2022 Volume 45 Issue 9 Pages 1300-1305
2022 Volume 45 Issue 9 Pages 1300-1305
Understanding a monoclonal antibody’s (MAb) physicochemical properties early in drug discovery is important for determining developability. Viscosity is important because antibodies with high viscosity have limited administration routes. Predicting the viscosity of highly concentrated MAb solutions is therefore essential for assessing developability. Here, we measured the viscosity and diffusion interaction coefficient (kDiff) of 3 MAbs under 15 different formulation conditions (pH and salt) and evaluated correlations between parameters. We also used a computational approach to identify the key factors underlying differences in concentration-dependent curves for viscosity among the MAbs and formulation conditions. Results showed that viscosity increased exponentially at high concentrations, and that this concentration-dependency could be predicted from kDiff. Attempts to set viscosity criterion for use by subcutaneous (SC) and intramuscular (IM) administration suggested that solutions with kDiff greater than −20 mL/g may be candidates. Computational analysis suggested that the presence of a large negative charge in the complementarity determining region (CDR) is a major factor underlying the difference in concentration-dependency among the three MAbs under different formulation conditions. Because it is possible to predict the administration form of antibody solutions, determination of kDiff at the early discovery stage may be essential for effective antibody development.
In the past several decades, a considerable number of therapeutic antibodies have been developed as highly successful biologics. Further, hundreds of candidate agents targeting a wide variety of diseases, including cancer, autoimmune diseases, allergies and inflammation are now in the clinical development stage.1) Assessing the developability of antibody drug candidates now requires evaluation of the physicochemical properties of numerous candidate antibodies in the initial stage of antibody drug discovery.2–6) However, mass production of a wide variety of candidates in the initial stage of drug development of an antibody is very difficult. Further, manufacturing methods and administration routes of candidate antibodies are limited unless the candidate possesses good physicochemical properties that ensure high developability of an antibody drug, and which would lead to maximization of the value of the antibody as a drug for patients.2)
In general, administration of antibody solutions via subcutaneous (SC) injection or intramuscular (IM) injection has less burden and is safer for patients and medical workers than intravenous (IV) administration.7) SC and IM administration requires highly concentrated solutions, such as those greater than 50 mg/mL, because of the limited amount of antibody solution for administration.8) However, highly concentrated antibody solutions are more likely to exhibit higher viscosity and aggregation.7,9) Expelling a viscous antibody solution from a syringe and needle can be difficult due to the high injection force required.10) For this reason, the viscosity of an antibody solution is used as an index of possibility of IM and SC administration.11) The importance of reducing viscosity is increasing with the progression of medical diversification and the inevitable rise in prescription of self-administration.11–13)
Measurement of the viscosity of an antibody solution generally requires an amount of sample which is frequently difficult to obtain, especially in the initial stage of drug development. One suggested cause of increased viscosity is reversible self-association of an antibody8,14–16) which may itself be due to van der Waals forces, electrostatic interactions, and hydrophobic interactions.14,16,17) These interactions are greatly affected by the surface properties of the monoclonal antibody (MAb), which are governed by the amino acid sequence and buffer conditions (solution composition, pH, salt concentration, and additives).18–21) Computational tools are useful in examining surface properties and have undergone rapid development in recent years.22) These approaches enable the calculation of conformational properties such as hydrophobic and hydrophilic areas on the protein surface, the conformational isoelectric point (pI), net charge and zeta potential without consuming materials. These properties are effective for predicting the viscosity of MAb solutions. For example, the hydrophobic surface area increases the aggregation propensity of antibodies and leads to high viscosity at high concentrations.23,24) To investigate protein–protein interactions, the second virial coefficient (B2) is an excellent measure of weak interactions among molecules.8,25) In fact, determination of B2 at relatively low concentrations (<10 mg/mL) is used as an index of the likelihood of high viscosity and aggregation at high concentrations of greater than 100 mg/mL.26,27) Experimentally, B2 can be determined from the analytical ultracentrifugation sedimentation equilibrium (AUC-SE),14,28) static light scattering (SLS),29–31) or self-interaction chromatography (SIC).32,33) While AUC-SE is a powerful tool for measuring the B2 of samples under various solution conditions and a wide range of concentrations,8,14,28) measurement is time-expensive. The diffusion interaction coefficient parameter (kDiff), which was suggested to correlated with B2,26) can be determined by dynamic light scattering (DLS)8,27,34–36) or by Taylor dispersion method correlates of B2.22,26,27) Additionally, because measuring kDiff for a large number of samples can be achieved in a short period of time using DLS, kDiff is a suitable parameter for an initial screen for antibody drug discovery.22) To date, the kDiff of antibody solutions has been mainly used to compare viscosity between or among antibodies in a specific formulation, but not changes in viscosity according to different antibody formulation.27)
Here, to assess whether kDiff can be used to evaluate the developability of antibody candidates, particularly in terms of viscosity at high concentrations, we measured the viscosity and kDiff of three MAbs under 15 buffer conditions and examined the correlation between viscosity and kDiff. In addition, the surface properties of three MAbs in each formulation condition were analyzed using a computational approach to identify the cause of the difference in viscosity and kDiff for each MAb.
Humanized monoclonal antibody A (IgG1 subclass, MAb-A), B (IgG1 subclass, MAb-B), and C (IgG1 subclass, MAb-C) were produced in mammalian cell culture and multiple purified at Astellas Pharma Inc., Tokyo, Japan. The concentration of the three MAbs were determined based on the absorbance at 280 nm using Nanodrop 2000 (Thermo Fisher Scientific, Boston, MA, U.S.A.) and molar absorption coefficients.38) These three antibodies were stored at below −80 °C.
Preparation of Concentrated MAb Solution SamplesMAb solutions were replaced with the target buffer to 15 buffers using PD-10 columns (GE Healthcare, Chicago, IL, U.S.A.) according to provided manual. The 15 different buffer conditions (20 mM sodium citrate for pH 4.5, 5 and 6. 20 mM sodium phosphate for pH 6 and 7. sodium chloride concentration are 10, 100, and 1000 mM in all pH ranges.) were set for viscosity and kDiff studies. Each MAb solution was subsequently concentrated to approximately 10, 50, 75, 100, 125, 150 and 200 mg/mL with VIVAPORE5 (Sartorius, Göttingen, Germany) in accordance with the manufacturer’s instructions. In some conditions, the concentrations did not reach 200 mg/mL; in these cases, the maximum concentration was used for analysis.
Viscosity MeasurementsThe viscosity of MAb solutions was measured using an Electro Magnetically Spinning Viscometer (EMS-1000, Kyoto Electronics Manufacturing, Tokyo, Japan). It was measured by rotating a 2-mm diameter aluminum ball in a test tube containing 0.3 mL of solution. The viscosity of MAb solutions at 10, 50, 75, 100, 125, and 150 mg/mL was then measured 10 times, respectively.
Determination of kDiffThe kDiff of MAb solutions was estimated using DLS with reference to a previous report.8) Diffusion coefficients were measured at 25 °C as a function of protein concentration using a DynaPro PlateReader (Wyatt, Santa Barbara, CA, U.S.A.). Dynamics software (Wyatt) was used to schedule and automate five 3-s acquisitions for each sample. Triplicate data were averaged to reduce systematic errors in sample preparation and analysis. The mutual diffusion coefficient, Dm, was determined for each MAb solution at protein concentrations of 1.0, 2.5, 5.0, 7.5, and 10 mg/mL in each buffer condition. The relationship between Dm and the diffusion interaction parameter (kDiff) is proportional to the self-diffusion coefficient (D0) as a function of the MAb concentration (c) according to the following equation:
 | (1) |
Molecular Operating Environment (MOE) software (Chemical Computing Group, Montreal, Canada) was used to computationally analyze the molecular properties using PDB data of the three MAbs provided by Astellas Pharma Inc., Tokyo, Japan. Structures were prepared with the proceeding application followed by Protonate3D39) and refined with the AMBER forcefield (Amber10: EHT installed in MOE).40) The temperature was set at 300 K, while the salt concentration and pH were changed according to the prepared formulation conditions. Subsequently, surface properties were determined using a protein properties calculator at standard parameters in MOE.41,42)
The viscosities of MAb-A, B, and C at 125 mg/mL in solutions with sodium chloride concentrations of 10, 100, and 1000 mM with citric (pH 4.5, 5, 6) and phosphate buffer (pH 6, 7) are shown in Fig. 1. We found that the viscosity of MAb-B increased as the pH changed from acidic to neutral and as the sodium chloride concentration decreased. Previous studies have shown that electrostatic interactions contribute to the increase in viscosity when there is some charge bias on the surface area of antibodies at a pH close to the pI of the antibodies.15,24) The contribution of electrostatic interactions to attractive intermolecular interactions is supported by experimental findings that viscosity decreases as salt concentration increases due to the shielding of surface charge by salt ions.35) Similarly to Mab-B, the viscosity of Mab-C increased slightly as the pH changed from acidic to neutral. In contrast to Mab-B, however, the viscosity of Mab-C increased as the sodium chloride concentration increased. These results suggest the contribution of hydrophobic interactions to self-association at higher salt concentrations.43) It should be noted that in phosphate buffer and 10 mM NaCl at pH 7, the viscosity of MAb-B was 72.20 cP, which was almost 10 times higher than that of MAb-C (7.22 cP). These results indicate that the viscosity of individual antibodies changes according to the pH and concentration of sodium chloride.

Each graph shows the mean value ± standard deviation.
Figure 2 shows the kDiff of MAb-A, B, and C at sodium chloride concentrations of 10, 100, and 1000 mM with citrate (pH 4.5, 5, 6) and phosphate buffer (pH 6, 7). These three antibodies have the same sequence in their constant regions but differ in their complementarity-determining regions (CDRs). The kDiff profiles of these three antibodies differed due to the differences in their CDR sequences. With regard to ionic strength, the range of kDiff at the three salt concentrations decreased with increasing ionic strength.27,44) In buffer containing 10, 100, and 1000 mM sodium chloride, kDiff ranged from −42.86 to 1.91 mL/g, −31.86 to −2.47 mL/g, and −11.71 to −5.00 mL/g, respectively. At 10 mM sodium chloride, the kDiff decreased as the pH approached the pI for all three antibodies. The kDiff profile according to the pH and salt concentration was similar between MAb-A and MAb-C, but differed from that of MAb-B. In MAb-B, large negative kDiff were obtained in 10 mM NaCl, which decreased with increasing NaCl concentration. These results again suggest that electrostatic interactions significantly contribute to the kDiff of MAb-B. The kDiff of MAb-B also suggests the possibility of specific patches of charge on the surface of MAb-B, such that attractive interactions occur among molecules at low sodium chloride concentrations and pH values that differ greatly from the pI, and interactions weaken at higher salt concentrations or at pH values approaching the pI. Meanwhile, the kDiff of MAb-A and C changed according to changes in pH and salt concentration, suggesting that they possessed weaker electrostatic interactions than MAb-B.

Each graph shows the mean value ± standard deviation.
To evaluate the intermolecular interactions force under the various conditions in this study, we estimated B2 by substituting values into Eq. 3 as follow. The kDiff can be represented by the molecular weight (M), B2, and the hydrodynamic term, which is composed of ξ1 (the frictional drag of the protein) and ν (molecular volume) as shown in the following equation:
 | (2) |
Saito et al.26) measured kDiff using DLS and B2 using area under the curve (AUC), and defined kDiff as follows:
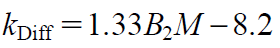 | (3) |
Connolly et al.27) and Lehermayr et al.36) reported similar correlations with slightly different coefficients. As shown in Fig. 3, B2 was negative only for MAb-B in 7 formulation conditions, indicating that attractive forces dominated in these conditions while repulsive forces dominated in all other conditions tested. Interestingly, the B2 values of MAb-A and MAb-C were positive for all 15 formulation conditions, corresponding to repulsive intermolecular interactions under these formulation conditions. Furthermore, at the sodium chloride concentration of 1000 mM, all kDiff values were between 0 and 5. This can be explained by previous suggestions26,44) that, at higher ionic strength, irrespective of an antibody’s surface properties, intermolecular electrostatic interactions can be shielded.

B2 was estimated using the equation of Saito et al.8)
To determine the molecular origins of the differences in profiles of MAb-B compared to MAb-A and MAb-C in solution, we evaluated the antibodies’ surface properties using MOE software. Given that differences of these three antibodies were only present in the amino acid sequences of the CDRs, calculation of surface charges was performed for Fragment variable (Fv) regions for comparison with the properties of the three MAbs. Table 1a shows the pI and net charge profiles. It should be noted that the net charge of MAb-B was the lowest among the three MAbs under all formulation conditions. Table 1b shows the patches of negative, positive, and hydrophobic surface areas identified using MOE. The analysis revealed a unique negative region in the CDR of MAb-B that was not observed in MAb A or MAb-C. Because antibodies have many positive surface areas in constant regions, especially at neutral pH, the negative surface areas of MAb-B may interact with these positive surface areas, leading to the formation of self-associations.24) Contribution of electrostatic interactions to the high viscosity of MAb-B is supported by the fact that the viscosity of MAb-B decreased as the salt concentration increased at all pH values.
| (a) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Antibody name | MAb-A | MAb-B | MAb-C | ||||||
| pH | 5 | 6 | 7 | 5 | 6 | 7 | 5 | 6 | 7 |
| Sequence-based pI | 8.88 | 7.15 | 9.31 | ||||||
| Structure-based pI | 9.07 | 8.29 | 9.61 | ||||||
| Net charge | 5.18 | 5.03 | 4.97 | 3.61 | 2.61 | 2.31 | 9.45 | 9.18 | 9.04 |
| (b) | |||||||||
| Description | MAb-A | MAb-B | MAb-C | ||||||
| Area of hydrophobic protein patches (Å2) | 640 | 600 | 620 | ||||||
| Area of positive protein patches (Å2) | 1420 | 1230 | 1600 | ||||||
| Area of negative protein patches (Å2) | 810 | 860 | 870 | ||||||
| Area of hydrophobic protein patches near CDRs (Å2) | 280 | 250 | 160 | ||||||
| Area of positive protein patches near CDRs (Å2) | 740 | 570 | 670 | ||||||
| Area of negative protein patches near CDRs (Å2) | 300 | 400 | 350 | ||||||
There were also some differences in the hydrophobic surface areas among the antibodies, although there was no significant increase in viscosity due to differences in the hydrophobic area. This suggests that these hydrophobic differences did not greatly affect viscosity in the high molecular concentration environments examined in this study.17,23)
Correlation between kDiff and ViscosityFigure 4 shows the relationship between the viscosities at three concentrations (100, 125, 150 mg/mL) and kDiff obtained under 15 formulation conditions for MAb-A, MAb-B, and MAb-C and estimated exponential fitting plots. Viscosity and kDiff correlate clearly exponentially (100 mg/mL: R2 = 0.79, 125 mg/mL: R2 = 0.88, 150 mg/mL: R2 = 0.86).

Each line is an approximate representation of the exponential functions.
Previous report suggested when the injection force was fixed at 30 N, liquids with a viscosity below 10 cP could be injected by a 30-gauge needle, while liquids with a viscosity of 25 cP could be expelled by a 27-gauge needle.10) Assuming that 30 N is the maximum force for injection and a 27-gauge needle is generally used for SC and IM administration, we set the criterion at 20 cP at the high concentration of 100–150 mg/mL with referring to the report by Burckbuchler.10) According to the correlation between viscosity and kDiff in Fig. 4, a MAb formulation with a kDiff greater than −20 mL/g is required for a viscosity below 20 cP. Thus, this study showed that the kDiff can be used to determine the eligibility of an antibody or formulation for SC and IM administration.
Because administration routes for viscous antibody candidates are limited, predicting the concentration-dependency of viscosity in the early development stage is an important part of evaluating the developability of a drug candidate. In addition, sufficient sample volume is seldom available in this initial stage, requiring low-consumption assays for evaluating the viscosity of candidates. To resolve these issues, we conducted comparative analysis of viscosity and the diffusion interaction parameter (kDiff) of 3 antibodies under 15 buffer conditions and investigated their correlations. We found a high correlation between kDiff and the viscosity of highly concentrated antibodies, suggesting that simple measurement of kDiff can enable prediction of the antibody’s concentration tendency. Setting a viscosity criterion for selection for SC and IM administration is also important for determining the prefillable syringe format of candidates at the late development stage. Based on previous findings, a viscosity of 20cP may be useful for determining the syringeability of a drug candidate.10) From the correlation between kDiff and viscosity observed in this study, a kDiff of −20 mL/g may be an alternative viscosity criterion.
From the estimation of B2 values, we found that attractive forces applied in MAb-B under some formulation conditions, whereas repulsive forces dominated in MAb-A and MAb-C under all formulation conditions. Using a computational method, we also found that the unique presence of a negatively charged area in the CDR region may cause the attractive forces and then increase an viscosity in Mab-B. We speculate that this negatively charged area forms electrostatic interactions with positively charged areas on the same CDR or the constant region. This hypothesis is supported by the finding that the viscosity decreased with increasing sodium chloride concentration or with adjustment to a pH that differed greatly from the pI. We found that it contributed less to the concentration-dependency of viscosity than the hydrophilic area although the hydrophobic areas are considered as contributing to high viscosity.17) Based on previous reports that examined the relationship between surface properties and viscosity,23,24) the contributions of the hydrophobic and hydrophilic areas to viscosity are dependent on the antibody species and formulation.
The authors thank the staff at Astellas Pharma Inc. (Tsukuba, Japan) and Osaka University for their help with the experiments.
This study was funded by Astellas Pharma Inc. and Osaka University.
This article contains supplementary materials.