2022 Volume 45 Issue 9 Pages 1389-1393
2022 Volume 45 Issue 9 Pages 1389-1393
Thymoquinone is a popular health-promoting antioxidant supplement, but it may induce toxicity to cells and organs because of its propensity to promote oxidation of biomolecules under some conditions. Furthermore, as hydroquinones have been found to exhibit more potent antioxidant and prooxidant activities than their parent quinones, the reduced metabolite thymohydroquinone may have stronger effects than thymoquinone. In this study, the antioxidant and prooxidant activities of thymoquinone and thymohydroquinone were assessed to determine whether they both act as antioxidants and induce oxidative damage to biomolecules as do other quinones. Using ESR spectroscopy, we demonstrated that thymohydroquinone exhibits more potent antioxidant activity than does thymoquinone. In addition, thymohydroquinone was found to act as a prooxidant to induce oxidative damage of isolated plasmid DNA in the presence of free Cu2+ or Fe2+–ethylenediaminetetraacetic acid (EDTA). Interestingly, the prooxidant effect of thymohydroquinone in the presence of Fe2+ was not observed in the absence of EDTA. Thymohydroquinone thus was demonstrated to have two biologically relevant activities: as an antioxidant and a prooxidant.
Thymoquinone (TQ), one of the active ingredients in black cumin seeds, is known to exhibit several biological activities, including anti-cancer1) and antioxidant effects.2) Quinones typically are highly reactive with biomolecules, so that they often induce cytotoxicity. For example, N-acetyl-4-benzoquinone imine, which has a quinonimine structure, is a metabolic byproduct that is formed when excessive doses of acetaminophen are consumed, and it induces toxicity to cells.
TQ, which is a p-quinone, exerts protective effects by inducing the activity of cytoprotective enzymes, resulting in protection against the cellular damage exerted by oxidative stress. In particular, TQ has been reported to upregulate multiple antioxidant cytoprotective enzymes via induction of mRNA expression and activation of enzyme activity.3) However, in addition to its antioxidant activity, TQ has also been shown to exert a prooxidant effect.4)
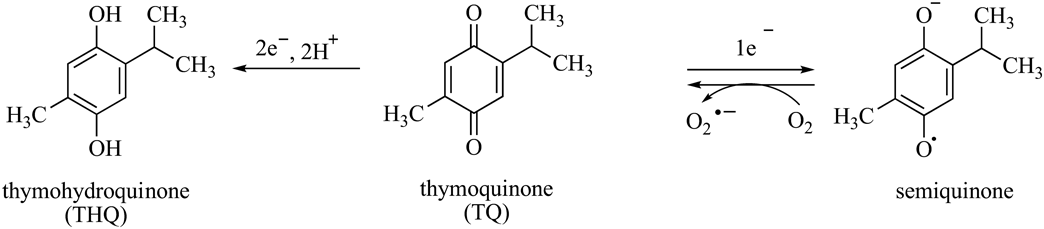
On a chemical level, TQ undergoes redox cycles that are composed of enzymatic or nonenzymatic reactions that metabolize TQ to thymohydroquinone (THQ) or semiquinone (Fig. 1). These reactions are accompanied by the generation of superoxide anion radicals (O2·−).3,5) THQ is also known to be an cytoprotective antioxidant, but semiquinone has been shown to cause cellular damage as a result of its prooxidant activity.3) Natural antioxidants derived from plants induce oxidative damage to DNA in the presence of transition metals, such as copper and iron.6,7) Accordingly, TQ shows prooxidant effects at low concentrations, especially in the presence of copper ions.4) THQ possesses a higher electron density than does TQ, so THQ may either induce more potent oxidative damage to DNA in the presence of transition metals or exert more potent antioxidant activity than does TQ.
In the present study, we tested the antioxidant and prooxidant activities of TQ and THQ to quantify their relationships and to provide further insight into the possible adverse effects of TQ supplementation. Antioxidant activities of TQ and THQ were evaluated using ESR spectroscopy, and their prooxidant effects were evaluated by observing oxidative damage to DNA using agarose gel electrophoresis.
The radical scavenging activities of TQ and THQ were evaluated using ESR spectroscopy. THQ was synthesized according to a previously reported procedure.8) The ESR assay was conducted with galvinoxyl radical (GO·) in the presence of test compounds. These reagents were dissolved in acetonitrile. Predetermined concentrations of test compounds were mixed with 50 µM GO· and then immediately introduced into a flat quartz cell, which was then placed in the ESR sample cavity. ESR observations were performed with a JES-RE1X spectrometer (JEOL, Tokyo, Japan) at the following instrument settings: modulation width, 0.2 mT; time constant, 0.03 s; and microwave frequency, 9.405 GHz.
Analysis of DNA Strand BreakageThe prooxidant activities of TQ and THQ in the presence of transition metals were evaluated by observing DNA cleavage activity using agarose gel electrophoresis. Reactions (20 µL total volume) were carried out in phosphate buffer (pH 7.0), containing pBR322 DNA, 12.5 µM metal, and test compound (10 µM TQ or THQ). Reactions were initiated by the addition of metal. Following incubation at 37 °C for 1 h, the reaction mixture was treated with 5 µL of loading buffer and applied to a 1.0% agarose gel. The loading buffer consisted of 100 mM Tris–borate-ethylenediaminetetraacetic acid (EDTA) buffer (pH 8.3; TBE), 0.1% bromophenol blue, and 30% glycerol. Horizontal gel electrophoresis was carried out in 50 mM TBE buffer containing 0.1% ethidium bromide. Gels were destained in water and photographed with UV transillumination.
The radical scavenging activities of TQ and THQ were evaluated in a non-aqueous system using GO· as the oxyl radical species. The antioxidant activity of TQ was weak, reducing the ESR signal of 50 µM GO· by only 14% even at a concentration of 100 µM (data not shown). However, in the case of THQ (Fig. 2), potent antioxidant activity could be observed at a concentration as low as 6.25 µM, and THQ at a concentration of 25 µM THQ completely scavenged 50 µM GO· (Table 1).
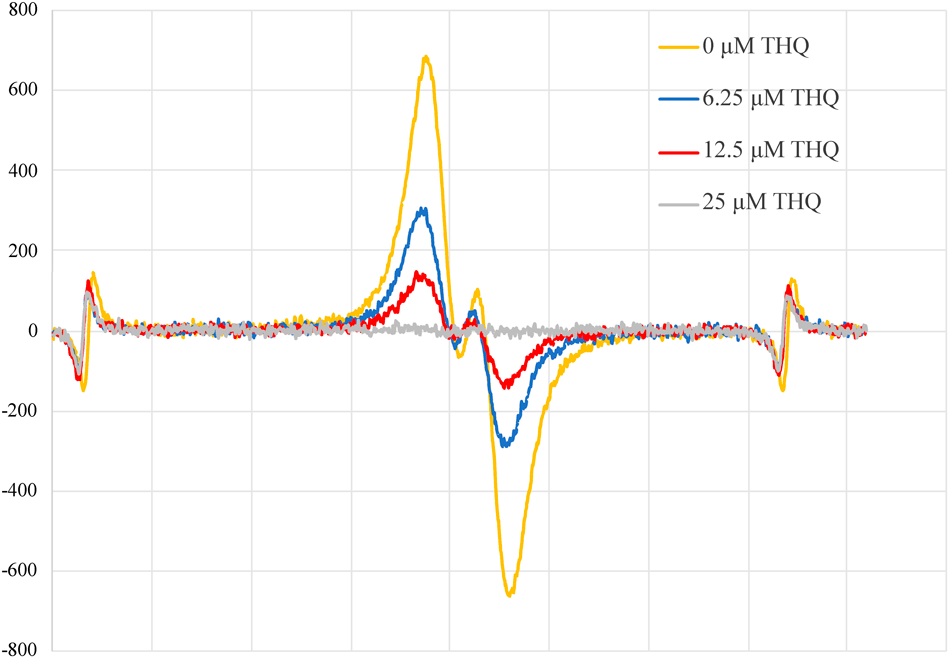
Electron spin resonance spectroscopy of 50 µM galvinoxyl radical was performed in the presence of the noted concentrations of THQ. Peaks at both ends of the spectra are markers of the reference ion (Mn2+).
| Ratio of peak heights (vs. Mn2+) | Activity ratio (vs. peak ratio in 50 µM GO·) | GO· scavenging activity (%) | |
|---|---|---|---|
| 50 µM GO· | 4.62 | 1 | 0 |
| 50 µM GO· + 6.25 µM THQ | 2.96 | 0.64 | 36 |
| 50 µM GO· + 12.5 µM THQ | 1.17 | 0.25 | 75 |
| 50 µM GO· + 25 µM THQ | Not detected | Not detected | 100 |
The peak heights from the electron spin resonance spectra (Fig. 2) were compared to the heights of the reference peaks on the left.
The plasmid pBR322 predominantly exists as supercoiled DNA (form I). It is converted to nicked DNA (form II) when oxidative damage is induced by reactive oxygen species (ROS), including ROS that are generated by the interaction of natural antioxidants with certain transition metals.9) We therefore investigated DNA cleavage mediated by the interaction of TQ or THQ with transition metals. Figure 3A shows the results of interactions between test compounds and metals. When the DNA was incubated with Fe2+, approximately half of the DNA was converted from form I to form II (lane 4). Similar results were found when the DNA was incubated with Fe2+ and 10 µM TQ (lane 7) or THQ (lane 10). However, when Cu2+ was added without TQ or THQ, no nicking or cleavage was observed (lane 6). A slight nicking was observed upon incubation of DNA with Cu2+ and TQ (lane 9). On the other hand, when DNA was incubated with Cu2+ and THQ, strong DNA damage was observed (lane 12). In fact, no remaining supercoiled DNA was detected, and some of the DNA was linearized to form III, indicating double-stranded cleavage.
Next, we used radical scavenging agents to investigate whether DNA cleavage that occurred in the presence of THQ and Cu2+ was induced by ROS-mediated oxidative damage or by an ROS-independent mechanism. Dimethyl sulfoxide (DMSO) is a commonly used scavenger of ·OH, and thiourea has been used as a scavenger of ·OH and copper–peroxide complexes.10,11) As shown in Fig. 3B, thiourea suppressed THQ/Cu2+-induced DNA cleavage (lane 7), but DMSO failed to suppress this DNA cleavage (lane 6). TQ exhibited weak DNA nicking in the presence of Cu2+ (lane 2), and this nicking was inhibited by thiourea (lane 4) but not by DMSO (lane 3).

(A) DNA cleavage by TQ and THQ with transition metals. (B) Effects of radical scavenging agents on DNA cleavage induced by TQ and THQ in the presence of Cu2+. (C) DNA cleavage by TQ and THQ with Fe2+ or Fe2+–EDTA. (D) Effects of radical scavenging agents on DNA cleavage induced by TQ and THQ in the presence of Fe2+ or Fe2+–EDTA.
Because DNA cleavage could be observed upon the addition of Fe2+ alone (Fig. 3A, lane 4), we concluded that Fe2+ directly binds to DNA in the process of inducing DNA cleavage. To test this conclusion by preventing direct interaction between DNA and Fe2+, EDTA was added to coordinate Fe2+, and the resulting DNA cleavage was assessed. As indicated by a comparison of lanes 5 and 6 in Fig. 3C, the degrees of DNA nicking in the presence of Fe2+–EDTA or in the presence of free Fe2+ were apparently equivalent. On the other hand, a comparison of lanes 10 and 12 suggests that DNA cleavage by Fe2+–EDTA was enhanced by the presence of THQ. The DNA cleavage that was induced by Fe2+ and Fe2+–EDTA and promoted by THQ could be inhibited by DMSO and thiourea (Fig. 3D, lanes 6, 7, 9, 10).
It is well known that TQ is an antioxidant, but THQ showed much more potent radical scavenging activity than did TQ in ESR assays. This finding is consistent with the fact that THQ, which is generated by the reduction of TQ, possesses a higher electron density than does TQ. Accordingly, THQ is able to provide one electron to GO· more readily than can TQ. These results suggest that THQ is likely to also act as an antioxidant in vivo in addition to the parent compound TQ.
In the analysis of DNA strand breakage, THQ was found to potently enhance the DNA-cleavage activity of Cu2+ in a thiourea-sensitive manner. Inhibition of this DNA cleavage activity by thiourea demonstrates that it was induced by copper-peroxide complexes.12) It has been reported that 5-alkyl-1,3-dihydrobenzene similarly cleaves DNA in a Cu2+-dependent manner,13) and we have reported that resveratrol in the presence of Cu2+ generates a copper-peroxide complex under aerobic conditions to induce DNA cleavage. Accordingly, we suggest that THQ in the presence of Cu2+ under aerobic conditions also generates a copper–peroxide complex that induces DNA cleavage. In this model, THQ, Cu2+ and plasmid DNA would form a complex14) and react with O2 to generate the Cu2+–hydroperoxyl complex (Cu2+–OOH) that would induce the oxidative damage to DNA (Chart 1).

The Cu+ formed by the one-electron reduction of Cu2+ by THQ reacts with molecular oxygen to produce Cu2+–O2·. Addition of one electron and one proton to Cu2+–O2· produces a Cu2+–hydroperoxyl complex (Cu2+–OOH). Reaction of Cu2+–OOH with plasmid DNA triggers rapid DNA cleavage.
When Fe2+ ions were incubated with DNA, oxidative damage to DNA was observed regardless of the presence of TQ and THQ. We conclude from these results that one electron from Fe2+ was donated to O2 to generate O2·-, which immediately reacted via a Haber–Weiss reaction to form ·OH, which directly induced oxidative damage of DNA. Furthermore, it was also demonstrated that THQ in the presence of Fe2+–EDTA exhibits much more potent DNA cleavage than that observed in the presence of Fe2+ alone. This result suggests that coordination of Fe2+ may be necessary to induce the maximal prooxidant effect of THQ. Ferrous ions rarely exist in the free state in biological systems but instead form complexes with chelators and proteins; our results thus suggest that the combination of THQ and iron-chelating proteins may induce oxidative damage in vivo.
In conclusion, we show that the THQ contributes to the antioxidant activity of its parent compound TQ. Further, THQ also exhibits a prooxidant effect to induce oxidative damage to DNA in the presence of Fe2+–EDTA and Cu2+. The enhancing by THQ of the oxidative damage induced by Fe2+–EDTA is of particular interest. Most ferrous ions in living organisms are complexed to proteins and chelators, so that this reaction may be readily induced in vivo. THQ, a reduced metabolite of TQ, is thus considered to have two biological activities: as an antioxidant and a prooxidant. Further study is warranted to investigate the impact of this mechanism on the use of thymoquinone as nutritional supplement.
This work was supported by MEXT KAKENHI Grant No. 20K15486.
The authors declare no conflict of interest.