Abstract
We previously demonstrated that per os administration and ad libitum ingestion of a magnesium chloride (MgCl2) solution had a prophylactic effect on dextran sulfate sodium (DSS)-induced colitis in mice, magnesium being considered to play a role in this preferable action. Magnesium oxide (MgO) is a commercially available magnesium formulation, but whether or not it prevents development of colitis is unknown. In this study, we investigated the effect of MgO administration on development of colitis in DSS-treated male C57BL/6J mice. Experimental colitis was induced by ad libitum ingestion of 1% (w/v) DSS, and the colitis severity was evaluated by disease activity index (DAI) scores, histological assessment and colonic expression of inflammatory cytokines. A 1 mg/mL MgO solution was administered to mice through ad libitum ingestion from a day before DSS treatment to the end of the experimental period of 12 d. In addition, the effects of DSS, MgO and their combination on the gut microbiota were investigated by 16S ribosomal RNA metagenome analysis. DSS-induced elevation of DAI scores was partially but significantly decreased by MgO administration, while MgO administration had no apparent effect on the shortened colonic length, elevated mRNA expression of colonic interleukin-1β and tumor necrosis factor-α, increased accumulation of colonic mast cells, or altered features of the gut microbiota in DSS-treated mice. Overall, we demonstrated that MgO had a prophylactic effect on the development of colitis in DSS-treated mice by preventing histological colonic damage, but not colonic inflammation or alteration of the gut microbiota.
INTRODUCTION
Colitis, i.e., ulcerative colitis and Crohn’s disease, is an intractable disease, and the number of patients is increasing worldwide year by year.1–3) Currently, patients receive symptomatic, but not curative, treatment with 5-aminosalicylic acid, steroids and immunosuppressants, because the mechanism underlying the development of colitis remains unclear.4) Recently, it appeared that activation of P2X7 receptors (P2X7R) expressed by colonic resident mast cells is the first step of the developmental cascade of colitis,5) and prophylactic blockade of P2X7R is a potential prophylactic/therapeutic approach for colitis.5–7) Previously, we found that magnesium is an antagonist for P2X7R,8) and its prophylactic administration to mice with colitis induced by dextran sulfate sodium (DSS) is effective to prevent its development.9) In this study, we used magnesium chloride (MgCl2), and its per os (p.o.) administration at the dose of 500 mg/kg once a day exerted a partial but significant preventive effect as to the development of colitis in DSS-treated mice without any adverse symptoms such as diarrhea, this preferable action being due to inhibition of P2X7R-immunopositive mast cell accumulation in the colon, followed by induction of colonic inflammatory injury.
In clinical situations, magnesium oxide (MgO) is a safe and effective medicine for gastritis/gastric ulcers and constipation. Orally administered MgO is considered to be converted into MgCl2 via a reaction with gastric acid in the stomach, and is expected to exert a prophylactic effect as to colitis development. However, there is no available information regarding whether MgO prevents the development of colitis or not.
Regarding the potential pathogenesis of colitis, it has recently been reported that the gut microbiota plays a critical role as a major environmental driver, because the microbiota is considered to be involved in regulation of the mucosal immune response and epithelial barrier function.4) Based on reports that dysbiosis is a causative factor for the development of ulcerative colitis,10,11) transplantation of microbiota from healthy subjects to ulcerative colitis cases is considered to be a novel therapeutic approach.12) There are reports on dysbiosis in murine experimental colitis,4) but there is no available information on the effects of therapeutic and/or preventive agents on the gut microbiota.
Therefore, we investigated the prophylactic effect of MgO ad libitum ingestion in mice with colitis induced by DSS treatment together with the underlying mechanisms including alteration of the gut microbiota. In this study, MgO was administered to mice by ad libitum ingestion of its aqueous solution to prevent transient high colonic magnesium concentrations, by which colonic adverse effects such as diarrhea are induced.
MATERIALS AND METHODS
AnimalsIn this study, we used 40 male C57BL/6JJmsSlc mice (7 weeks-old, Japan SLC, Hamamatsu, Japan). After 1-week acclimation, the mice were randomly divided into 4 groups (10 mice/group) and 5 mice in the same group were housed in a cage with food (MF; Oriental Yeast Co., Ltd., Tokyo, Japan) and water available ad libitum under controlled conditions of 22 ± 1 °C with a 12–12 h light–dark cycle in a specific pathogen-free facility. All experiments were performed according strictly to ARRIVE guidelines, and were authorized by the Experimental Animal Research Committee of Kyoto Pharmaceutical University (Authorization Nos. DEB-15-001 and DEB-20-001, from 2015 to 2022).
Induction of Colitis According to our previous study,13) to induce experimental colitis in C57BL/6J mice, we used DSS (dextran sulfate sodium salt, colitis grade, Cat. No. 160110, Lot No. S2839, molecular weight: 36000–50000; MP Biomedicals, LLC, OH, U.S.A.). DSS was dissolved in double distilled water (DW) at the concentration of 1% (w/v), and mice had access to the DSS solution ad libitum for 11 d (from Day 0 to Day 11).
Daily evaluation of the colitis severity was performed with disease activity index (DAI) scores, which are the sums of the scores for body weight loss (0 ≦ 5%, 1 = 5–10%, 2 = 11–15%, 3 = 16–20%), stool consistency (0 = normal, 1 = soft but still formed, 2 = very soft, 3 = diarrhea), and rectal bleeding (0 = no blood, 1 = occult blood in stool, 2 = trace of blood visible in stool, 3 = rectal bleeding), and scoring was performed blindly by at least two independent investigators.9,13)
In the following experiments, 10 mice in each group were divided equally (5 mice/group) into histological and biochemical examinations, and their DAI scores were almost the same between the two examinations (DAI scores on Day 11 were 0.6 ± 0.894 and 0.4 ± 0.548 in control group, 5 ± 0.707 and 3.6 ± 1.14 in DSS group, 0.6 ± 0.548 and 0.2 ± 0.447 in MgO group, and 3.6 ± 1.67 and 3.4 ± 1.52 in MgO + DSS group, respectively).
MgO AdministrationWe previously found that p.o. administration of 500 mg/kg of MgCl2 once a day (0.1 mmol/mouse/d) for an 11 d experimental period had a prophylactic effect on DSS-induced colitis in C57BL/6N mice.9) Based on the average volume of daily water intake of a mouse (approximately 4 mL/d), the concentration of the MgO solution for ad libitum administration was determined to be 1 mg/mL in DW, i.e., MgO was administered at the dose of approximately 0.1 mmol/mouse/d. As for preparation of the aqueous MgO solution, MgO was firstly dissolved in a 1M HCl solution, and the resulting solution was neutralized (pH 7–8) by adding an appropriate volume of a 1M NaOH solution. By this preparation procedure, NaCl is generated in the solution. Strong salty taste is known to induce aversion for ingestion in mammals including mice, but the daily intake volume of MgO solution was almost comparable with that of water (the averaged volumes of 5 mice/cage were 3.88 and 4.68 mL/d/mouse, respectively). In the case of administration of MgO to DSS-treated mice, DSS was dissolved in the MgO solution at the concentration of 1 w/v%. Previously, we confirmed that simultaneous administration of MgO and DSS dissolved in a solution had no or negligible effects on colitis induction in mice.9)
Macroscopic and Histological Assessment of ColitisOn Day 11, just after evaluation of body weight change and stool conditions for DAI scoring, 5 mice were assigned for histological assessment for colitis severity. Under deep anesthesia with an intraperitoneal injection of a mixture of medetomidine (0.3 mg/kg), midazolam (4 mg/kg), and butorphanol (5 mg/kg), mice were transcardially perfused with 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (pH 7.4, Sorensen’s buffer) containing 0.2% picric acid, followed by removal of the entire colon (from the caecum to the anus). After determination of the colon length followed by additional overnight immersion of the tissues in 4% PFA at 4 °C, the tissues were immersed in a 30% sucrose solution at 4 °C overnight and then cut into 10 µm frozen sections with a microtome (CM1850; Leica Biosystems Nussloch GmbH, Nussloch, Germany). Thereafter, the tissues were stained with haematoxylin–eosin (H&E), and histologically evaluated under a light microscope (BX40; Olympus, Tokyo, Japan), photomicrographs being taken with a digital camera (Moticam 2000; Shimadzu, Kyoto, Japan).
As reported previously,9,14) using a minimum of 2 sections of different parts of the distal colon per mouse, histological scoring was performed for 3 parameters: severity of inflammation (0 = rare inflammatory cells in the lamina propria, 1 = increased number of granulocytes in the lamina propria, 2 = confluence of inflammatory cells extending into the submucosa, and 3 = transmural extension of the inflammatory infiltrate), crypt damage (0 = intact crypts, 1 = loss of the basal one-third, 2 = loss of the basal two-thirds, 3 = entire crypt loss, 4 = a change of the epithelial surface with erosion, and 5 = confluent erosion), and ulceration (0 = absence of ulceration, 1 = 1 or 2 foci of ulceration, 2 = 3 or 4 foci of ulceration, and 3 = confluent or extensive ulceration). The scoring was done blindly by two independent investigators.
ImmunohistochemistryAccording to the previous studies,9,15) the colonic sections obtained for histological assessment described above were washed with Sorensen’s buffer and then incubated in a blocking buffer (1% donkey serum, 0.3% Triton X-100, 0.3% bovine serum albumin and 0.05% sodium azide-containing phosphate buffer) for 1 h at room temperature. After washing with phosphate buffer, the sections were incubated with primary antibodies diluted with the blocking buffer at 4 °C overnight. The primary antibodies used were mouse anti-mast cell tryptase antibodies (dilution ratio: 1 : 50; Cat. No. sc-59587, Santa Cruz Biotechnology, Inc., TX, U.S.A.), and rabbit anti-P2X7R antibodies (dilution ratio: 1 : 100; Cat. No. APR-004, Alomone Labs, Jerusalem, Israel). After washing with phosphate buffered saline, the sections were incubated with donkey anti-mouse immunoglobulin G (IgG) antibodies conjugated with Alexa Fluor® 594, and donkey anti-rabbit IgG antibodies conjugated with Alexa Fluor® 488 (dilution ratio: 1 : 1000 with the blocking buffer containing Hoechst33258 (1 mg/mL); Cat. Nos. A21203 and A21206, respectively, Thermo Fisher Scientific Inc., MA, U.S.A.) at 4 °C overnight. The sections were mounted on glass slides and then enclosed with Vectorshield® (Vector Laboratories, CA, U.S.A.), and photomicrographs were taken under a laser confocal microscope (LSM800; Carl Zeiss, Oberkochen, Germany). The antigen specificities of the antibodies for mast cell tryptase and P2X7R were confirmed in negative control experiments with the primary antibodies omitted. Using photomicrographs of a minimum of 5 sections of different parts of the distal colon per mouse, the numbers of mast cell tryptase- and P2X7R-immunopositive cells were blindly determined by at least two independent investigators.
Real-Time PCRcDNA was prepared from the colonic epithelial tissues obtained from 5 mice in a group, the remainders being used for histological examination, and real-time quantitative PCR was performed using the primer sets of Il1b (forward, 5′-CTCAATGGACAGAATATCAACCAAC-3′, reverse, 5′-GGTGTGCCGTCTTTCATTACAC-3′), Tnfa (forward, 5′-CAGCCGATGGGTTGTACCTT-3′, reverse, 5′-TGTGGGTGAGGAGCACGTAGT-3′), and Actb (forward, 5′-CCTGAGGAGCACCCTG-3′, reverse, 5′-TCCGGAGTCCATCACA-3′), as reported previously. The expression levels of mRNAs were corrected as to that of β-actin and are given as % of the control.13,16)
16S Ribosomal RNA (rRNA) Metagenome AnalysisTotal DNA was obtained from cecum feces, and the V3-V4 hypervariable region of 16S rRNA was amplified, followed by sequencing on an Illumina MiSeq platform (Illumina, Inc., CA, U.S.A.). Bioinformatic sequence analysis was carried out using a QIIME II pipeline (ver. 2021.2) with SILVA data base release 13_8.13,17)
Statistical AnalysisEach experimental result is given as the mean ± standard deviation (S.D.), and statistical significance was evaluated by two-way repeated measures ANOVA (Fig. 1) or one-way ANOVA, followed by the Tukey post hoc multiple comparisons test (Figs. 2–4), and Kruskal–Wallis test (Fig. 5A), a p value of 0.05 or less being considered statistically significant. Exact p, F, H and n values for all statistical analyses in each experiment are shown in Table 1.
Table 1. Exact
p,
F and
n Values for All Statistical Analyses in Each Experiment
| Figure | Statistical analysis | F or H value | p–Value | Post hoc | p-Value | n |
|---|
| 1A | Two-way ANOVA | Fmain effect of treatment (3,108) = 38.7 | <0.001 | Control vs. DSS | <0.001 | 10, 10 |
| | | | Control vs. MgO | <0.001 | 10, 10 |
| | | | Control vs. MgO + DSS | 0.0011 | 10, 10 |
| | | | DSS vs. MgO | <0.001 | 10, 10 |
| | | | DSS vs. MgO + DSS | <0.001 | 10, 10 |
| | | | MgO vs. MgO + DSS | 0.484 | 10, 10 |
| | Fmain effect of time (11,396) = 2.86 | 0.0013 | | | |
| | Finteraction (33,396) = 4.46 | <0.001 | | | |
| 1B | Two-way ANOVA | Fmain effect of treatment (3,108) = 138 | <0.001 | Control vs. DSS | <0.001 | 10, 10 |
| | | | Control vs. MgO | 0.134 | 10, 10 |
| | | | Control vs. MgO + DSS | <0.001 | 10, 10 |
| | | | DSS vs. MgO | <0.001 | 10, 10 |
| | | | DSS vs MgO + DSS | 0.0236 | 10, 10 |
| | | | MgO vs. MgO + DSS | <0.001 | 10, 10 |
| | Fmain effect of time (8,288) = 13.5 | <0.001 | | | |
| | Finteraction (24,288) = 7.15 | <0.001 | | | |
| 1C | Two-way ANOVA | Fmain effect of treatment (3,108) = 44.4 | <0.001 | Control vs. DSS | <0.001 | 10, 10 |
| | | | Control vs. MgO | 1 | 10, 10 |
| | | | Control vs. MgO + DSS | <0.001 | 10, 10 |
| | | | DSS vs. MgO | <0.001 | 10, 10 |
| | | | DSS vs. MgO + DSS | 0.205 | 10, 10 |
| | | | MgO vs. MgO + DSS | <0.001 | 10, 10 |
| | Fmain effect of time (8,288) = 12.5 | <0.001 | | | |
| | Finteraction (24,288) = 4.35 | <0.001 | | | |
| 1D | Two-way ANOVA | Fmain effect of treatment (3,108) = 159 | <0.001 | Control vs. DSS | <0.001 | 10, 10 |
| | | | Control vs. MgO | 0.457 | 10, 10 |
| | | | Control vs. MgO + DSS | <0.001 | 10, 10 |
| | | | DSS vs. MgO | <0.001 | 10, 10 |
| | | | DSS vs. MgO + DSS | <0.001 | 10, 10 |
| | | | MgO vs. MgO + DSS | <0.001 | 10, 10 |
| | Fmain effect of time (8,288) = 23.8 | <0.001 | | | |
| | Finteraction (24,288) = 10.3 | <0.001 | | | |
| 2A | One-way ANOVA | F(3,108) = 17.7 | <0.001 | Control vs. DSS | <0.001 | 5, 5 |
| | | | Control vs. MgO | 0.109 | 5, 5 |
| | | | Control vs. MgO + DSS | <0.001 | 5, 5 |
| | | | DSS vs. MgO | 0.0102 | 5, 5 |
| | | | DSS vs. MgO + DSS | 0.999 | 5, 5 |
| | | | MgO vs. MgO + DSS | 0.0116 | 5, 5 |
| 2B | One-way ANOVA | F(3,108) = 6.28 | 0.0051 | Control vs. DSS | 0.0232 | 5, 5 |
| | | | Control vs. MgO | 1.00 | 5, 5 |
| | | | Control vs. MgO + DSS | 0.0454 | 5, 5 |
| | | | DSS vs. MgO | 0.0253 | 5, 5 |
| | | | DSS vs. MgO + DSS | 0.986 | 5, 5 |
| | | | MgO vs. MgO + DSS | 0.0494 | 5, 5 |
| 2C | One-way ANOVA | F(3,108) = 56.7 | <0.001 | Control vs. DSS | <0.001 | 5, 5 |
| | | | Control vs. MgO | 0.956 | 5, 5 |
| | | | Control vs. MgO + DSS | <0.001 | 5, 5 |
| | | | DSS vs. MgO | <0.001 | 5, 5 |
| | | | DSS vs. MgO + DSS | 0.545 | 5, 5 |
| | | | MgO vs. MgO + DSS | <0.001 | 5, 5 |
| 3B | One-way ANOVA | F(3,108) = 18.9 | <0.001 | Control vs. DSS | <0.001 | 5, 5 |
| | | | Control vs. MgO | 0.997 | 5, 5 |
| | | | Control vs. MgO + DSS | 0.663 | 5, 5 |
| | | | DSS vs. MgO | <0.001 | 5, 5 |
| | | | DSS vs. MgO + DSS | <0.001 | 5, 5 |
| | | | MgO vs. MgO + DSS | 0.548 | 5, 5 |
| 4A | One-way ANOVA | F(3,108) = 8.02 | 0.0017 | Control vs. DSS | 0.0043 | 5, 5 |
| | | | Control vs. MgO | 0.980 | 5, 5 |
| | | | Control vs. MgO + DSS | 0.0337 | 5, 5 |
| | | | DSS vs. MgO | 0.0094 | 5, 5 |
| | | | DSS vs. MgO + DSS | 0.739 | 5, 5 |
| | | | MgO vs. MgO + DSS | 0.0707 | 5, 5 |
| 4B | One-way ANOVA | F(3,108) = 38.8 | <0.001 | Control vs. DSS | <0.001 | 5, 5 |
| | | | Control vs. MgO | 0.992 | 5, 5 |
| | | | Control vs. MgO + DSS | <0.001 | 5, 5 |
| | | | DSS vs. MgO | <0.001 | 5, 5 |
| | | | DSS vs. MgO + DSS | 0.460 | 5, 5 |
| | | | MgO vs. MgO + DSS | <0.001 | 5, 5 |
| 4C | One-way ANOVA | F(3,108) = 34.9 | <0.001 | Control vs. DSS | <0.001 | 5, 5 |
| | | | Control vs. MgO | 0.548 | 5, 5 |
| | | | Control vs. MgO + DSS | <0.001 | 5, 5 |
| | | | DSS vs. MgO | <0.001 | 5, 5 |
| | | | DSS vs. MgO + DSS | 0.996 | 5, 5 |
| | | | MgO vs. MgO + DSS | <0.001 | 5, 5 |
| 5A | Kruskal–Wallis | H = 9.51 | 0.0232 | Control vs. DSS | 0.0143 | 4, 5 |
| | | | Control vs. MgO | 0.0864 | 4, 5 |
| | | | Control vs. MgO + DSS | 0.0500 | 4, 5 |
| | | | DSS vs. MgO | 0.0758 | 5, 5 |
| | | | DSS vs. MgO + DSS | 0.465 | 5, 5 |
| | | | MgO vs. MgO + DSS | 0.0758 | 5, 5 |
RESULTS
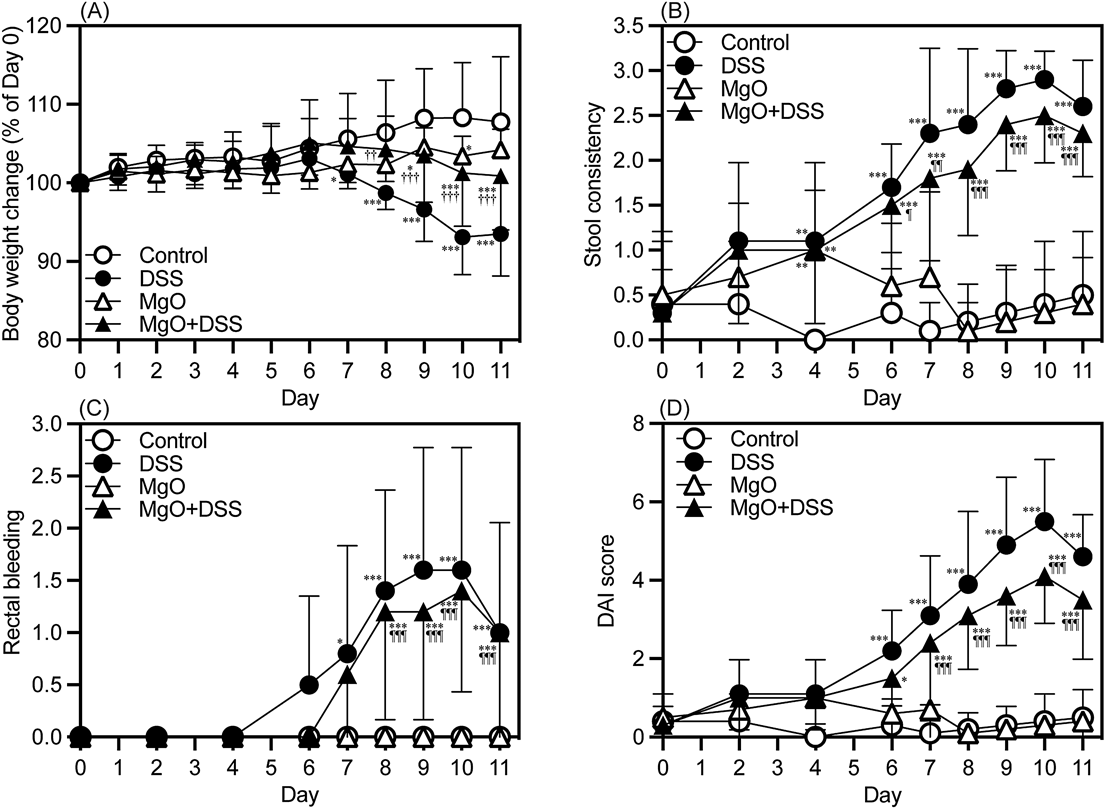
Evaluation of colitis development in mice is shown in Fig. 1. Two-way repeated measures ANOVA analyses revealed that the body weight gain, and stool consistency, rectal bleeding and DAI scores in DSS-treated mice were significantly lower (p < 0.001) and higher (p < 0.001 each), respectively, than those in control mice (Table 1). The alteration in body weight gain, stool consistency and DAI scores in DSS-treated mice was partially but significantly ameliorated by MgO ingestion (p < 0.001, p = 0.0236 and p < 0.001, respectively, Table 1). While there was no apparent difference in the colitis severity between the control and MgO groups, ad libitum ingestion of the MgO solution slightly decreased the body weight gain of MgO group mice compared with control ones (p < 0.001, Table 1), this being due to decreased water intake (data not shown).
To evaluate histological damage to the colons of MgO-, DSS- and MgO + DSS-administered mice, the colons were stained with H&E (Fig. 2). Since there was no apparent difference in histological features of the colons between the control and MgO groups, MgO alone was found to induce no or negligible tissue damage to the colon. In contrast, DSS treatment induced histological injury to the colon evident as crypt loss (arrowhead) accompanied by infiltration of inflammatory cells such as neutrophiles (arrow), this being reflected by the quantitative histological scores. On administration of MgO, the alteration in the colons of DSS-treated mice was almost completely abolished.
Comparison of the colon length among the four groups (Fig. 3A) revealed the DSS- and MgO + DSS groups had significantly shorter colons than the control group did (p < 0.001 for both groups), and there was no difference between the control and MgO groups. Expression of mRNAs for interleukin (IL)-1β and tumor necrosis factor (TNF)-α in the colons of DSS-treated mice was significantly greater than that in control mice, and administration of MgO to DSS-treated mice induced no detectable alteration in their expression (Figs. 3B, C).
Figure 4A shows immunohistochemical evaluation of mast cell accumulation in the colons of mice. The numbers of colonic mast cell tryptase- and P2X7R-immunopositive cells were significantly greater in DSS-treated mice than in control ones (p < 0.01 and p < 0.001, respectively) (Figs. 4B, C), approximately 10% of the mast cells accumulated in the colon being P2X7R-immunopositive (Fig. 4D). MgO administration had no detectable effect on colonic accumulation of mast cells and P2X7R-immunopositive cells in either naïve or DSS-treated mice.
16S rRNA metagenome analysis revealed that DSS treatment altered the α- and β-diversity of the gut microbiota, which were evaluated by Faith PD significance (Fig. 5A) and weighted Unifrac distance PCoA (Fig. 5B) analyses, respectively, and MgO administration had no apparent effect on the α- and β-diversity in either naïve or DSS-treated mice (Fig. 5). Similar alteration was found in the relative composition of bacterial genera in the gut microbiota (Table 2), and the abundance of Alistipes spp., Alloprevotella spp., Roseburia spp., etc. was reduced, while the abundance of Bacteroides spp., etc. was elevated in the DSS- and MgO + DSS groups compared with those in the control ones. On the other hand, MgO ingestion significantly increased the relative composition of the Lachnospiraceae NK4A136 group and Desulfovibrio spp. compared with the case of the control group.
Table 2. Comparison of Relative Abundance of Bacterial Genera in the Gut Microbiota among the Control, DSS, MgO and MgO + DSS Groups
| Rank | Bacterial genus | Control (n = 4) | DSS (n = 5) | MgO (n = 5) | MgO + DSS (n = 5) | F(3,48) | p |
|---|
| 1 | Unclassified Lachnospiraceae | 22.2 ± 2.00 | 17.5 ± 4.83 | 21.5 ± 2.55 | 17.9 ± 3.14 | 2.39 | 0.109 |
| 2 | Alistipes | 11.1 ± 1.83 | 1.35 ± 0.884*** | 9.59 ± 2.00††† | 0.740 ± 0.347***,¶¶¶ | 69.1 | <0.001 |
| 3 | Muribaculum | 8.73 ± 2.19 | 9.44 ± 1.82 | 9.31 ± 1.90 | 9.45 ± 1.57 | 0.140 | 0.935 |
| 4 | Unclassified Muribaculaceae | 8.41 ± 0.587 | 6.91 ± 1.72 | 6.87 ± 0.793 | 6.40 ± 2.18 | 1.42 | 0.277 |
| 5 | Uncultured Muribaculaceae | 7.26 ± 0.831 | 7.06 ± 1.23 | 7.85 ± 1.09 | 7.81 ± 0.737 | 0.752 | 0.538 |
| 6 | Bacteroides | 5.36 ± 0.505 | 19.0 ± 8.45** | 6.70 ± 1.09†† | 20.0 ± 4.32**,¶¶ | 11.7 | <0.001 |
| 7 | Ruminococcaceae UCG-014 | 3.85 ± 0.915 | 4.30 ± 2.28 | 2.02 ± 0.269 | 3.51 ± 1.16 | 2.50 | 0.0994 |
| 8 | A2 | 3.13 ± 0.875 | 0.00162 ± 0.00363*** | 2.07 ± 0.987††† | 0 ± 0***,¶¶¶ | 26.8 | <0.001 |
| 9 | Alloprevotella | 2.74 ± 0.962 | 0.865 ± 1.36 | 2.10 ± 1.25 | 0.375 ± 0.526* | 4.65 | 0.0172 |
| 10 | Lachnospiraceae NK4A136 group | 2.72 ± 1.05 | 1.87 ± 0.951 | 5.15 ± 1.46*,††† | 2.67 ± 0.584¶ | 8.94 | 0.0012 |
| 20 | Anaerotruncus | 0.734 ± 0.354 | 0.184 ± 0.0966** | 0.611 ± 0.219† | 0.112 ± 0.169**,¶ | 9.15 | 0.0011 |
| 26 | G(-) bacterium cTPY-13 | 0.518 ± 0.273 | 0.00885 ± 0.0198*** | 0.445 ± 0.102††† | 0 ± 0***,¶¶¶ | 20.1 | <0.001 |
| 28 | Desulfovibrio | 0.451 ± 0.188 | 0.555 ± 0.152 | 1.80 ± 0.763**,†† | 0.842 ± 0.389¶ | 8.71 | 0.0014 |
| 29 | Acetatifactor | 0.408 ± 0.237 | 0.0622 ± 0.0455** | 0.354 ± 0.157† | 0.0219 ± 0.0102**,¶¶ | 9.85 | <0.001 |
| 30 | Roseburia | 0.378 ± 0.134 | 0 ± 0** | 0.317 ± 0.232†† | 0 ± 0**,¶¶ | 10.6 | <0.001 |
| 31 | ASF356 | 0.286 ± 0.0573 | 0.0629 ± 0.0682* | 0.218 ± 0.184 | 0.0530 ± 0.0919* | 4.63 | 0.0174 |
| 32 | Uncultured Desulfovibrionaceae | 0.282 ± 0.134 | 0.275 ± 0.0304 | 0.104 ± 0.0139*,†† | 0.180 ± 0.0701 | 6.57 | 0.0047 |
| 36 | Uncultured Peptococcaceae | 0.219 ± 0.0533 | 0.479 ± 0.174* | 0.205 ± 0.0371†† | 0.361 ± 0.120 | 6.27 | 0.0057 |
| 38 | Turicibacter | 0.193 ± 0.219 | 1.45 ± 0.287*** | 0.377 ± 0.414†† | 2.20 ± 0.415***,†,¶¶¶ | 34.0 | <0.001 |
| 43 | Blautia | 0.146 ± 0.0922 | 0.0141 ± 0.0162** | 0.0843 ± 0.0269 | 0.0270 ± 0.0254** | 7.54 | 0.0026 |
| 45 | UC5-1-2E3 | 0.117 ± 0.0780 | 0 ± 0** | 0.0554 ± 0.0444 | 0 ± 0** | 7.78 | 0.0023 |
| 61 | Ruminococcus 1 | 0.0364 ± 0.0451 | 3.02 ± 0.912** | 0.123 ± 0.0996†† | 3.32 ± 1.62***,¶¶¶ | 16.3 | <0.001 |
| 80 | Clostridium sensu stricto 1 | 0.00537 ± 0.0107 | 1.27 ± 0.788** | 0.158 ± 0.114†† | 1.33 ± 0.437**,¶¶ | 10.6 | <0.001 |
Bacterial genera that were ranked in the top ten as to abundance and showed significant differences in relative composition among the four groups are shown. The total number of bacterial genera detected was 113. Each value represents the mean ± S.D. * p < 0.05, ** p < 0.01, *** p < 0.001 (vs. Control). † p < 0.05, †† p < 0.01, ††† p < 0.001 (vs. DSS). ¶ p < 0.05, ¶¶ p < 0.01, ¶¶¶ p < 0.001 (vs. MgO).
DISCUSSION
In this study, we found that ad libitum ingestion of a MgO solution ameliorated partially but significantly the decrease of body weight gain and colitis severity caused by DSS treatment, and almost completely prevented the colonic injury caused by DSS treatment, while there was no apparent alteration in the DSS-induced shortened colon length, increased expression of colonic inflammatory cytokines, increased colonic accumulation of mast cells, or altered diversity and composition of the microbiota. Overall, prophylactic administration of MgO prevents development of the physical symptoms of colitis, at least in part, through protection of the colon from histological injury.
Based on the findings obtained here, MgO is judged to have a prophylactic action on DSS-induced colitis, at least in part, through prevention of the development of colonic injury, as MgCl2 does. However, differing from the case of MgCl2,9) MgO did not have a detectable effect on the DSS-induced colonic inflammatory immune response. Although there is no reasonable explanation for the different preventive mechanisms, differences in magnesium disposition, but not magnesium formulations, might be involved. Because both MgCl2 and MgO were dissolved probably as magnesium ions in an aqueous solution, their intestinal absorption is considered to be the same. On the other hand, p.o. administration caused transient great elevation of the luminal and blood magnesium concentrations, while ad libitum ingestion results in low but stable concentrations in both the lumen and blood. It is reasonable to consider that the luminal concentration of magnesium ions is a determinant of the inhibitory effect on the inflammatory immune response induced by colonic resident immune cells such as mast cells, while the blood concentration of magnesium determines the preventive effect as to colonic injury induced by infiltration of immune cells such as neutrophiles. The key step in the extravasation of neutrophiles is binding of leukocyte integrins such as lymphocyte function-associated antigen-1, macrophage-1 antigen and very late antigen-4 to their endothelial ligands such as intercellular adhesion molecule 1 (ICAM-1), ICAM-2 and vascular cell adhesion molecule 1 (VCAM-1),18) and the expression of VCAM-1 is reported to depend inversely on the magnesium level.19) Overall, it is suggested that the transient high luminal and blood magnesium concentrations on p.o. administration could inhibit both colonic inflammation and injury, while the stable low magnesium concentrations in blood, especially vicinity of colonic endothelial cells, on ad libitum ingestion prevent colonic injury induced by infiltration of systemic immune cells, but the luminal magnesium concentrations are not enough to inhibit activation of luminal resident immune cells. This explanation is considered to be supported by the finding that there was no alteration in stool consistency scores between control and MgO-ingested mice, and that DSS-induced alterations of the diversity and composition of bacterial genera in the gut microbiota was not affected by the co-administration of MgO.
Previously, we found that the colonic magnesium concentration after administration/ingestion of a magnesium solution depended on the concentrations of the administered magnesium solution.9) Since the magnesium concentration in the dosing MgCl2 solution of 500 mg/kg p.o. was 25 mg/mL/50 g body weight (263 mM), this being approximately 10-fold higher than that in the dosing MgO solution of 1 mg/mL ad libitum ingestion (25 mM), the colonic luminal magnesium concentration after the p.o. administration was estimated to be approximately 10-fold greater than that after the ad libitum ingestion. Thus, it is considered that the colonic luminal magnesium concentration after ad libitum ingestion of a MgO solution is not enough to inhibit the activation of P2X7R expressed by colonic resident mast cells, the first step in induction of the colonic inflammatory response.5)
In clinical situation, MgO administration is known to induce rarely hypermagnesemia especially in patients with renal dysfunction and constipation. In our preliminary experiments, we measured blood magnesium concentrations in mice after 10 d ad libitum ingestion of MgO solution (1 mg/mL, the same concentration with this study), and the concentrations were comparable with the case of control (569 ± 128 (n = 3) and 629 ± 49.0 (n = 4) ng/mL, respectively, t = 0.760, p = 0.482), there being no or only negligible induction of hypermagnesemia under this experimental condition.
Ad libitum ingestion of MgO solution exerted prophylactic effect on development of DSS-induced colitis, but there was no difference in rectal bleeding scores between DSS and MgO + DSS groups. Although there is no reasonable explanation on this, we speculated that the preventive effect of MgO on DSS-induced colitis might be not adequate to inhibit rectal bleeding as indicated by no effect on DSS-induced shortened colon length.
The decreased levels of Alistipes spp., Alloprevotella spp. and Roseburia spp., and the increased level of Bacteroides spp. are well-known characteristic features in DSS-induced colitis,20–24) and the same alterations were found in this study. In particular, Alistipes spp. and Bacteroides spp. are reported to be involved in regulation of mucus barrier function through protective and dysfunctional effects, respectively.25,26) On the other hand, the relative abundance of the Lachnospiraceae NK4A136 group and Desulfovibrio spp. was increased in MgO-ingested mice, the former and latter playing roles in maintenance of gut mucus barrier function via the production and sulphation, respectively, of mucin.27,28) Therefore, it is suggested that magnesium ingestion might exert a protective effect on colonic mucus integrity.
In this study, we found a prophylactic effect of ad libitum ingestion of magnesium. Thus, taken together the finding of no apparent adverse effect of MgO ingestion such as diarrhea, the daily ingestion of magnesium in magnesium-rich foods such as nuts and seeds, and the use of magnesium-containing supplements and magnesium-rich hard mineral water are expected to prevent and/or ameliorate the development of colitis symptoms.
Acknowledgments
We thank N. J. Halewood for his helpful and critical proofreading of this manuscript.
Conflict of Interest
The authors declare no conflict of interest.
REFERENCES
- 1) Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet, 369, 1627–1640 (2007).
- 2) Podolsky DK. Inflammatory bowel disease. N. Engl. J. Med., 347, 417–429 (2002).
- 3) Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology, 142, 46–54 e42, quiz, e30 (2012).
- 4) Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu. Rev. Immunol., 28, 573–621 (2010).
- 5) Kurashima Y, Amiya T, Nochi T, Fujisawa K, Haraguchi T, Iba H, Tsutsui H, Sato S, Nakajima S, Iijima H, Kubo M, Kunisawa J, Kiyono H. Extracellular ATP mediates mast cell-dependent intestinal inflammation through P2X7 purinoceptors. Nat. Commun., 3, 1034 (2012).
- 6) Marques CC, Castelo-Branco MT, Pacheco RG, Buongusto F, do Rosario A Jr, Schanaider A, Coutinho-Silva R, de Souza HS. Prophylactic systemic P2X7 receptor blockade prevents experimental colitis. Biochim. Biophys. Acta., 1842, 65–78 (2014).
- 7) Wan P, Liu X, Xiong Y, Ren Y, Chen J, Lu N, Guo Y, Bai A. Extracellular ATP mediates inflammatory responses in colitis via P2X7 receptor signaling. Sci. Rep., 6, 19108 (2016).
- 8) Fujiwara M, Ohbori K, Ohishi A, Nishida K, Uozumi Y, Nagasawa K. Species difference in sensitivity of human and mouse P2X7 receptors to inhibitory effects of divalent metal cations. Biol. Pharm. Bull., 40, 375–380 (2017).
- 9) Ohbori K, Fujiwara M, Ohishi A, Nishida K, Uozumi Y, Nagasawa K. Prophylactic oral administration of magnesium ameliorates dextran sulfate sodium-induced colitis in mice through a decrease of colonic accumulation of P2X7 receptor-expressing mast cells. Biol. Pharm. Bull., 40, 1071–1077 (2017).
- 10) Baek SJ, Kim SH, Lee CK, Roh KH, Keum B, Kim CH, Kim J. Relationship between the severity of diversion colitis and the composition of colonic bacteria: a prospective study. Gut Liver, 8, 170–176 (2014).
- 11) Martin-Subero M, Anderson G, Kanchanatawan B, Berk M, Maes M. Comorbidity between depression and inflammatory bowel disease explained by immune-inflammatory, oxidative, and nitrosative stress; tryptophan catabolite; and gut-brain pathways. CNS Spectr., 21, 184–198 (2016).
- 12) Yoshimatsu Y, Mikami Y, Kanai T. Bacteriotherapy for inflammatory bowel disease. Inflamm. Regen., 41, 3 (2021).
- 13) Komoto M, Asada A, Ohshima Y, Miyanaga K, Morimoto H, Yasukawa T, Morito K, Takayama K, Uozumi Y, Nagasawa K. Dextran sulfate sodium-induced colitis in C57BL/6J mice increases their susceptibility to chronic unpredictable mild stress that induces depressive-like behavior. Life Sci., 289, 120217 (2022).
- 14) Theiss AL, Vijay-Kumar M, Obertone TS, Jones DP, Hansen JM, Gewirtz AT, Merlin D, Sitaraman SV. Prohibitin is a novel regulator of antioxidant response that attenuates colonic inflammation in mice. Gastroenterology, 137, 199–208.e6 (2009).
- 15) Nishida K, Dohi Y, Yamanaka Y, Miyata A, Tsukamoto K, Yabu M, Ohishi A, Nagasawa K. Expression of adenosine A2b receptor in rat type II and III taste cells. Histochem. Cell Biol., 141, 499–506 (2014).
- 16) Iwamura M, Honda R, Nagasawa K. Elevation of the blood glucose level is involved in an increase in expression of sweet taste receptors in taste buds of rat circumvallate papillae. Nutrients, 12, 990 (2020).
- 17) Kamimura Y, Kuwagaki E, Hamano S, Kobayashi M, Yamada Y, Takahata Y, Yoshimoto W, Morimoto H, Yasukawa T, Uozumi Y, Nagasawa K. Reproducible induction of depressive-like behavior in C57BL/6J mice exposed to chronic social defeat stress with a modified sensory contact protocol. Life Sci., 282, 119821 (2021).
- 18) Filippi MD. Neutrophil transendothelial migration: updates and new perspectives. Blood, 133, 2149–2158 (2019).
- 19) Bernardini D, Nasulewic A, Mazur A, Maier JA. Magnesium and microvascular endothelial cells: a role in inflammation and angiogenesis. Front. Biosci., 10, 1177–1182 (2005).
- 20) Ding S, Ma Y, Liu G, Yan W, Jiang H, Fang J. Lactobacillus brevis alleviates DSS-induced colitis by reprograming intestinal microbiota and influencing serum metabolome in murine model. Front. Physiol., 10, 1152 (2019).
- 21) Li AL, Ni WW, Zhang QM, Li Y, Zhang X, Wu HY, Du P, Hou JC, Zhang Y. Effect of cinnamon essential oil on gut microbiota in the mouse model of dextran sodium sulfate-induced colitis. Microbiol. Immunol., 64, 23–32 (2020).
- 22) Liang YN, Yu JG, Zhang DB, Zhang Z, Ren LL, Li LH, Wang Z, Tang ZS. Indigo naturalis ameliorates dextran sulfate sodium-induced colitis in mice by modulating the intestinal microbiota community. Molecules, 24, 4086 (2019).
- 23) Liu B, Piao X, Niu W, Zhang Q, Ma C, Wu T, Gu Q, Cui T, Li S. Kuijieyuan decoction improved intestinal barrier injury of ulcerative colitis by affecting TLR4-dependent PI3K/AKT/NF-kappaB oxidative and inflammatory signaling and gut microbiota. Front. Pharmacol., 11, 1036 (2020).
- 24) Sun Z, Li J, Dai Y, Wang W, Shi R, Wang Z, Ding P, Lu Q, Jiang H, Pei W, Zhao X, Guo Y, Liu J, Tan X, Mao T. Indigo naturalis alleviates dextran sulfate sodium-induced colitis in rats via altering gut microbiota. Front. Microbiol., 11, 731 (2020).
- 25) Evrensel A, Unsalver BO, Ceylan ME. Neuroinflammation, gut-brain axis and depression. Psychiatry Investig., 17, 2–8 (2020).
- 26) Jakobsson HE, Rodriguez-Pineiro AM, Schutte A, Ermund A, Boysen P, Bemark M, Sommer F, Backhed F, Hansson GC, Johansson ME. The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep., 16, 164–177 (2015).
- 27) Surana NK, Kasper DL. Moving beyond microbiome-wide associations to causal microbe identification. Nature, 552, 244–247 (2017).
- 28) Lennon G, Balfe A, Bambury N, Lavelle A, Maguire A, Docherty NG, Coffey JC, Winter DC, Sheahan K, O’Connell PR. Correlations between colonic crypt mucin chemotype, inflammatory grade and Desulfovibrio species in ulcerative colitis. Colorectal Dis., 16, O161–O169 (2014).