2022 Volume 45 Issue 9 Pages 1394-1397
2022 Volume 45 Issue 9 Pages 1394-1397
Euglena gracilis is a microalga that has recently attracted attention because of its bioactivities. Paramylon (PM), a major β-1,3-glucan, constitutes 70–80% of the cells of the E. gracilis EOD-1 strain. Dectin-1 is a pattern recognition receptor that recognizes β-glucan. However, it is unclear whether PM binds to dectin-1. In this study, we investigated the reactivity of EOD1PM with dectin-1 by analyzing the binding of soluble murine and human dectin-1–Fc fusion protein (m dectin-1 Fc, h dectin-1 Fc) to EOD1PM using flow cytometry and enzyme-linked immunosorbent assay (ELISA). m Dectin-1 Fc bound to EOD1PM particles when m dectin-1-Fc is added. Furthermore, the binding specificity was examined in a competitive reaction following addition of a soluble antigen. It was found that the binding of m dectin-1-Fc to EOD1PM was not inhibited by the addition of dextran or ovalbumin but by the addition of solubilized EOD1PM or Candida cell wall- solubilized β-glucan. In addition, the h dectin-1–Fc fusion protein was found to specifically bind to EOD1PM. These results suggest that dectin-1 recognizes and binds to the β-glucan structure of EOD1PM. Dectin-1 is expressed in leukocytes as a β-glucan receptor and is involved in the expression of various biological activities; therefore, the dectin-1 pathway may be involved in the biological activity of EOD1PM.
Euglena gracilis is a type of microalga that has recently attracted attention because of its functionality as a food material and biofuel raw material. Euglena gracilis is rich in nutrients such as vitamins, minerals, amino acids, and fatty acids, and the cells contain paramylon (PM), a β-1,3-glucan. Particularly, the E. gracilis EOD1 strain shows good proliferative properties and is composed of 70–80% PM.1)
PM derived from the E. gracilis EOD-1 strain (hereinafter EOD1PM) has been reported to suppress increases in blood glucose levels and lower blood cholesterol levels in diet-induced obesity mouse models.2) In humans, continuous intake of foods containing EOD1PM has been reported to reduce physical and mental fatigue in daily life3) and maintain testosterone levels.4) Furthermore, ingestion of EOD1PM shows immunomodulatory effects such as inducing soluble immunoglobulin (Ig) A production on the mucosal surface of human cells, an increase in IgA antibody levels in response to PM, and an improvement in health-related QOL.5)
β-Glucan is widely present in nature and can be found in fungi (e.g., mushrooms and yeasts), grains (e.g., barley), and algae. Previous studies have reported that β-glucan exhibits immunomodulatory activities such as macrophage activation and cytokine production induction.6) Dectin-1, a C-type lectin expressed in dendritic cells and macrophages, is a pattern recognition receptor that recognizes β-glucan.7) Furthermore, dectin-1 is involved in inducing cytokine production via β-glucan and protects against fungal infections. β-Glucan exists in various structural conformations, and their physical properties affect binding of dectin-1.
Euglena gracilis and its component PM have been reported to exhibit various biological activities in the host; however, it is unclear whether PM binds to dectin-1. Therefore, we investigated the reactivity of EOD1PM to dectin-1.
Candida and Aspergillus cell wall-solubilized β-glucan (CSBG and ASBG, respectively) and Agaricus-derived polysaccharide fraction were prepared as previously reported.8–10) Dextran was purchased from Pharmacia (Stockholm, Sweden), and ovalbumin was purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). EOD1PM was donated by Kobelco Eco-Solutions Co., Ltd. (Hyogo, Japan).
Preparation of Soluble Dectin-1–Fc Fusion ProteinSoluble murine and human dectin-1 Fc fusion proteins (m dectin-1 Fc, h dectin-1 Fc) were prepared according to previous reports11) the fusion proteins comprised the Fc portion of human IgG1 and carbohydrate recognition receptor of dectin-1.
Examination of Dectin-1 Binding to EOD1PM ParticlesEOD1PM suspension (125 µg/mL) was blocked with 2% fetal bovine serum in phosphate-buffered saline containing 0.1% NaN3. Soluble murine or human dectin-1–Fc was added to the buffer, and the solution was incubated at 4 °C for 60 min. Next, to detect the bound soluble dectin-1, a detection antibody (anti-human IgG-fluorescein isothiocyanate, 1 : 200; BioLegend, CA, U.S.A.) was added, followed by incubation at 4 °C for 60 min. After fixation, the binding of dectin-1–Fc fusion protein to EOD1PM was examined using flow cytometry (FACSCanto, Becton Dickinson, NJ, U.S.A.).
Examination of Dectin-1 Binding to Solubilized EOD1PMEOD1PM was solubilized in 1 M NaOH (10 mg/mL) and diluted in 0.1 M bicarbonate buffer (25 μg/mL, pH 9.5). The solubilized EOD1PM was coated on an enzyme-linked immunosorbent assay (ELISA) plate and then incubated at 4 °C overnight. After washing with phosphate-buffered saline containing 0.05% Tween-20 (PBST pH 7.4; FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) and blocked with 1% bovine serum albumin in PBST at 37 °C for 60 min. m Dectin-1 Fc or h dectin-1 Fc was added, and the solution was incubated at 37 °C for 60 min. Next, to detect the bound m dectin-1 Fc or h dectin-1 Fc, a detection antibody (anti-human IgG-horseradish peroxidase (1 : 2000; Sigma-Aldrich)) was added, followed by incubation at 37 °C for 60 min. After washing with PBST, the substrate 3,3′,5,5′-tetramethylbenzidine (KPL Inc., MD, U.S.A.) was added for color development. After stopping the color development reaction with phosphoric acid, the absorbance (450 nm/630 nm) was measured.
To investigate the binding of dectin-1 to EOD1PM particles, we used the soluble m dectin-1 Fc fusion protein. When the m dectin-1 Fc was added to EOD1PM, the peak shifted to the right depending on the concentration of m dectin-1 Fc added (Fig. 1a). This result suggests that m dectin-1 Fc binds to EOD1PM. Binding of m dectin-1 Fc to EOD1PM particles was inhibited by addition of solubilized EOD1PM (Fig. 1b). In contrast, the binding of it was not inhibited when dextran and ovalbumin were added to the reaction mixture (Fig. 1c). Thus, m dectin-1 Fc may recognize and specifically bind to the β-glucan structure of EOD1PM. Furthermore, upon addition of CSBG, which contains β-1,3-glucan as the main chain and β-1,6-glucan as the side chain, the binding of m dectin-1 Fc was inhibited, and cross-reactivity of murine dectin-1 between EOD1PM and CSBG was exhibited.
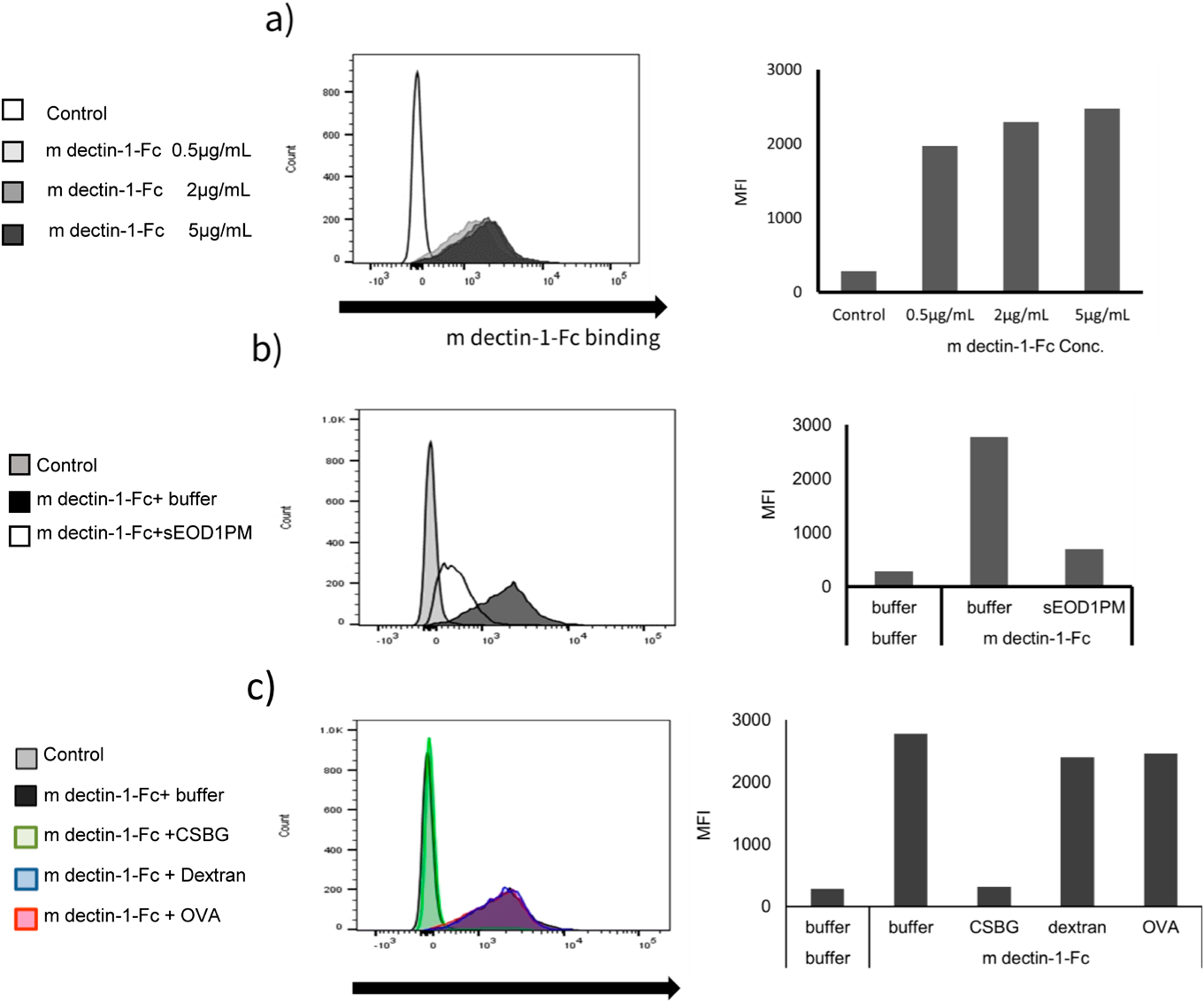
(a) Reactivity of the murine dectin-1 Fc. EOD1PM particles were blocked, reacted with murine dectin-1 Fc, and analyzed by flow cytometry; (b, c) Specificity of the murine dectin-1 Fc. EOD1PM particles were blocked, reacted with mixture of murine dectin-1 Fc and competitive soluble antigen (b : sEOD1PM, c : CSBG (Candida cell wall-solubilized β-glucan), dextran, OVA), and analyzed by flow cytometry.
To quantitatively examine the binding of soluble m dectin-1 to EOD1PM, we performed ELISA using solubilized EOD1PM as a solid-phase antigen. The results suggested that the absorbance and extent of binding to EOD1PM increased according to the concentration of m dectin-1 Fc added. The binding level was equivalent to that of CSBG (Fig. 2a). The binding to solubilized EOD1PM also suggests that it is reactive to the primary β-glucan structure of EOD1PM. In addition, analysis of the reaction specificity showed that binding of m dectin-1 to immobilized EOD1PM was suppressed by addition of solubilized EOD1PM and by addition of CSBG and ASBG, the main structures of which are mostly β-1,3-glucan (Fig. 2b). In contrast, the binding of murine dectin-1 to solubilized EOD1PM was not suppressed by dextran, yeast mannan, and ovalbumin. These results suggest that the β-glucan structure is involved in binding of murine dectin-1 to EOD1PM.
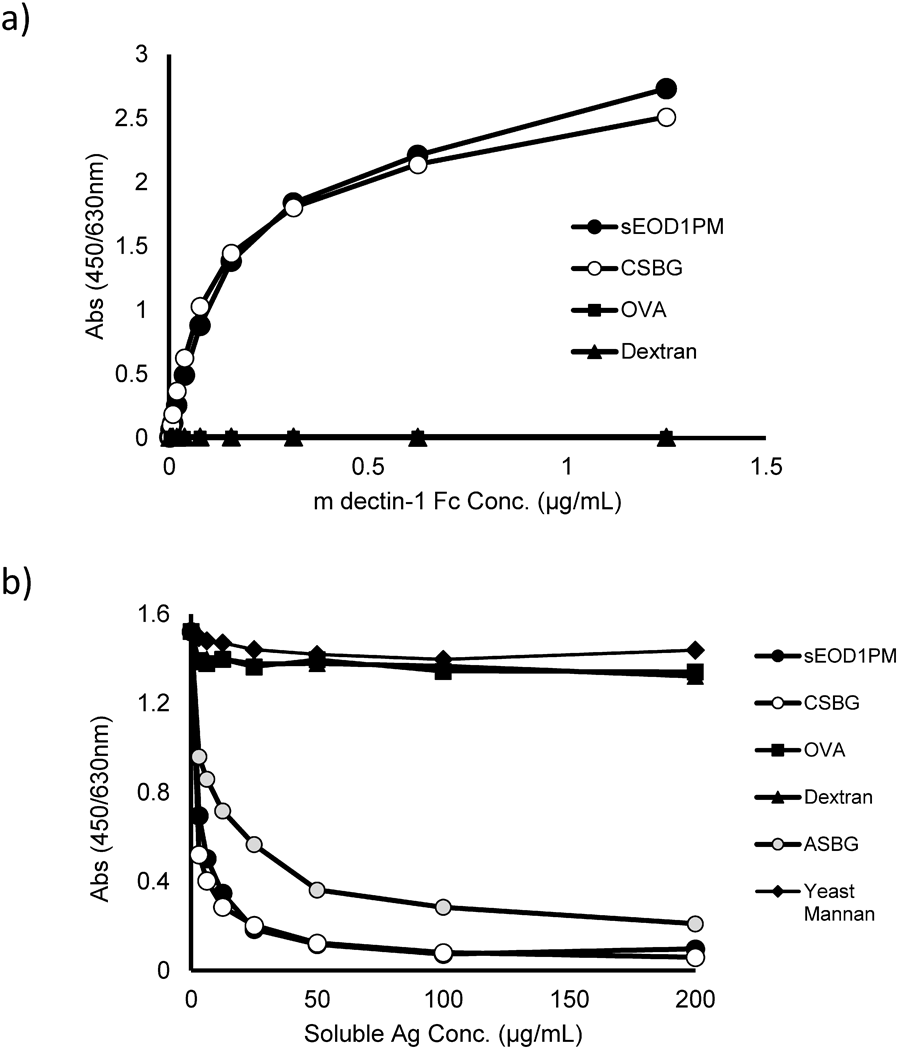
(a) Reactivity of murine dectin-1. An ELISA plate was coated with sEOD1PM and blocked. Murine dectin-1 Fc was diluted, and the amount of plate-bound Ig was determined using the detection antibody. (b) Specificity of murine dectin-1. An ELISA plate was coated with sEOD1PM and blocked. Murine dectin-1 Fc was mixed with serially diluted competitive soluble antigen and then applied to the ELISA plate. The amount of plate-bound Ig was determined using the detection antibody.
Furthermore, the binding of h dectin-1 to EOD1PM particles was investigated (Fig. 3). A concentration-dependent shift in fluorescence intensity was observed, suggesting that h dectin-1 Fc binds to EOD1PM particles. In addition, examination of the binding of h dectin-1 Fc using ELISA with soluble EOD1PM showed that h dectin-1 Fc binds to solubilized EOD1PM in the same manner as CSBG (Fig. 4a). We also investigated the binding specificity of h dectin-1 Fc to EOD1PM using competitive ELISA (Fig. 4b); binding competition with CSBG and ASBG was observed, whereas competition with dextran, yeast mannan, and ovalbumin was not observed.
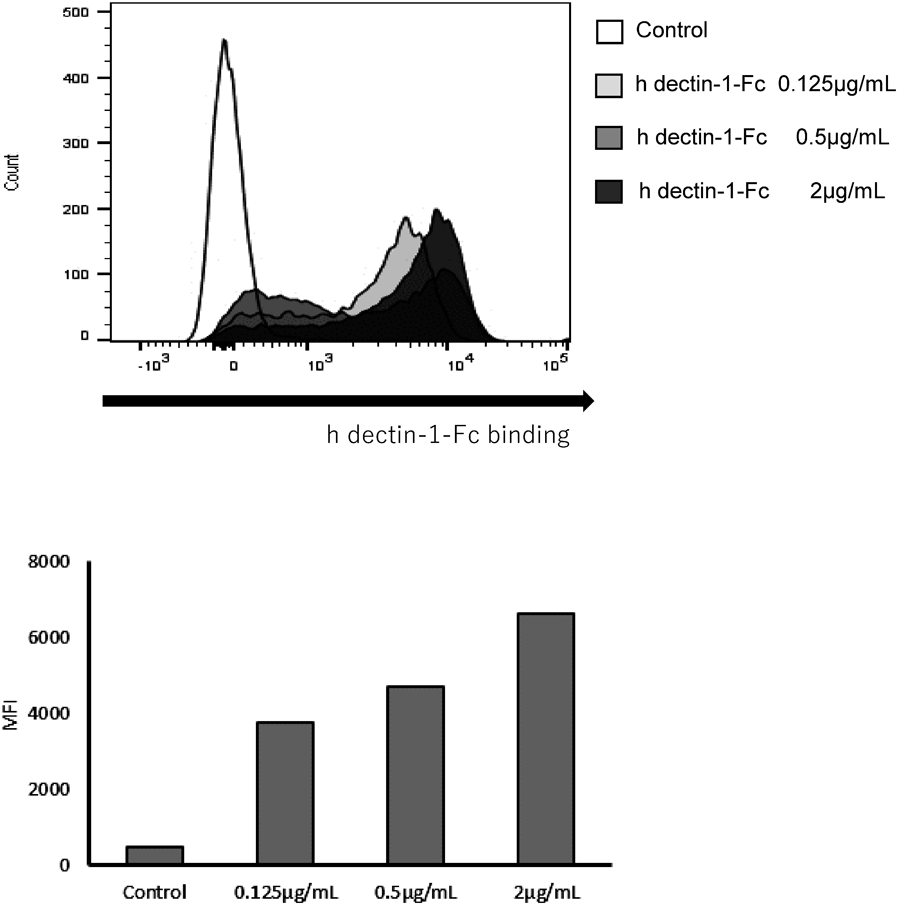
Reactivity of human dectin-1 Fc. EOD1PM particles were blocked, reacted with human dectin-1 Fc, and analyzed by flow cytometry.

(a) Reactivity of human dectin-1. An ELISA plate was coated with sEOD1PM and blocked. Human dectin-1 Fc was diluted, and the amount of plate-bound Ig was determined using the detection antibody. (b) Specificity of human dectin-1. An ELISA plate was coated with sEOD1PM and blocked. Human dectin-1 Fc was mixed with serially diluted competitive soluble antigen and then applied to the ELISA plate. The amount of plate-bound Ig was determined using the detection antibody.
The results of this study reveal that m dectin-1 and h dectin-1 directly bind to EOD1PM. A previous report on the binding of h dectin-1 to PM was based on the binding absorption experiment of human dectin-1.12) Our results reveal that the dectin-1 directly binds to PM particles, specifically to the β-1,3-glucan structure of PM. It has been reported that PM shows immunomodulatory effects such as survival protection against influenza virus infection and anti-tumor activity in mice as well as in humans,13,14) but whether it can bind to murine dectin-1 is unknown. In this study we showed that mouse dectin-1 binds directly to PM in a dose-dependent manner. Additionally, we examined the binding specificity of dectin-1 to PM using a competitive reaction after the addition of soluble antigen. Soluble EOD1PM and CSBG had different degrees of competition for the binding to PM particles. Dectin-1 is reactive to β-1,3-glucan. However, these findings suggested that the structural differences among glucans, such as β-1,6-glucan side chains, affect the reactivity of dectin-1.
Dectin-1 is expressed in phagocytic cells such as dendritic cells, macrophages, and neutrophils in humans; recognizes β-glucan; and exerts biological activities.6) Furthermore, dectin-1 is expressed in epithelial cells of the intestinal tract and is involved in intracellular signal transduction and chemokine production via Syk.15) PM activates macrophages and induces tumor necrosis factor-α and nitric oxide production in vitro.16) Additionally, PM ingestion increases the production of secretory IgA on the mucosal surface and improves the QOL and fatigue in humans. Our results suggest that ingested PM can bind to dectin-1 expressed in epithelial cells and immunocompetent cells of the intestinal tract,17,18) allowing its active expression through them. Thus, EOD1PM, a food ingredient, binds to a specific receptor and exerts various activities.
Three of the authors (N.O., N.N., and M.T.) are salaried employees of the Kobelco Eco-Solutions Co., Ltd. The research funding for this study was provided by the Kobelco Eco-Solutions Co., Ltd.