gluconate (CaGlu, CALCICOL®, Nichi-Iko Pharmaceutical Co., Ltd., Toyama, Japan), FESIN® (20 mg/mL saccharated ferric oxide injection, Nichi-Iko Pharmaceutical Co., Ltd.), and MAGSENT® (10% MgSO4, 10% glucose injection, TOA Pharmaceuticals Co., Ltd., Toyama, Japan) were used (Table 1). KCl and MgSO4 were diluted in saline solution. NaCl was diluted with injection water.
| Agents (electrolyte) | Concentration (% (w/v)) | Electrolyte concentration (mEq/mL) |
|---|---|---|
| Saline | 0.9 | 0.15 |
| Potassium chloride (K+) | 0.3 | 0.04 |
| Sodium chloride (Na+) | 10.0 | 1.71 |
| 3.0 | 0.51 | |
| Calcium chloride (Ca2+) | 2.0 | 0.36 |
| Calcium gluconate (Ca2+) | 8.5 | 0.39 |
| Magnesium sulfate (Mg2+) | 12.3 | 1.00 |
| 0.6 | 0.05 | |
| MAGSENT® (Mg2+) | 10.0 | 0.81 |
| FESIN® (Fe2+) | 2.0 | 20 mg/mL |
| BFLUID® | — | — |
| Intralipos® | 20.0 | — |
All experiments were carried out in accordance with the Guide for Animal Experimentation from the Committee of Research Facilities for Laboratory Animal Sciences of Hiroshima University (Permit No. A18-111).
Seventy-nine male Wistar rats (7 weeks old; body weight, 250–280 g) were purchased from Japan SLC, Inc. (Shizuoka, Japan). Rats were housed in individual cages in a temperature-controlled room at 23 °C and relative humidity (50%) on a 12 h light–dark cycle. They were fed a standard laboratory diet (MF, Oriental Yeast Company, Tokyo, Japan) and water ad libitum for more than 1 week before the experiments.
Extravasation ModelsRats were anesthetized with medetomidine (0.15 mg/kg), midazolam (2.0 mg/kg), and butorphanol (2.5 mg/kg) injected intraperitoneally. Extravasation studies were performed in accordance with previous reports.9) Briefly, rats were randomly assigned to 12 experimental groups. The fur on their backs was shaved with electrical clippers (Thrive 2100; Daito Electric Machine Industry Co., Ltd., Osaka, Japan). Twenty-four hours after fur removal, rats without wounds were injected intradermally with 100 µL of electrolyte injections or infusions (the minimum volume at which lesions can be observed macroscopically). In the negative control group, 100 µL saline was injected intradermally. Intradermal injections using a 26-G needle were performed after pinching the dorsal skin at the center of a fur-free site 7 cm from the ear. Two injections were administered on the axisymmetric dorsal sides of each rat. Right-hand side lesions were macroscopically monitored until the injury healed completely or on day 30. Left-hand side lesions were punch-biopsied under anesthesia using a dermal punch (4 mm diameter; Maruho, Osaka, Japan) 24 h or 30 d after intradermal injection, in accordance with the peak time of lesion severity according to a previous report on histopathological evaluation of extravasation injury.5)
Evaluation of Skin Injury and Its ClassificationExtravasation injuries to the skin were evaluated macroscopically according to a previously described method.9) Briefly, the widest diameter and the corresponding perpendicular diameter of the skin lesions were measured using a caliper. The area of the lesion sites was calculated in cm2 as the product of the diameters.9) Each lesion site was inspected every day during the first week after intradermal injection, and then every 5 d from day 7. Four lesion parameters (erythema, induration, ulceration, and necrosis) were assessed. The area under the lesion–time curve (AUC) was calculated in cm2 ∙ d using the trapezoidal method.9) The AUC, peak area of the lesion, and damage duration were analyzed until the injury healed completely or on day 30.
For histopathological evaluation, tissue samples obtained by biopsy were fixed in 10% formaldehyde before dehydration, then 5-µm thick sections from the paraffin-embedded tissue blocks were stained with hematoxylin and eosin and evaluated under a light microscope (BX51; Olympus, Tokyo, Japan). Each sample was analyzed by independent pathologists who were blinded to the experimental procedures.
Each electrolyte solution and infusion were classified into three categories depending on the degree of skin injury. Agents that caused ulcers in all cases were classified as “vesicant”. Agents that did not cause ulcers and had an average AUC >0.23 (limit of detection calculated from the saline group) were classified as “irritants”. Agents with an AUC ≤0.23 were classified as “non-tissue-damaging agents”.
Statistical AnalysesData are presented as the mean ± standard deviation (S.D.). Differences among treatment groups were analyzed using ANOVA, followed by the Student–Newman–Keuls multiple-comparison post hoc test. A value of p < 0.05 was considered to indicate statistically significant differences. An unpaired Student’s t-test was used to compare 3% NaCl vs. 10% NaCl or 2% CaCl2 vs. 8.5% CaGlu.
Saline-injected rats showed slight erythema at injection sites that disappeared within 0.8 d. The macroscopic findings of the electrolytes and infusions during extravasation are shown in Table 2. Similar to saline, 0.3% KCl and 0.6% MgSO4 showed almost no injury, and there was no significant difference from saline in peak area, AUC, or damage duration. For 3% NaCl, erythema, induration, and ulceration were observed in some rats, and there was a significant difference in the peak area (p < 0.01) and damage duration (p < 0.01). Erythema, induration, and ulceration were observed in all individuals in 10% NaCl, and there were significant differences in peak area (p < 0.01), AUC (p < 0.01), and damage duration (p < 0.01). Comparing the peak area, AUC, and damage duration of 3% and 10% NaCl extravasation, significant differences were observed in peak area (p < 0.05), AUC (p < 0.05), and damage duration (p < 0.01). Injuries increased in a concentration-dependent manner. In the examination of calcium, 2% CaCl2, and 8.5% CaGlu showed erythema, induration, ulceration, and calcification in all cases, and did not completely heal, even after 30 d. Therefore, the AUC and damage duration could not be evaluated. In addition, there was a significant difference in peak areas (p < 0.01). When the peak areas of 2% CaCl2 and 8.5% CaGlu were compared using an unpaired Student's t-test, a significant difference was found (p < 0.05), and the damage caused by 2% CaCl2 was more severe. Erythema, induration, and ulceration were observed with 12.3% MgSO4 and MAGSENT®, and there were significant differences in peak area (p < 0.01), AUC (p < 0.01), and damage duration (p < 0.01). Although erythema, induration, and ulceration, were observed in all cases with FESIN®, it was difficult to calculate the peak area, AUC, and damage duration due to pigmentation present in all cases preventing accurate measurements.
| Agents (electrolyte) | Macroscopic findings | Histopathological findings | Peak area (cm2) | AUC (cm2·d) | Damage duration (d) | |
|---|---|---|---|---|---|---|
| 24 h | 30 d | |||||
| Saline | NC or E | NC | — | 0.05 ± 0.08 | 0.05 ± 0.08 | 0.8 ± 1.1 |
| 0.3% Potassium chloride (K+) | NC or E | NC | — | 0.05 ± 0.10 | 0.03 ± 0.05 | 0.8 ± 1.6 |
| 10% Sodium chloride (Na+) | E, I, U | ES, IIC, DCF, NEC | — | 0.74 ± 0.17**# | 3.83 ± 0.32**# | 19.2 ± 2.0**## |
| 3% Sodium chloride (Na+) | E, I | ES, IIC, DCF, NEC | — | 0.28 ± 0.15** | 0.38 ± 0.21 | 5.2 ± 3.3** |
| 2% Calcium chloride (Ca2+) | E, I, U, C | ES, IIC, DCF, NEC, phlebitis | ES, IIC, DCF, NEC, C | 1.37 ± 0.24**† | −a) | −b) |
| 8.5% Calcium gluconate (Ca2+) | E, I, U, C | ES, IIC, DCF, NEC, phlebitis | ES, IIC, DCF, NEC, C | 0.90 ± 0.10** | −a) | −b) |
| 12.3% Magnesium sulfate (Mg2+) | E, I, U | ES, DCF | — | 0.35 ± 0.12** | 1.58 ± 0.67** | 9.0 ± 5.2** |
| 0.6% Magnesium sulfate (Mg2+) | NC or E | NC | — | 0.06 ± 0.08 | 0.05 ± 0.06 | 1.0 ± 1.3 |
| MAGSENT® (Mg2+) | E, I, U | ES | — | 0.75 ± 0.16** | 3.15 ± 0.33** | 15.0 ± 3.2** |
| FESIN® (Fe2+) | E, I, U, P | ES, IIC, DCF, NEC | Siderophage | —b) | —b) | —b) |
| BFLUID® | E, I | NC | — | 0.36 ± 0.06** | 0.58 ± 0.24 | 3.7 ± 1.0 |
| Intralipos® | E, I, U | ES, IIC | — | 1.02 ± 0.25** | 2.59 ± 0.85** | 6.0 ± 2.4** |
AUC, area under the lesion–time curve; NC, no change; E, erythema; I, induration; U, ulceration; C, calcification; P, pigmentation; ES, epidermal shedding; IIC, infiltration of inflammatory cells; DCF, degeneration of collagen fibers; NEC, necrosis. Each agent was administered intradermally at a volume of 100 µL. Lesions were monitored until the injury completely healed or on day 30. Each value represents the mean ± standard deviation of the results from three to nine rats. * p < 0.05, ** p < 0.01 compared with saline treated rats; #p < 0.05, ##p < 0.01 compared with 3% sodium chloride; †p < 0.05, compared with 8.5% calcium gluconate. a) Not completely healed, b) Unmeasurable.
BFLUID® showed erythema and induration, and the peak area was significantly different (p < 0.01); however, there was no significant difference in AUC and damage duration. Intralipos® showed erythema, induration, and ulceration, with significant differences in peak area (p < 0.01), AUC (p < 0.01), and damage duration (p < 0.01).
Histopathological EvaluationIn saline-treated rats, skin tissue specimens showed normal skin cells with intact architecture (Fig. 1). The architecture of the skin cells was normal, and no histological changes were observed after injection of 0.3% KCl. Degeneration of collagen fibers and necrosis were observed in NaCl in a concentration-dependent manner. In 10% NaCl, many inflammatory cells infiltrated around the fur follicles, and the dermis was necrotic compared with 3% NaCl. In 2% CaCl2 and 8.5% CaGlu, epidermal shedding, infiltration of inflammatory cells into the dermis and cutaneous muscle, degeneration of collagen fibers, necrosis, and phlebitis were observed. The findings on day 30 showed calcification instead of phlebitis. Rats injected with magnesium sulfate and MAGSENT® were observed to be injured in a magnesium concentration-dependent manner. FESIN® was confirmed to induce epidermal shedding, inflammatory cell infiltration, collagen fiber degeneration, and necrosis. On day 30, although the tissue structure was maintained, siderophages throughout the tissue and pigmentation were observed.
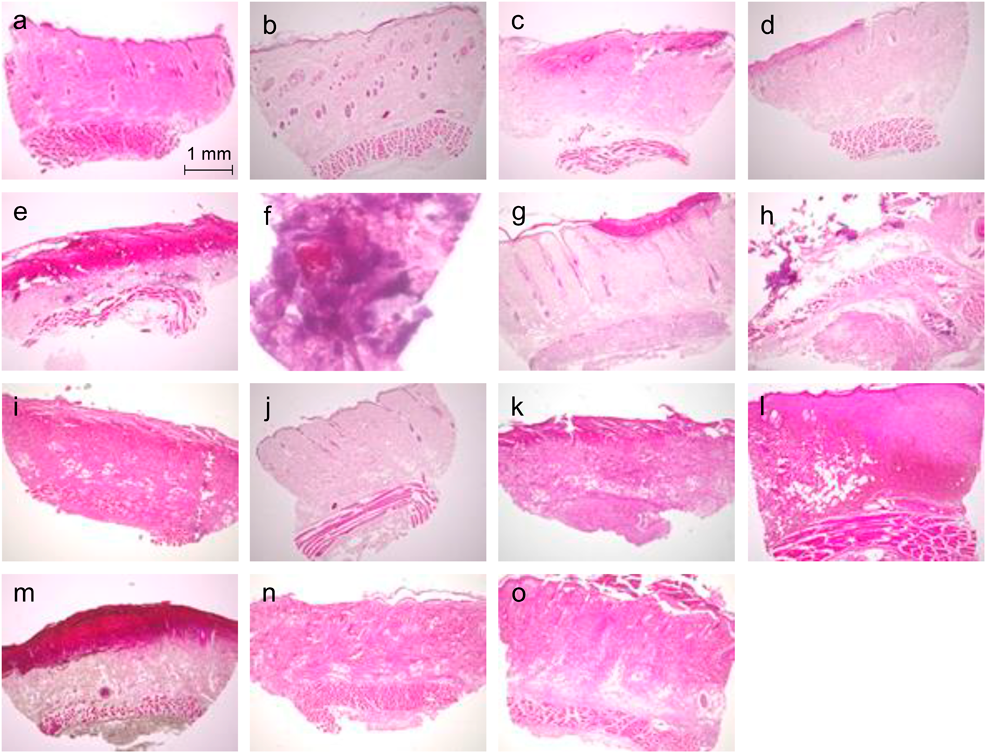
Skin tissues were biopsied at 24 h or 30 d after having been injected intradermally with 100 µL of each solution. Hematoxylin and eosin stain: (a–e, g–o) magnification 4×; (f) magnification 20×. a: saline (negative control), b: 0.3% potassium chloride, c: 10% sodium chloride, d: 3% sodium chloride, e: 2% calcium chloride, f: 2% calcium chloride (day 30), g: 8.5% calcium gluconate, h: 8.5% calcium gluconate (day 30), i: 12.3% magnesium sulfate, j: 0.6% magnesium sulfate, k: MAGSENT®, l: FESIN®, m: FESIN® (day 30), n: BFLUID®, o: Intralipos®.
No histological changes were observed with BFLUID®; however, epidermal shedding and infiltration of inflammatory cells were observed with Intralipos®.
Based on these results, the risk classification for extravasation was as follows: 10% NaCl, 2% CaCl2, 8.5% CaGlu, 12.3% MgSO4, MAGSENT®, FESIN®, and Intralipos® are “vesicants”; 3% NaCl and BFLUID® are “irritants”; and 0.3% KCl and 0.6% MgSO4 are “non-tissue-damaging agents” (Table 3).
| Agents | Vesicants | Irritants | Non-tissue-damaging agents |
|---|---|---|---|
| Electrolyte solution | 10% Sodium chloride | 3% Sodium chloride | 0.3% Potassium chloride |
| 2% Calcium chloride | 0.6% Magnesium sulfate | ||
| 8.5% Calcium gluconate | |||
| 12.3% Magnesium sulfate | |||
| MAGSENT® | |||
| FESIN® | |||
| Infusion | Intralipos® | BFLUID® |
Vesicants are agents that cause tissue necrosis or ulcers even at small volumes of extravasation, owing to their inherent toxicity to cells. Irritants do not cause necrosis or ulcers, and the average area under the lesion–time curve (AUC) was more than 0.23 (limit of detection calculated from the saline group). Non-tissue-damaging agents are agents with an AUC ≤0.23.
NaCl was administered peripherally at a concentration of 3%, which caused only mild inflammation after intradermal administration; however, at a concentration of 10%, ulceration and necrosis were observed. This result supports the recommendation of administration from the central vein. Concomitantly, NaCl concentrations of 3% and 10% could be classified as an irritant and a vesicant, respectively, in regards to the severity of skin injuries. In our previous study, when the risk of leakage of hyperosmolarity agents was examined using glucose and mannitol, these could be classified as irritants when the osmotic pressure ratio (to saline) was ≥1.9 and vesicants when the osmotic pressure ratio was ≥4.9.6) Since the osmotic pressure ratio of 10% NaCl is approximately 6, it is considered likely that the osmotic pressure is also involved in the induction of injury.
KCl solutions (2 mEq/mL) have been reported as having a high risk of skin injury due to extravasation,7,10) but in this experiment KCl did not induce skin injury when it was used at the maximum concentration (K+ 0.04 mEq/mL) described in the package insert. It has been reported that subcutaneous administration of 0.6 mEq/mL K+ solution in guinea pigs resulted in continued erythema for 30 min without induration or necrosis.11) However, in the present study, a 0.66 mEq/mL solution resulted in induration and erythema with necrosis at the central part of administration within 24 h. Furthermore, when a 0.5 mEq/mL K+ solution was intradermally administered, no skin damage was observed macroscopically (data not shown). Although the osmotic pressure ratio of the 0.5 mEq/mL K+ solution was 4.5, no macroscopic changes were observed; therefore, the osmotic pressure of the potassium solution may fluctuate rapidly within the skin after injection. Thus, no injury is expected after extravasation of a ≤0.04 mEq/mL K+ solution.
Calcium solutions (0.446–1.36 mEq/mL) cause serious and prolonged injury due to calcification, regardless of the osmotic pressure ratio, suggesting that it is important to recognize the potential risk of extravasation of the drug. In fact, extravasation injuries caused by calcium solutions have been reported most frequently among noncytotoxic agents.10)
The Ca2+ concentration of 2% CaCl2 is very close to that of 8.5% CaGlu (0.36 and 0.39 mEq/mL, respectively), but the peak area of 2% CaCl2 was significantly larger than that of 8.5% CaGlu. This injury is thought to be caused by the reaction of phosphorus and calcium in the skin to form hydroxyapatite, causing calcification conditions, such as calcinosis cutis. Heckler and McCraw reported that the degree of calcium-related cutaneous necrosis depends on the free Ca2+ concentration, not the total Ca2+ concentration.12) Therefore, it is thought that 2% CaCl2 caused more severe injuries because of the higher free Ca2+ concentration.
Comparing only Mg2+ concentrations, the 0.6% magnesium sulfate (Mg2+ 0.05 mEq/mL) solution, which is widely used for electrolyte supplementation, did not induce injury, but an undiluted solution (Mg2+ 1 mEq/mL) induced necrosis. MAGSENT® (Mg2+ 0.81 mEq/mL), a drug used in the treatment of eclampsia, caused injury with a higher peak area, AUC, and damage duration than 12.3% MgSO4 (Mg2+ 1 mEq/mL), despite its low Mg2+ concentration. This is because MAGSENT® has a very high osmotic pressure ratio of 3.5–6.0, as MgSO4 is dissolved in 10% glucose. It is thought that extravasation injury is caused not only by the toxicity of the electrolyte itself, but also in large part by the osmotic pressure. Therefore, it is necessary to evaluate the solution components as a whole in a clinical setting.
FESIN® (saccharated iron oxide) was classified as a vesicant with ulceration and iron pigmentation. The package insert of FESIN® stated that extravascular leakage of FESIN® can cause pigmentation around the leakage site, and pigmentation was observed in all cases of intradermal administration of FESIN® in this study. It was difficult to measure the peak area, AUC, and damage duration due to pigmentation; therefore, the degree of injury could not be evaluated appropriately.
From our previous report, agents can be classified as irritants when the osmotic pressure ratio is ≥1.9, and as vesicants when the osmotic pressure ratio is ≥4.96); therefore, the classification of BFLUID® as an “irritant” in this study is reasonable. Since Intralipos® was classified as a vesicant even though the osmotic pressure ratio was approximately 1 according to the package insert, caution should be exercised in the extravasation of fat emulsion.
In our previous studies, we have shown that osmotic pressure and vasoconstrictive effects are indicators of the degree of injury after extravascular leakage.6) In addition, studies using mice have reported acid- or base-induced skin injury.13) These reports suggest that physical changes (hyperosmolarity, acid-base, or vasoconstrictive effects) may be involved as factors causing cellular injury in the extravascular leakage model used in this study. However, it was difficult to model individual physical changes in the drugs and concentrations used in this study because the conditions that could occur in a clinical setting were reproduced. Further studies are needed to elucidate the mechanism of cellular injury caused by the drugs used in this study.
In this study, we found that electrolyte solutions and infusions can be classified into vesicants, irritants, and non-tissue-damaging agents according to their composition. Therefore, it is necessary to understand the risk of injury for each drug and to use appropriate treatments for each drug in cases of leakage. It is believed that the characteristic symptoms and severity of the extravasation of each drug revealed in this study will be useful for preparing guidelines for treatment of extravasation. It is possible to prevent or decrease the frequency and severity of extravasation by being alert and taking the appropriate measures according to the severity of the extravasation injury.
The authors declare no conflict of interest.