2022 Volume 45 Issue 9 Pages 1398-1402
2022 Volume 45 Issue 9 Pages 1398-1402
Vancomycin (VCM) is a standard treatment for bacterial meningitis. However, little is known about the transferability of VCM to cerebrospinal fluid (CSF), thus evidence of the transferability of VCM to CSF during bacterial meningitis is needed. In this study, we evaluated the concentration of VCM in the plasma and CSF of postoperative neurosurgical patients with bacterial meningitis and evaluated the factors that affect the transferability of VCM to CSF. The concentrations of VCM in plasma (trough) and CSF were determined in eight patients (four males and four females) with bacterial meningitis who were treated with VCM using HPLC. The ratio of the VCM concentrations in CSF/plasma was also calculated by estimating the blood VCM concentration at the same time as the VCM concentration in CSF was measured. The results showed that the VCM concentration in CSF was 0.9–12.7 µg/mL and the CSF/plasma VCM concentration ratio was 0.02–0.62. We examined the effect of drainage on the transferability of VCM to CSF, which showed that the VCM concentration in CSF and the CSF/plasma VCM concentration ratio were significantly higher in patients not undergoing drainage than in patients who were undergoing drainage. The CSF protein and glucose concentrations, which are diagnostic indicators of meningitis, were positively correlated with the VCM concentration in CSF and the CSF/plasma VCM concentration ratio. Thus, VCM transferability to CSF may be affected by changes in the status of the blood–brain barrier and blood–cerebrospinal fluid barrier due to drainage or meningitis.
Bacterial meningitis is a severe infectious disease that develops when bacteria invade the subarachnoid space, which is surrounded by the arachnoid and pia mater, two of the meninges that cover and protect the central nervous system. Because there is no robust immune response in the medullary cavity, bacteria that reach it multiply rapidly in a hematogenous or direct manner, causing severe inflammation. Bacterial meningitis sometimes occurs in patients after neurosurgical operation. Pseudomonas aeruginosa, Staphylococcus aureus and Staphylococcus epidermidis are the major causative bacteria of bacterial meningitis after neurosurgery.1) Since the resistant rate of these bacteria in Japan is high, treatments by third-generation cephem antibiotics are limited.2) Thus, vancomycin (VCM) is a key drug for the treatment of bacterial meningitis.3) In fact, the Japanese guidelines for the clinical management of bacterial meningitis 2014 recommend the combination of a carbapenem antibiotic and VCM for postoperative patients with bacterial meningitis.2)
Effective treatment of bacterial meningitis depends on the concentration of the antibacterial agents not only in the blood but also at the site of infection, the cerebrospinal fluid (CSF). Under normal conditions, the blood–brain barrier limits the transfer of drugs into the CSF.4) During meningitis, drug transfer into the CSF fluctuates due to a variety of factors, including disruption of the blood–brain barrier caused by pathogen-activated inflammatory mediators, which increases drug transfer; decreased production and excretion of CSF; and inhibition of the transporters that excrete drugs in the CSF, which reduces drug excretion and alters drug concentration and retention time.5) Drug transfer was shown to decrease as meningitis inflammation improved, and drainage resulted in drug loss in the CSF.6,7) The physicochemical properties of drugs, such as the protein binding rate, molecular weight, and lipophilicity, affect drug transfer to CSF.8) Therefore, identifying the factors that control the CSF transfer of antibacterial agents, whose concentration in CSF is key to successful treatment, is important for developing treatment strategies for bacterial meningitis. However, there are few reports on the factors that affect the transfer of VCM to CSF during meningitis. Therefore, the purpose of this study was to evaluate the VCM concentration in the plasma and CSF of postoperative neurosurgical patients with bacterial meningitis who were treated with VCM and to determine the factors that contribute to its fluctuation.
Eight postoperative neurosurgical patients with bacterial meningitis who were admitted to Showa General Hospital and provided consent were included in the study. All patients intravenously received VCM for over 60 min. The blood and CSF samples used were residuals of specimens collected for diagnostic and treatment evaluation. The sampling timings of blood and CSF samples were at the trough and 0.4–20.9 h after VCM administration, respectively. All specimens were numbered for anonymization and sent to the Faculty of Pharmacy, Keio University, where they were stored at −80 °C until use. Clinical data were collected from electronic medical records and anonymized by assigning a number corresponding to the specimen number. This study was approved by the Ethics Committee of Showa General Hospital (Approval No. REC-116) and the Research Ethics Committee of the Faculty of Pharmacy, Keio University (Approval No. 161111-1) and was conducted in accordance with the Ethical Guidelines for Medical Research Involving Human Subjects.
Measurement of VCM Concentrations in Plasma and CSF Using HPLCThe concentration of VCM in blood and CSF was determined using HPLC. The detailed methods are described below.
ReagentsVancomycin hydrochloride was purchased from Funakoshi (Tokyo, Japan). Acetonitrile, HPLC grade, was obtained from Nacalai Tesque (Kyoto, Japan). All other reagents were of analytical grade and were purchased from Wako Pure Chemical Corporation (Osaka, Japan).
Specimen PretreatmentSamples were pretreated using a spin column (Mono spin C18; GL Sciences, Tokyo, Japan). Briefly, 150 µL of the sample was added to a conditioned spin column and centrifuged (4 °C, 2400 × g, 3 min). After washing with 500 µL of purified water, 150 µL of 30% acetonitrile was added, and the eluate was collected by centrifugation (4 °C, 2400 × g, 1 min) and was used as the sample for measurement.
HPLC Measurement ConditionsVCM was measured using a Chromaster® HPLC system equipped with a Chromaster 5160 Pump, Chromaster 5310 Column Oven, and Chromaster 5430 Diode Array Detector (HITACHI, Tokyo, Japan). A LaChrom LM Type A Column (100 × 4.6 mm) and LaChrom LM Type A Guard Columns were used. The mobile phase consisted of 10 mM acetate (pH 4.7) (solution A) and acetonitrile (solution B) at a flow rate of 2.0 µL/min, which was applied in the following gradient: 0–3 min: 95% solution A +5% solution B, 3–3.1 min: 70% solution A +30% solution B, 3.1–4.6 min: 40% solution A +60% solution B, and 4.6–7 min: 95% solution A +5% solution B. The column temperature was 40 °C, and the absorbance was measured at 235 nm. Under these conditions, the retention time of VCM was approximately 2.4 min, and the measurement trueness of VCM in plasma and CSF (0.5, 10, and 100 µg/mL) were 97.6–101.4% and 92.9–113.4%, respectively, with daily variations of 1.1–6.9% and 0.7–10.2%, respectively. The quantitative range of this measurement method was 0.5–100 µg/mL.
Calculation of the CSF/Plasma VCM Concentration RatioThe pharmacokinetic parameters (healthy subjects) on the VCM’s interview form (https://www.info.pmda.go.jp/go/interview/1/270161_6113400A1189_1_016_1F.pdf) were set as the initial values, and the distribution volume and disappearance rate constant were calculated for each patient by simulating the trends in plasma drug concentration based on actual measurements using a two-compartment model in Phoenix WinNonlin®. Based on the trends in plasma drug concentration, the ratio of the VCM concentrations in CSF/plasma was calculated by predicting the plasma concentration at the time the CSF concentration was measured.
Statistical AnalysisStatistical analysis of the VCM concentration in CSF, the CSF/plasma VCM concentration ratio, and the effects of drainage was performed using the Mann–Whitney U test. Spearman rank correlation coefficient was used to determine the correlations of the VCM concentration in CSF and CSF/plasma VCM concentration ratio with laboratory CSF values (CSF cell number, CSF protein concentration, and CSF glucose concentration). The calculated correlation coefficient (R) was interpreted as follows: 0<|R|≤0.2, almost no correlation; 0.2<|R|≤0.4, weak correlation; 0.4<|R|≤0.7, strong correlation; 0.7<|R|≤1.0, very strong correlation. The level of significance was set at 0.05.
The characteristics and laboratory results of the eight patients (four males and four females) from whom consent was obtained are shown in Table 1. The mean age and weight were 60.9 ± 16.2 years and 56.8 ± 6.6 kg, respectively. Serum creatinine levels during treatment were 0.5 ± 0.1 mg/dL, and estimated glomerular filtration rate (eGFR) was 71.8–234.1 mL/min/1.73 m2. No patient had renal dysfunction, and no renal dysfunction was observed after VCM administration.
| Diagnosis | Sex | Age (years) | Weight (kg) | Serum creatinine (mg/dL) | eGEF (mL/min/1.73 m2) | |
|---|---|---|---|---|---|---|
| 1 | Postoperative meningitis following hydrocephalus and ventricular drainage | Female | 47 | 51 | 0.36–0.41 | 125.9–145.2 |
| 2 | Postoperative meningitis following left putamen hemorrhage | Male | 78 | 65 | 0.73–0.78 | 71.8–76.7 |
| 3 | Postoperative meningitis following subarachnoid hemorrhage | Male | 45 | 52 | 0.48–0.57 | 120.3–145.2 |
| 4 | Postoperative meningitis following subarachnoid hemorrhage | Female | 48 | 52.8 | 0.41–0.42 | 121.9–125.2 |
| 5 | Postoperative meningitis following subarachnoid hemorrhage | Male | 40 | 53 | 0.32–0.41 | 178.5–234.1 |
| 6 | Postoperative meningitis following subarachnoid hemorrhage | Female | 77 | 50 | 0.42 | 106.5 |
| 7 | Postoperative meningitis following subarachnoid hemorrhage | Male | 45 | 52 | 0.48–0.57 | 120.3–145.2 |
| 8 | Postoperative meningitis following subarachnoid hemorrhage | Female | 81 | 65 | 0.38–0.47 | 96.3–117.1 |
eGFR: estimated glomerular filtration rate.
The duration of VCM administration, number of specimens (blood and CSF) collected, presence or absence of drainage, and concomitant antibacterial agents administered for each patient are shown in Table 2. The duration of VCM administration was 5–21 d, and five patients (cases 4–8) underwent drainage. Seven patients were concomitantly treated with other antibacterial agents.
| Duration of VCM administration (days) | Number of plasma samples | Number of CSF samples | Drainage | Bacteria | Concomitant antibacterial agents | |
|---|---|---|---|---|---|---|
| 1 | 18 | 6 | 6 | No | Methicillin-resistant Staphylococcus epidermidis | — |
| 2 | 17 | 3 | 4 | No | — | Cefepime and metronidazole |
| 3 | 11 | 2 | 3 | No | — | Ceftriaxone, ampicillin, and metronidazole |
| 4 | 10 | 6 | 2 | Yes | — | Ceftazidime |
| 5 | 15 | 3 | 2 | Yes | — | Ceftriaxone |
| 6 | 11 | 2 | 6 | Yes | — | Meropenem |
| 7 | 5 | 2 | 2 | Yes | — | Cefepime |
| 8 | 21 | 3 | 8 | Yes | — | Cefepime |
Cases 2–8 were prophylactically administered VCM.
The VCM concentration ranges were 9.6–20.6 µg/mL in plasma and 0.9–12.7 µg/mL in CSF. The plasma pharmacokinetic parameters of each patient were calculated based on the VCM concentration in plasma and the estimated VCM concentration in plasma at the time of CSF sample collection. The CSF/plasma VCM concentration ratio, calculated using the estimated plasma VCM concentration and the measured CSF VCM concentration, was 0.02–0.62.
Effect of Drainage on the Transferability of VCM to CSFWe compared the transferability of VCM to CSF (the VCM concentration in CSF and the CSF/plasma VCM concentration ratio) with and without drainage. The results showed that the VCM concentration in CSF and the CSF/plasma VCM concentration ratio were significantly higher in cases without drainage (cases 1–3) than in cases with drainage (cases 4–8; Fig. 1).
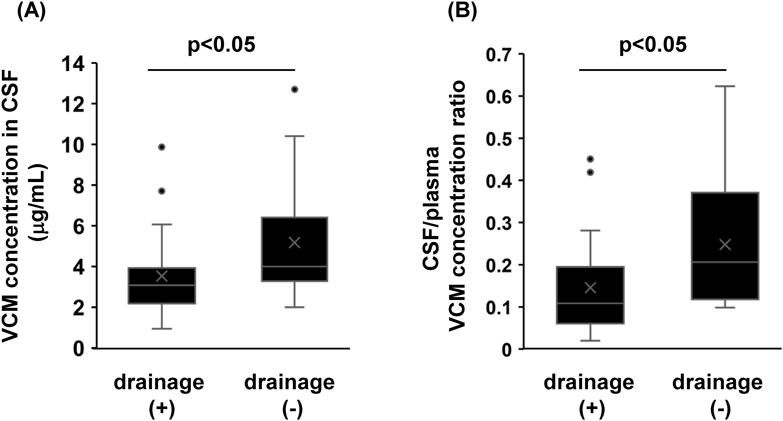
Cases with drainage (n = 20) and without drainage (n = 13).
Since drainage was shown to affect the transferability of VCM to the CSF, we investigated the correlation of the VCM concentration in CSF and the CSF/plasma VCM concentration ratio with the diagnostic indices of meningitis (cell count, protein concentration, and glucose concentration in CSF) in cases without drainage (cases 1–3). The results showed R of 0.30 and 0.42 for CSF protein concentration with the VCM concentration in CSF and with CSF/plasma VCM concentration ratio, respectively, indicating a positive correlation (Fig. 2B). CSF glucose concentration was also positively correlated with the VCM concentration in CSF and the CSF/plasma VCM concentration ratio, with R values of 0.49 and 0.25, respectively (Fig. 2C). However, there was no positive correlation between the number of cells in CSF and VCM transfer to CSF (Fig. 2A).

Patients who underwent drainage were excluded from the analysis (n = 13).
Since pharmacokinetics in plasma and CSF differ, it is important to evaluate CSF transferability as the ratio of the area under the CSF drug concentration-time curve/area under the plasma drug concentration-time curve.9) However, due to the difficulty of collecting samples to accurately predict the area under the CSF drug concentration-time curve, many studies have evaluated the transfer rates as the CSF/plasma drug concentration ratios.10,11) Therefore, in this study, we calculated the CSF/plasma VCM concentration ratio and evaluated the factors that contribute to the variability in the transferability of VCM to CSF.
Li et al. reported a strong positive correlation between drug clearance from CSF and drug excretion by drainage.7) Therefore, we hypothesized that drainage would increase VCM excretion from CSF and decrease the VCM concentration in CSF. We evaluated the effect of drainage on VCM concentration in CSF, which showed that patients who underwent drainage had significantly lower VCM concentrations in CSF and CSF/plasma VCM concentration ratios than patients who did not undergo drainage (Fig. 1). There are two possible reasons for this difference in VCM concentration between patients with and without drainage: increased excretion due to drainage and increased excretion of CSF flowing from the brain to the blood.12) However, the volume of distribution of VCM in plasma (11.87 L) has been reported to be much larger than that in CSF (0.039 L),6,13) and the effect of outflow from the brain towards the blood on the behavior of the plasma VCM concentration is negligible. Therefore, drainage may be the main factor contributing to the observed decrease in the VCM concentration in CSF. Drainage is an essential treatment in cases with CSF or blood retention in the brain or an abscess. In addition, increasing the dose of VCM to maintain the VCM concentration in CSF carries a risk renal dysfunction. Therefore, if the therapeutic efficacy of VCM is inadequate in postoperative neurosurgical patients with bacterial meningitis undergoing drainage, a change to a drug with better transferability, such as linezolid,14) should be considered.
When bacterial meningitis is suspected, the cell count, protein concentration, and glucose concentration in CSF are essential examination parameters; Increases in the CSF cell count and protein concentration and a decrease in the glucose concentration are diagnostic criteria for bacterial meningitis. Recently, a correlation between the transferability of cephem antibacterial agents to CSF and CSF laboratory test values (i.e., protein and glucose concentrations) was found.15) Therefore, we evaluated the correlation between CSF laboratory test values and the transferability of VCM to CSF in cases 1–3, to exclude the effect of drainage shown in Fig. 1. The results showed a positive correlation between CSF protein concentration and both the CSF VCM concentration and CSF/plasma VCM concentration ratio (Fig. 2B). During meningitis, disruption of the blood–brain barrier due to inflammation increases its permeability to serum proteins and their influx into the CSF, resulting in an increased protein concentration in CSF.16) Although VCM is considered to have low CSF transferability due to its high molecular weight and high water solubility, at the onset of meningitis, its translocation to CSF is enhanced due to disruption of the blood–brain barrier, which may be correlated with the protein concentration in CSF. We detected a positive correlation between CSF glucose concentration and CSF transferability of VCM (Fig. 2C). During meningitis, the concentration of glucose in CSF is believed to decrease due to glycolysis by pathogenic bacteria and the increased numbers of neutrophils in CSF and decreased numbers of glucose transporters due to blood–brain barrier disruption. Therefore, we expected that the glucose concentration would increase with remission of meningitis; however, we obtained unexpected results. A possible explanation is described below. In bacterial meningitis, damage at the site of infection can progress even after the bacteria have been eradicated by antibacterial agents,17) and there is a time lag between improvement of meningitis and recovery of the blood–brain barrier. As a result, the decrease in pathogen numbers and increase in CSF glucose concentration, along with the continuing high VCM permeability at the blood–brain barrier, resulted in a positive correlation between CSF glucose concentration and CSF transfer of VCM. Thus, considering that the change in CSF glucose concentration has a time lag with the change in the blood–brain barrier status, the CSF protein concentration better reflects the transferability of VCM to CSF. This suggests that if improvement of meningitis results in a decrease in CSF protein levels, the transferability of VCM to CSF will be reduced. Therefore, even if meningitis improves, the dose of VCM should remain the same.
Our study suggests that drainage and disruption of the blood–brain barrier and blood–cerebrospinal fluid barrier affect the transfer of VCM to CSF. In addition, the protein and glucose concentrations in CSF, which are diagnostic parameters of meningitis, were correlated with the transferability of VCM to CSF. However, study findings both refute10) and support18–20) our results. This is because this study has several limitations: (i) the number of cases in our study is small, (ii) the estimated VCM concentrations in blood were used to calculate CSF/plasma VCM concentration ratio. Thus, it is necessary to repeat the study with a higher number of cases in the future to reach a conclusion.
This work was performed by a research expense distributed from Keio University.
The authors declare no conflict of interest.