2022 Volume 45 Issue 9 Pages 1347-1353
2022 Volume 45 Issue 9 Pages 1347-1353
Abacavir (ABC)-induced hypersensitivity (AHS) is strongly associated with human leukocyte antigen (HLA)-B*57 : 01 expression. Previous studies have demonstrated the feasibility of applying the HLA-transgenic mouse model in this context. ABC-induced adverse reactions were observed in HLA-B*57 : 01 transgenic (B*57 : 01-Tg) mice. Moreover, regulating immune tolerance could result in severe AHS that mimics symptoms observed in the clinical setting, which were modeled in CD4+ T cell-depleted programmed death-1 receptor (PD-1) knockout B*57 : 01-Tg (B*57 : 01-Tg/PD-1−/−) mice. Here, we aimed to examine whether thymus and activation-regulated chemokine (TARC)/CCL17 level can be used as a biomarker for AHS. Serum TARC levels increased in HLA-B*57 : 01-transgenic mice following oral administration of ABC; this increase was associated with the severity of skin toxicity. In ABC-fed CD4+ T cell-depleted B*57 : 01-Tg/PD-1−/− mice, TARC was detected in the epidermal keratinocytes of the ear. Skin toxicity was characterized by the infiltration of CD8+ T cells partially expressing C-C chemokine receptor type 4, which is the primary receptor for TARC. In vivo TARC neutralization effectively alleviated the symptoms of ear skin redness and blood vessel dilatation. Moreover, TARC neutralization suppressed the infiltration of CD8+ T cells to the ear skin but did not affect the ABC-induced adaptive immune response. Therefore, TARC was involved in ABC-induced skin toxicity and contributed to the recruitment of CD8+ T cells to skin. This evidence suggests that serum TARC level may be a functional biomarker for AHS.
Adverse drug reactions may be life-threatening, causing a significant burden on the medical system and hindering drug development; preventing them may help improve human health. These reactions are typically classified as non-immune- and immune-related. Non-immunologic adverse drug reactions are generally dose-dependent and predictable, accounting for 75–80% of cases; in contrast, immunologic adverse drug reactions are idiosyncratic, dose-independent, and associated with low morbidity.1,2) Idiosyncratic adverse drug reactions (IADRs) are difficult to predict.
IADRs occur in one or several organs, including the skin, liver, kidney, bone marrow, or other organs, presenting as Stevens–Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN),3) drug-induced liver injury,4) agranulocytosis,5) and aplastic anemia.6) Although severe IADRs are rare, the immune function of the skin increases the risk of IADRs.7) Carbamazepine (CBZ) may cause serious cutaneous reactions at a rate of 1 to 4.1 per 10000 treatment-naïve patients.8) The incidence rate of CBZ-SJS/TEN is less than 0.06%.9) Non-steroidal anti-inflammatory drugs account for 0.81% of allergic or pseudo-allergic reactions, mainly in the skin, as reported by the Comprehensive Hospital Drug Monitoring in Berne and St. Gallen and the Spontaneous Adverse Drug Reactions Center of Switzerland.10) Abacavir (ABC) triggers hypersensitivity reactions (approximately 3.7% incidence) that include fever, rash, and gastrointestinal disorders within 6 weeks of administration.11–13) The delayed onset of IADRs may be the result of an adaptive immune response.14)
Some IADRs are strongly associated with human leukocyte antigen (HLA) alleles. For example, HLA-B*57 : 01 is a strong risk factor for ABC-induced hypersensitivity (AHS).15) CBZ-induced SJS/TEN is associated with HLA-B*15:02 in both European and Asian populations, while HLA-A*31 : 01 is a risk factor for CBZ-induced drug reaction with eosinophilia and systemic symptoms syndrome.16) Previous studies have examined the contribution of HLA to the onset of IADRs. Shirayanagi et al. developed a phage display technique to detect structural alterations in a HLA complex under drug exposure.17) Transferring cytotoxic T lymphocytes, which express T cell receptors observed in CBZ-SJS/TEN patients, to HLA-B*15:02-transgenic mice treated with CBZ resulted in skin toxicity.18) HLA-B*57 : 01 is at the center of research into ABC-induced adverse reactions.19) Cardone et al.20) and Susukida et al.21) reported that CD4+ T cell depletion reduced ABC tolerance in HLA-B*57 : 01 transgenic (B*57 : 01-Tg) mice, activating CD8+ T cells in ABC-sensitized skin. Furthermore, CD4+ T cell-depleted programmed death-1 receptor (PD-1) knockout B*57 : 01-Tg (B*57 : 01-Tg/PD-1−/−) mice with AHS exhibited severe skin toxicity and infiltration of CD8+ T cells into the ear.22) In this model, the symptom was associated with CD8+ T cell infiltration;22) but the underlying mechanism of CD8+ T cell infiltration into the ear in AHS is unknown.
Thymus and activation-regulated chemokine (TARC), also known as CCL17, is a chemokine constitutively expressed in the thymus and may also be induced during allergic inflammation in dendritic cells,23) keratinocytes,24) endothelial cells, and fibroblasts.25) TARC is involved in many skin diseases, and serum TARC levels in patients with atopic dermatitis, bullous pemphigoid, and mycosis fungoides are higher than those in healthy controls.26) TARC assessments are covered by national insurance in Japan because they reflect the severity of atopic dermatitis.27) C-C chemokine receptor type 4 (CCR4) has a high-affinity for TARC and is expressed in type 2 helper T cells, regulatory T cells, and cutaneous lymphocyte antigen-positive skin-homing T cells.28,29) CCR4 plays an important role in recruiting T cells to the skin to induce skin inflammation.30,31) Meanwhile, blocking the TARC-CCR4 axis may suppress CD8+ T cell migration to the skin during vitiligo.32)
Herein, we hypothesized that TARC is involved in ABC-induced skin toxicity in HLA-transgenic mice. This study aimed to investigate whether TARC expression was induced in the mouse model and whether skin toxicity severity positively correlated with serum TARC levels.
ABC sulfate was purchased from Carbosynth Ltd. (Compton, Berkshire, U.K.). In vivo anti-mouse CD4 monoclonal antibody (mAb) (clone GK1.5) was purchased from BioLegend (San Diego, CA, U.S.A.). Anti-mouse CCL17/TARC mAb (clone 110904) and isotype control immunoglobulin G2A (IgG) (MAB006) were purchased from R&D Systems, Inc. (Minneapolis, MN, U.S.A.).
Animals and TreatmentHLA-transgenic and B*57 : 01-Tg/PD-1−/− mice were generated as described previously.21,22) Male mice (8–16 weeks old) received either a normal diet or 1% (w/w) ABC-containing diet for a week. To achieve CD4+ T cell depletion, the mice received two intraperitoneal (i.p.) injections (days −3 and 1) of 0.25 mg/body anti-CD4 mAb or phosphate-buffered saline (PBS) as the vehicle control, as previously described.22) For in vivo TARC neutralization, mice were administered anti-TARC mAb i.p. at 20 µg/body on days −1, 1, 3, and 5. Anti-TARC mAb treatment appeared functionally equivalent to TARC-deficiency in mice.33) Concurrently, mice received IgG as a positive control. Animals were treated according to the guidelines issued by the National Institutes of Health. All procedures were approved by the Animal Care Committee of Chiba University.
Measurement of Serum TARCMouse blood was collected from inferior vena cava. Serum was then separated by centrifugation at 3000 × g at 4 °C for 30 min after 15 min in a BD Microtainer (Becton, Dickinson and Company, Tokyo, Japan). Serum TARC levels were measured using a Mouse CCL17/TARC DuoSet enzyme-linked immunosorbent assay kit (R&D Systems) following the manufacturer’s instructions.
Hematoxylin–Eosin (H&E), and Immunohistochemistry (IHC) StainingAfter a week of ABC administration, ear biopsies were obtained and embedded in Tissue-Tek® O.C.T. Compound (Sakura Finetek, Tokyo, Japan) and cryopreserved using liquid N2-cold hexane. The tissue samples were sliced into 5-µm-thick sections using a Leica CM3050S cryotome (Leica Biosystems, Wetzlar, Germany). For H&E staining, the sections were fixed in 4% paraformaldehyde in phosphate buffer (Nacalai Tesque, Kyoto, Japan) for 10 min and stained with hematoxylin for 2 min and eosin (both obtained from Muto Pure Chemicals Co., LTD., Tokyo, Japan) for 4 min. Entellan® (Merck Millipore, Billerica, MA, U.S.A.) was used to mount the stained sections.
For IHC experiments, the sections were fixed with 4% paraformaldehyde or acetone for 5 min. The samples were then blocked with 5% fetal bovine serum in PBS for 30 min at 25 °C. Each sample was incubated for 1 h at 25 °C with the following antibodies diluted by PBS containing 0.1% bovine serum albumin: rat anti-CD8a mAb (clone YTS169.4; Abcam, Cambridge, U.K.; dilution of 1 : 250), rabbit anti-TARC mAb (Abcam; dilution of 1 : 100), mouse anti-cytokeratin 14 mAb (Abcam; dilution of 1 : 100), and rabbit CCR4 polyclonal Ab (Thermo Fisher; dilution of 1 : 100). The sections were then incubated for 1 h at 25 °C with the corresponding Alexa Fluor-conjugated secondary Ab (Abcam; dilution of 1 : 250), rhodamine phalloidin (Thermo Fisher; dilution of 1 : 300), and Hoechst 33342 nuclear stain (Thermo Fisher; dilution of 1 : 1000). Vectashield (Vector Laboratories Inc., Burlingame, CA, U.S.A.) was used to mount the stained sections. All samples were imaged using a BZ-X700 fluorescence microscope (Keyence, Osaka, Japan).
Flow Cytometry Analysis for Effector Memory CD8+ T CellsThe CD8+ T cells counts and frequency of effector memory CD8+ T cells (CD44highCD62Llow) were measured using flow cytometry. The draining auricular lymph node (LN) and splenocyte were isolated and stained with PE-Cy/7 anti-mCD8a Ab (53-6.7), PE anti-mouse/humanCD44 Ab (IM7), and fluorescein isothiocyanate (FITC) anti-mCD62L Ab (MEL-14) (BioLegend) for 30 min. The staining was stopped by adding 2 mL 2% fetal bovine serum in PBS. 180 µL cell suspension was performed for flow cytometry analysis on an EC800 flow cytometry (Sony, Tokyo, Japan). The data were analyzed using the Flow Logic software (Bay Bioscience Co., Ltd., Tokyo, Japan).
Statistical AnalysisData are presented as mean ± standard error of the mean (S.E.M.). Significance was determined using Bonferroni’s test for multiple comparisons following one-way ANOVA (GraphPad Software, San Diego, CA, U.S.A.). Statistical significance was set at p-values of <0.05. All experiments were performed at least three times and were reproducible.
Mice received either a normal (vehicle group) or ABC-containing diet for a week. In this model, immune tolerance-regulated HLA-transgenic mice were generated by mating with PD-1 knockout mice; subsequently, mouse CD4+ T cells were depleted to achieve an AHS phenotype consistent with that observed in clinical practice.22) A biomarker for AHS is yet to be established; herein, we focused on TARC levels, as their increase is observed in many skin diseases.26) After drug treatment, mouse serum was separated and TARC levels were measured. TARC levels did not increase in ABC-fed B*57 : 01-Tg mice without immune tolerance modulation (Fig. 1), and AHS did not appear in these mice before.22) CD4+ T cell-depleted B*57 : 01-Tg mice fed ABC exhibited an increase in serum TARC compared with the vehicle group or CD4+ T cell-depleted B*57 : 03-Tg mice fed ABC (HLA allotype control) (Fig. 1). Furthermore, CD4+ T cell-depleted B*57 : 01-Tg/PD-1−/− mice fed ABC exhibited the largest increase in serum TARC levels among all groups examined (Fig. 1). Further, serum TARC levels did not increase in B*57 : 03-Tg mice with reduced immune tolerance, indicating that elevated serum TARC was observed only in the HLA-B*57 : 01 genotype.
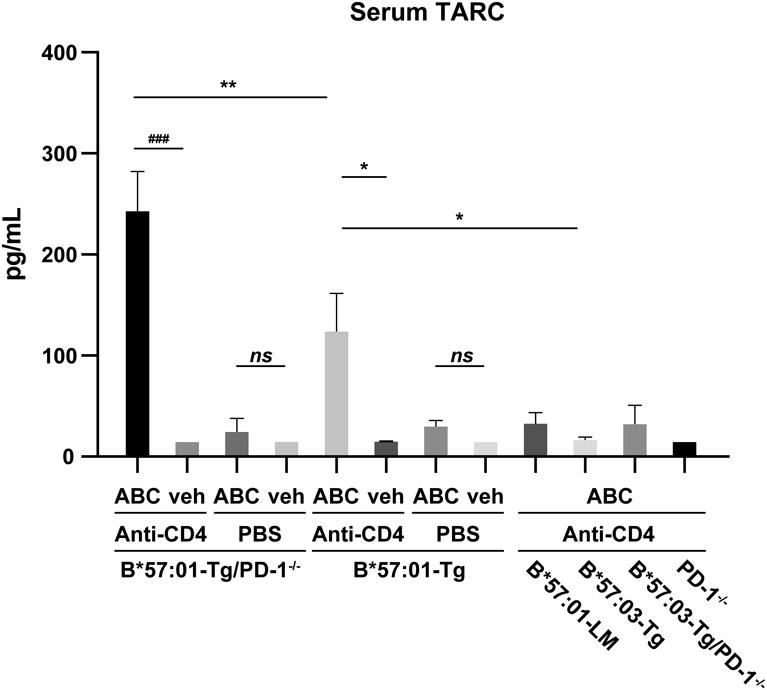
Transgenic (Tg) mice and their littermates (LM) received ABC (1% (w/w)) or normal diet (veh) for a week, alongside anti-CD4 mAb treatment (0.25 mg i.p.) or phosphate-buffered saline (PBS) treatment. Serum TARC level was measured on day 7. Data represent the mean ± S.E.M. (n = 3–7). For values below the range detectable by the assay, we used the lower limit of quantification. ns, not significant. * p < 0.05, ** p < 0.01 for comparisons with ABC-fed CD4+ T cell-depleted B*57 : 01-Tg mice; ### p < 0.001, ABC-fed CD4+ T cell-depleted B*57 : 01-Tg/PD-1−/− mice compared with the veh group; one-way ANOVA with Bonferroni’s multiple comparisons correction.
We confirmed that serum TARC levels increased in the AHS mouse model. Then, we investigated the TARC expression in thymus, liver, spleen, and ear skin. We observed ABC-fed CD4+ T cell-depleted B*57 : 01-Tg/PD-1−/− mice because they presented with the highest elevation of serum TARC (Fig. 1). IHC staining revealed that TARC existed in thymus with or without ABC administration and ABC did not significantly increase the TARC expression. In the liver and spleen, whether mice were fed ABC or not, TARC was not produced. While TARC was observed in the ear skin in ABC-fed mice, it was barely detectable in the vehicle group (Fig. 2a).
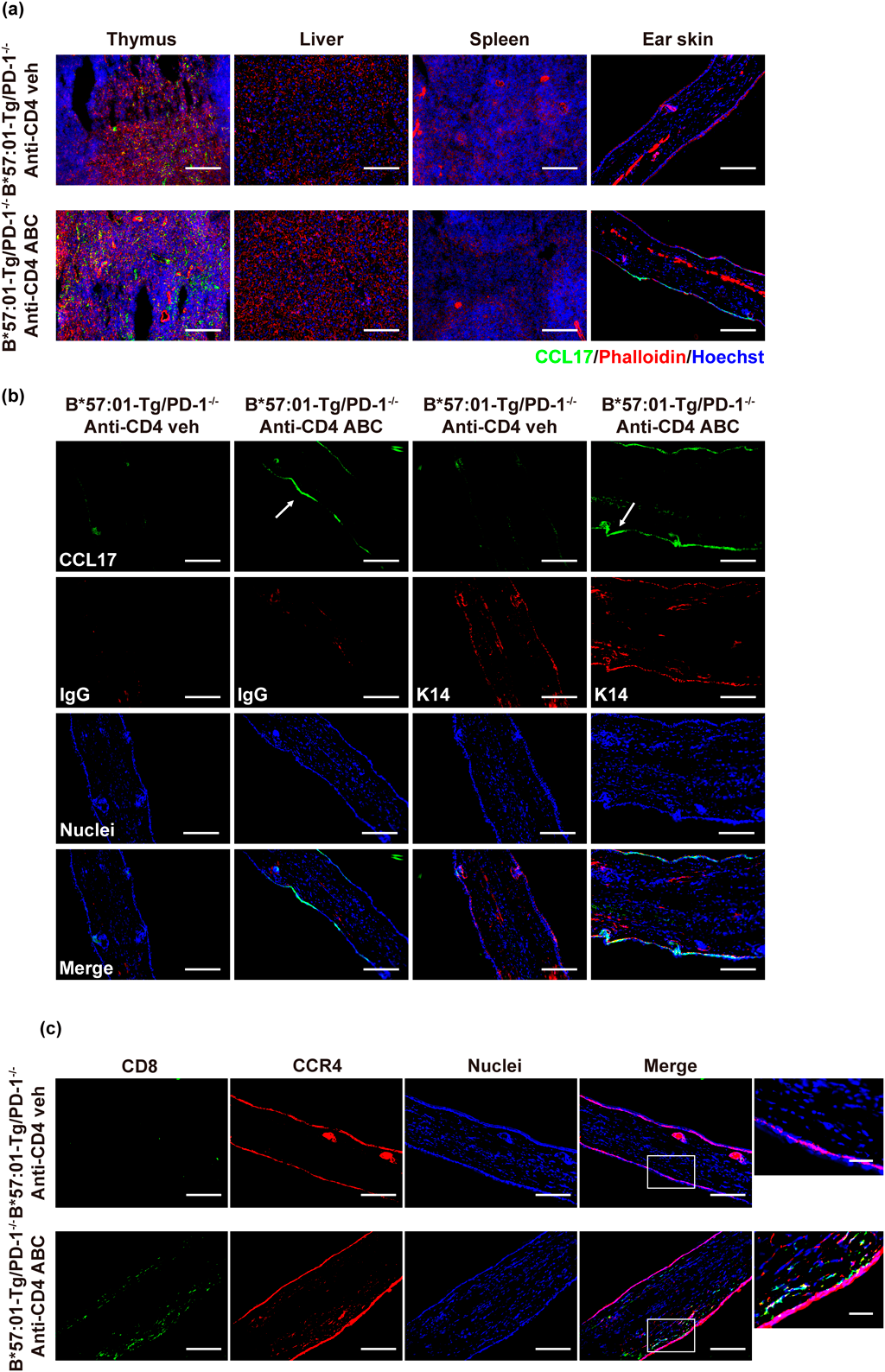
Anti-CD4 mAb-treated B*57 : 01-Tg/PD-1−/− mice received ABC (1% (w/w)) or normal diet (veh) for a week. (a) Sections from the thymus, liver, spleen, and ear skin were subjected to immunohistochemistry (IHC) and stained with CCL17 (green) and phalloidin (red). Each scale bar represents 100 µm. (b, c) The ear sections were co-stained by (b) CCL17 and IgG (left two panels) or CCL17 and cytokeratin 14 (K14) (right two panels) (arrows mark TARC expression) and (c) CD8 (green) and CCR4 (red). Nuclei were stained by Hoechst 33342. Each scale bar represents 100 µm. Higher magnitude images are also shown on the right (each scale bar represents 25 µm). Data are representative of three independent experiments.
More than 90% of cells in the epidermis are keratinocytes.34) We co-stained cytokeratin 14, a marker identified in keratinocytes, with TARC. The result indicated that TARC was mostly present in the epidermal keratinocytes of the mouse ear (Fig. 2b). ABC-induced skin toxicity displayed CD8+ T cell infiltration in CD4+ T cell-depleted B*57 : 01-Tg/PD-1−/− mice.22) CCR4 is the primary receptor of TARC, making TARC a chemoattractant for T cells expressing CCR4.29) Hence, we co-stained ear biopsies with anti-CD8a and anti-CCR4 Abs. The infiltrating CD8+ T cells partially expressed CCR4 (Fig. 2c). These results indicated that TARC was expressed in the ear epidermal keratinocytes and that infiltrating CD8+ T cells partially expressed CCR4 in ABC-induced skin toxicity in CD4+ T cell-depleted B*57 : 01-Tg/PD-1−/− mice.
TARC Neutralization Suppressed ABC-Induced Ear Skin Toxicity and CD8+ T Cells InfiltrationTARC was present in the ear epidermal keratinocytes of mice presenting with pathogenic skin hypersensitivity. We investigated whether TARC played a role in the regulation of skin inflammation. CD4+ T cell-depleted B*57 : 01-Tg/PD-1−/− mice received an anti-TARC mAb or IgG as a control treatment. The mice were fed either an ABC-containing diet or a normal diet for a week. The ears were examined for red rashes and vasodilatation, which were observed in the ABC-administered mice co-treated with IgG (Fig. 3). This finding is consistent with that of our previous study.22) The symptoms were suppressed by anti-TARC mAb treatment (Fig. 3). H&E staining of the ear biopsies also showed that TARC neutralization inhibited immune cell infiltration compared to IgG treatment (Fig. 3). Furthermore, mice treated with control IgG exhibited ABC-induced CD8+ T cell infiltration in their ears, whereas TARC neutralization suppressed this infiltration (Fig. 3). These results demonstrated that ABC-induced TARC expression enhanced immune response and aggravated skin hypersensitivity in CD4+ T cell-depleted B*57 : 01-Tg/PD-1−/− mice.
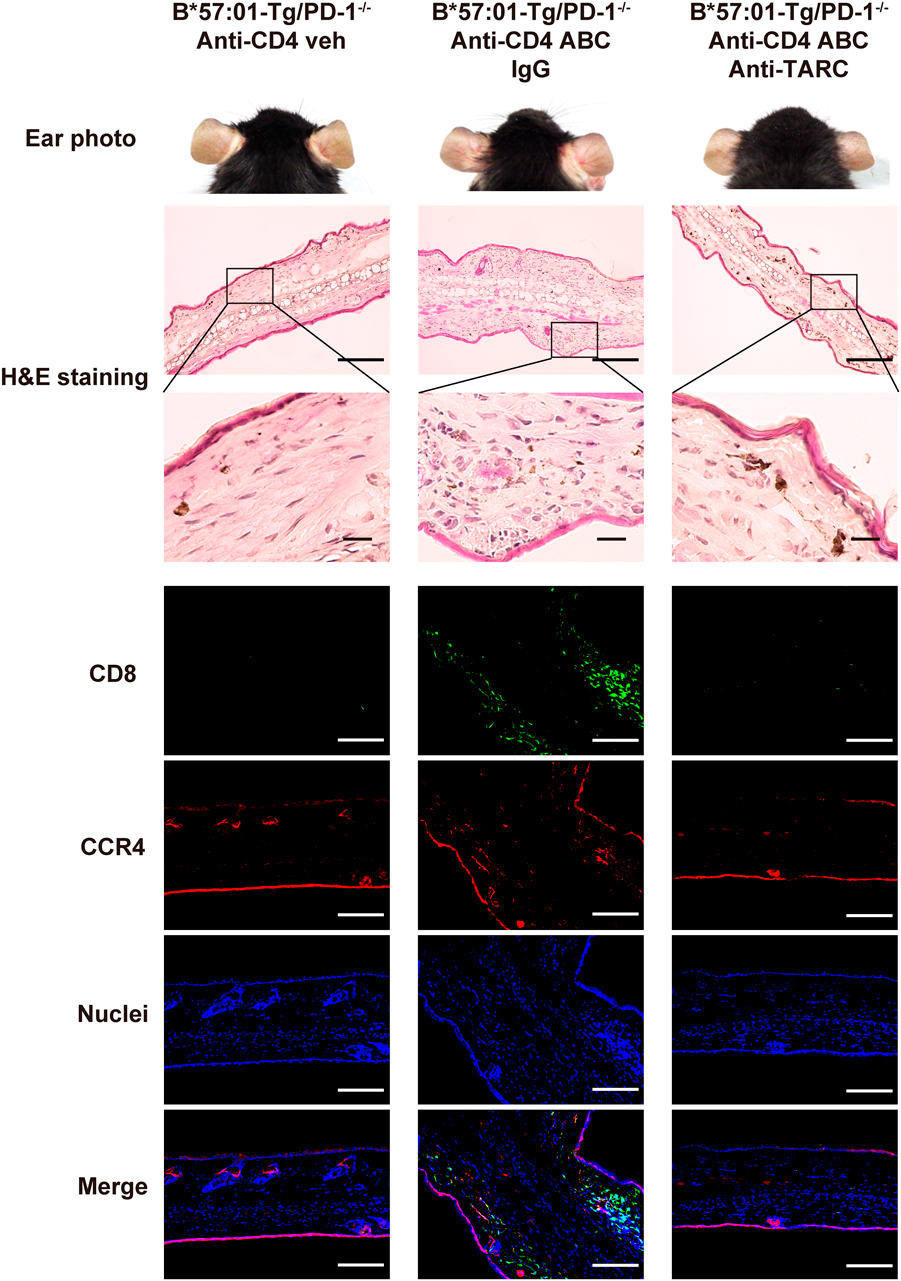
Anti-CD4 mAb-treated B*57 : 01-Tg/PD-1−/− mice received normal diet (veh) and were used as negative control. ABC-fed CD4+ T cell-depleted B*57 : 01-Tg/PD-1−/− mice were treated with anti-TARC mAb or IgG. Representative images of the ears at end of the diet (day 7). Ear sections underwent hematoxylin–eosin (H&E) or IHC staining. In H&E staining, each scale bar represents 200 µm. Higher magnitude images are also shown on the below (each scale bar represents 25 µm). IHC stained samples were co-stained with anti-CD8a (green) and anti-CCR4 (red) antibodies and Hoechst 33342 (blue; nuclei staining). In IHC staining, each scale bar represents 100 µm. Data are representative of three independent experiments.
In addition, we observed the systemic immune response by isolating cells from the auricular LN, axillary and iliac (AX/I) LN, and spleen of CD4+ T cell-depleted B*57 : 01-Tg/PD-1−/− mice administered ABC under TARC neutralization. First, we confirmed that TARC neutralization did not change the counts of CD8+ T cells in auricular LN (Fig. 4a). Reportedly, ABC-fed CD4+ T cell-depleted B*57 : 01-Tg/PD-1−/− mice exhibited systemic immune activation characterized by the increase of percentage of effector memory CD8+ T cells (phenotype of CD44highCD62Llow) in the auricular LN and spleen.22) We then examined effector memory T cells among CD8+ T cells and found that concurrent ABC and IgG administration resulted in an increase in percentage of effector memory CD8+ T cells in auricular LN (Fig. 4b). Similar results were also observed in the AX/I LN and spleen. These findings are consistent with those of Susukida et al.22) TARC neutralization caused an elevation in the percentage of effector memory CD8+ T cells induced by ABC administration similar to that seen in IgG control group (Fig. 4b). Taken together, TARC neutralization did not inhibit ABC-induced systemic immune activation, suggesting that TARC did not affect systemic adaptive immune activation.
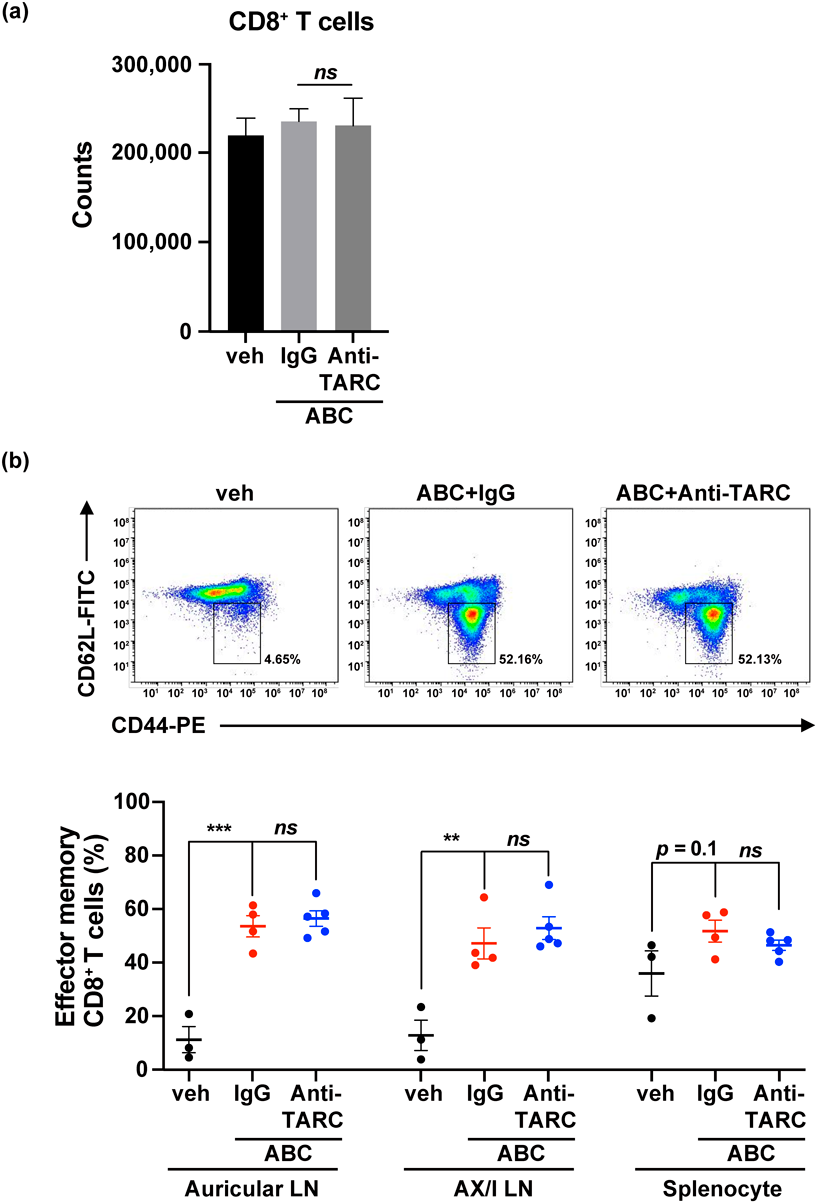
The counts of CD8+ T cells and effector memory T cells among CD8+ T cells were measured using flow cytometry. Lymphocytes isolated from the auricular LN, axillary and iliac (AX/I) LN, and spleen were co-stained with anti-CD8a, anti-CD44, and anti-CD62L antibodies. The phenotype of effector memory CD8+ T cells was CD44highCD62Llow. (a) The counts of CD8+ T cells in auricular LN in 180 µL cell suspension. Data represent the mean ± S.E.M. (n = 3–5). ns, not significant. (b) Representative dot plots depict effector memory T cells in gated CD8+ T cells classified by the phenotype of CD44 and CD62L expression in the auricular LN, as well as by the percentage of effector memory T cells among CD8+ T cells in the auricular LN, AX/I LN, and splenocytes. Each plot represents an individual mouse, and the reported values represent mean ± S.E.M. (n = 3–5). ns, not significant. ** p < 0.01, *** p < 0.001; one-way ANOVA with Bonferroni’s multiple comparisons correction.
AHS is rare; however, in some forms, it can be life-threatening, e.g., in SJS/TEN.35) Studying AHS is difficult because of the lack of a suitable animal model. AHS is strongly associated with HLA-B*57 : 01 in clinical cases and ABC-induced skin hypersensitivity has been studied in HLA-B*57 : 01-transgenic mice.19–22) In this study, we investigated the mechanism of the onset of skin toxicity in HLA-transgenic mice, confirming the involvement of TARC.
TARC is involved in many skin diseases.26) Serum TARC levels in patients with atopic dermatitis are higher than those in healthy controls.36) Among all the immune tolerance-deficient mouse models, only CD4+ T cell-depleted B*57 : 01-Tg and B*57 : 01-Tg/PD-1−/− mice exhibited skin toxicity reported by Susukida et al.22) Presenting with an ear rash, we confirmed that serum TARC levels increased in these two mouse models (Fig. 1). As Susukida et al. described, ABC administration for 21 d induced mild skin toxicity in CD4+ T cell-depleted B*57 : 01-Tg but severe skin toxicity was induced in CD4+ T cell-depleted B*57 : 01-Tg/PD-1−/− after administration for 7 d.22) In our study, ABC-fed CD4+ T cell-depleted B*57 : 01-Tg/PD-1−/− exhibited significantly higher serum TARC levels than ABC-fed CD4+ T cell-depleted B*57 : 01-Tg mice, indicating that the increase of TARC was consistent with the degree of skin toxicity. Mice carrying with HLA-B*57 : 03 allele did not exhibit ear skin toxicity and TARC elevation. This genetic propensity for serum TARC elevation is consistent with the clinical characteristics of ABC-induced adverse reactions, which occur at relatively high rates in patients who carry HLA*B-57 : 01.15) In addition, TARC was significantly higher in the ear skin compared with vehicle group, when exhibited ABC-induced skin toxicity symptoms (Fig. 2a).
TARC is produced by many cells during inflammation, including endothelial cells,25) keratinocytes,24) and bronchial epithelial cells.37) In skin diseases, TARC is likely derived from dendritic cells or keratinocytes.26,38) Tsuda et al. reported that the transformed keratinocyte cell line, HaCaT, produced TARC when exposed to inflammatory cytokines.39) The present findings (Fig. 2b) suggest that TARC may be mostly generated by keratinocytes. However, TARC is also present in cells that did not express Cytokeratin 14. Upon cytokine exposure, TARC was also produced in the epidermis of skin, Langerhans cells and immature dendritic cells that play a role in immune response.38) These cells might also be responsible for the synthesis of TARC in our study. Nevertheless, identifying the source and mechanisms of TARC production was outside the scope of this study and should be examined in future studies.
CCR4 is the primary receptor of TARC.29) CCR4-expressing T cells have been associated with various skin diseases. For example, Casciano et al.40) and Sgambelluri et al.41) proposed that CCR4-expressing memory CD8+ T cells are associated with inflammation in patients with psoriasis. In addition to the expression of TARC in keratinocytes, we found CCR4-expressing CD8+ T cells in mouse ears (Fig. 2c). A previous study reported that CCR4-expressing T cells recognize TARC and home to dermis.42) CCR4-expressing T cells migrated from the blood to the skin produce many kinds of cytokines, such as interleukin (IL)-4, interferon-γ, IL-2 and tumor necrosis factor-α.43,44) These cytokines further contributed to CD8+ T cell infiltration and activation.45) Based on these findings, we suspect that the expression of TARC may induce recruitment of CCR4-expressing CD8+ T cells to dermis and then cytokines cause CCR4-CD8+ T cells migrate to the dermis in ABC-induced skin toxicity. In our study, TARC neutralization suppressed toxic symptoms and inhibited CD8+ T cell infiltration (Fig. 3). This finding suggests that the TARC axis was involved in skin hypersensitivity. CD8+ T cells infiltrating the ear in a TARC-dependent manner supports the hypothesis that TARC recruits CD8+ T cells to the skin. Despite detecting TARC in epidermal tissue where it helped recruit CD8+ T cells, this study did not confirm whether this recruitment depended on interactions between TARC and CCR4.
In previous studies, AHS in HLA-transgenic mice resulted in a systemic immune response with an increase in percentage of effector memory CD8+ T cells.21,22) Here, TARC neutralization did not suppress systemic effector memory CD8+ T cell accumulation and the number of CD8+ T cells in auricular LN did not change (Fig. 4). This suggests that TARC neutralization did not decrease the number of CD8+ T cells but inhibited CD8+ T cell infiltration into the ear. Furthermore, TARC was not involved in the early sensitization phase of the ABC-induced adaptive immune response, but rather played a major role in the development of cutaneous reaction during the induction phase. Although extensive studies are required before recommending TARC measurements for clinical use, serum TARC levels may help diagnose ABC-induced skin hypersensitivity.
In conclusion, this study provided preliminary evidence on the pathogenesis of ABC-induced rash, which mimicked that observed in the clinical setting. This evidence suggests that ABC-induced skin hypersensitivity may be determined by TARC expression, which recruits CD8+ T cells to the skin and contributes to inflammation.
This work was supported by a Grant-in-Aid for Scientific Research (B) (21H02640 and 19H03386) from Japan Society for the Promotion of Science and JST SPRING (JPMJSP2109). The authors would like to thank Takeda Science Foundation.
The authors declare no conflict of interest.