2022 Volume 45 Issue 9 Pages 1378-1384
2022 Volume 45 Issue 9 Pages 1378-1384
Pyridoxine (VB6) is a vitamin that is essential to maintain the homeostasis of the human body by contributing to various metabolic reactions. In the skin, although some studies have shown that VB6 is involved in regulating homeostasis through the attenuation of intracellular oxidative stress, there are few reports regarding the effects of VB6 on the prevention or improvement of skin aging. Thus, we conducted this study to determine the potential anti-skin pigmentation effect of VB6 focusing on the phagocytosis of melanosomes (MSs) by keratinocytes. The phagocytosis of MSs by keratinocytes is activated by oxidative stress and is an important factor of skin pigmentation and the eventual appearance of pigmented spots. First, we confirmed the antioxidant property of VB6 that enhanced the expression of several intracellular antioxidants via nuclear erythroid factor 2-related factor 2 (Nrf2). Although the incorporation of fluorescent beads (FBs), which are used as pseudo-MSs, into keratinocytes was increased under higher oxidation conditions caused by UVB and by the depletion of intracellular glutathione, treatment with VB6 suppressed the increased incorporation of FBs into those keratinocytes via Nrf2 activation. Furthermore, VB6 restored the decreased expression of differentiation marker proteins in keratinocytes caused by FB incorporation. Taken together, the results show that VB6 has the potential to prevent the appearance of pigmented spots by suppressing the activation of phagocytosis in keratinocytes caused by oxidative stress, and by restoring the differentiation of keratinocytes disrupted by FB incorporation.
Pyridoxine (VB6), which is a water-soluble vitamin, is known to act as a coenzyme for enzymes involved in amino acid metabolism and nutrition, and which contributes to maintain the homeostasis of the body.1,2) In the skin, the depletion of VB6 causes some disorders like atopic dermatitis and seborrheic dermatitis.3,4) These facts suggest that VB6 is necessary to maintain homeostasis in the skin.
Meanwhile, the skin is always exposed to oxidative stress because of its location at the surface of the human body. External circumstances, such as sunlight, air pollution, high temperature and low humidity, stimulate the generation of reactive oxygen species (ROS).5–8) Among those stresses, the UV radiation in sunlight generates ROS excessively through the elevation of calcium influx in keratinocytes and results in accelerating the appearance of skin aging such as wrinkles, sagging and pigmented spots, like solar lentigos.9–12) Among the features of skin aging, faces that have many pigmented spots give a strong impression of aging and may reduce their social activities. Thus, to prevent or improve the appearance of pigmented spots is an important challenge to rescue the decreased social activity for all human society.
Pigmented spots are recognized as limited areas of skin hyperpigmentation. Although the reasons for the appearance of pigmentation in limited areas is still unknown, the mechanisms involved in the formation of pigmented spots is similar to the mechanism regulating skin pigmentation.12) In general, although skin pigmentation occurs due to UV exposure or inflammation, increases of melanin synthesis by the activation of melanocytes are essential in pigmentation.13) Additionally, the diffusion of melanosomes (MSs), in which melanin is produced and stored in melanocytes, into the epidermis due to their incorporation into keratinocytes by phagocytosis has been recognized as one determinant of skin color and also is a critical process of skin pigmentation.14) Recently, oxidative stress was shown to enhance the phagocytosis of MSs into keratinocytes.15,16) In fact, UVB, which is a strong generator of ROS, enhances the phagocytosis of MSs isolated from melanocytes and of fluorescent beads (FBs), which are used as pseudo-MSs.16,17) MSs incorporated into keratinocytes by phagocytosis are exfoliated from the skin surface by desquamation according to the differentiation of keratinocytes.18) Thus, the prevention or improvement of pigmented spots can be achieved not only by inactivating melanocytes and melanin synthesis but also by suppressing the phagocytosis of MSs and accelerating the terminal differentiation of keratinocytes to exfoliate MSs from the skin.
Interestingly, it has been revealed that VB6 has the ability to attenuate intracellular oxidative stress not only by directly scavenging ROS, but also by enhancing glutathione (GSH) synthesis through the activation of nuclear erythroid factor 2 (NE-F2)-related factor 2 (Nrf2) in keratinocytes.19,20) The binding of Nrf2 to an antioxidant response element after translocation from the cytoplasm into the nucleus up-regulates multiple types of antioxidant-related genes, including γ-glutamylcysteine synthetase (γ-GCS), which is the rate-limiting enzyme of GSH synthesis, catalase, heme oxigenase-1 (HO-1) and nicotinamide adenine dinucleotide phosphate (NADPH) quinone oxidoreductase 1 (NQO1).21,22) Thus, these considerations suggest that VB6 is an important antioxidant to keep the redox balance in keratinocytes, and prompted us to characterize the potential of VB6 as an anti-skin aging substance focusing on the prevention or improvement of pigmented spots.
In this study, we evaluated the effects of VB6 focusing on the phagocytosis of MSs into keratinocytes and its influence on the differentiation of keratinocytes in order to investigate the potential of VB6 to have an anti-skin pigmentation.
HuMedia-KG2 was purchased from Kurabo Industries (Osaka, Japan). Buthionine sulfoximine (BSO) and pyridoxine hydrochloride were purchased from Nacalai Tesque (Kyoto, Japan). Fluorescent beads (FluoSpheres® carboxylate-modified 0.2 µm), the BCA protein assay kit, Nrf2-small interfering RNA (siRNA), non-targeting siRNA, Lipofectamine RNAiMAX, the Power SYBR Green Cells-to-CT kit and Mammalian Protein Extraction Reagent were purchased from Thermo Fisher Scientific (Waltham, MA, U.S.A.). Hoechst 33342 was purchased from Dojindo (Kumamoto, Japan). The anti-keratin 10 polyclonal antibody and the anti-actin polyclonal antibody were purchased from Abcam (Cambridge, U.K.). The anti-involucrin polyclonal antibody and the anti-loricrin polyclonal antibody were purchased from Proteintech (Rosemont, IL, U.S.A.). EzWestBlue was purchased from ATTO (Tokyo, Japan).
Cell CultureNormal human epidermal keratinocytes (NHEKs) derived from the foreskins of Asian and Caucasian donors were obtained from Kurabo (Osaka, Japan) and were cultured in HuMedia-KG2 at 37 °C in a 5% CO2 atmosphere.
RNA InterferenceNHEKs were seeded into multi-well plates in HuMedia-KG2, and were treated with 100 nM Nrf2-siRNA (siNrf2) or non-targeting siRNA (siControl) using Lipofectamine RNAiMAX for 24 h.
Real-Time PCRTotal RNAs were extracted from NHEKs and cDNAs were synthesized using a Power SYBR Green Cells-to-CT kit. Real-time PCR was performed with SYBR Green Master Mix using an Eco Real-Time PCR System (Illumina, San Diego, CA, U.S.A.). Data were analyzed according to the ΔΔCt method.
Incorporation of FBs into KeratinocytesAfter treatment of NHEKs, 100 µM BSO pretreated NHEKs or siNrf2-treated NHEKs with samples for 24 h, the cells were irradiated with UVB at 20 mJ/cm2. At 4 h after incubation with FBs,16) the incorporation of FBs into cells was observed using a Floid® Cell Imaging Station, and was quantified by the measurement of fluorescence intensity (Ex; 535 nm, Em; 590 nm). The UVB source used was a TL20W/12RS UVB broadband lamp (Philips, Eindhoven, Holland, Netherlands) and radiation energies were measured with a UVX radiometer (UVP, Upland, Canada). In addition, protein concentrations were determined using a BCA protein assay kit.
Western BlottingCellular proteins were extracted with a protein extraction reagent containing protease inhibitors. The extracted proteins were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) on 10% polyacrylamide gels, and then were transferred to polyvinylidene difluoride membranes. The membranes were then incubated with primary antibodies specific for keratin 10 (1 : 1000), involucrin (1 : 1000), loricrin (1 : 1000) and β-actin (1 : 2000). Immunoreactive protein bands were then visualized using EzWestBlue and were analyzed using Image J.
Statistical AnalysisAll data are expressed as means ± standard deviation (S.D.). Comparisons between two groups or multiple groups were performed by Student’s t-test or Tukey’s test respectively. A p-value <0.05 is considered statistically significant.
Our previous study revealed that VB6 stimulated the nuclear translocation of Nrf2 and up-regulated the mRNA expression level of γ-GCS in keratinocytes.20) Here, in order to confirm in detail the antioxidant actions of VB6 in keratinocytes and the involvement of Nrf2, mRNA expression levels of other antioxidants, such as HO-1 and NQO1 that are encoded downstream of Nrf2, in VB6 treated NHEKs were examined using a siNrf2 knockdown system (Fig. 1a). mRNA expression levels of HO-1 and NQO1 were significantly up-regulated at 24 h after treatment with VB6 (Figs. 1b, c). However, the up-regulation of those mRNA expression levels by VB6 was abolished by Nrf2 knockdown in NHEKs (Figs. 1b, c). These results indicated that VB6 enhanced the intracellular antioxidant ability due to the up-regulation of mRNA expression levels of HO-1 and NQO1 via Nrf2.
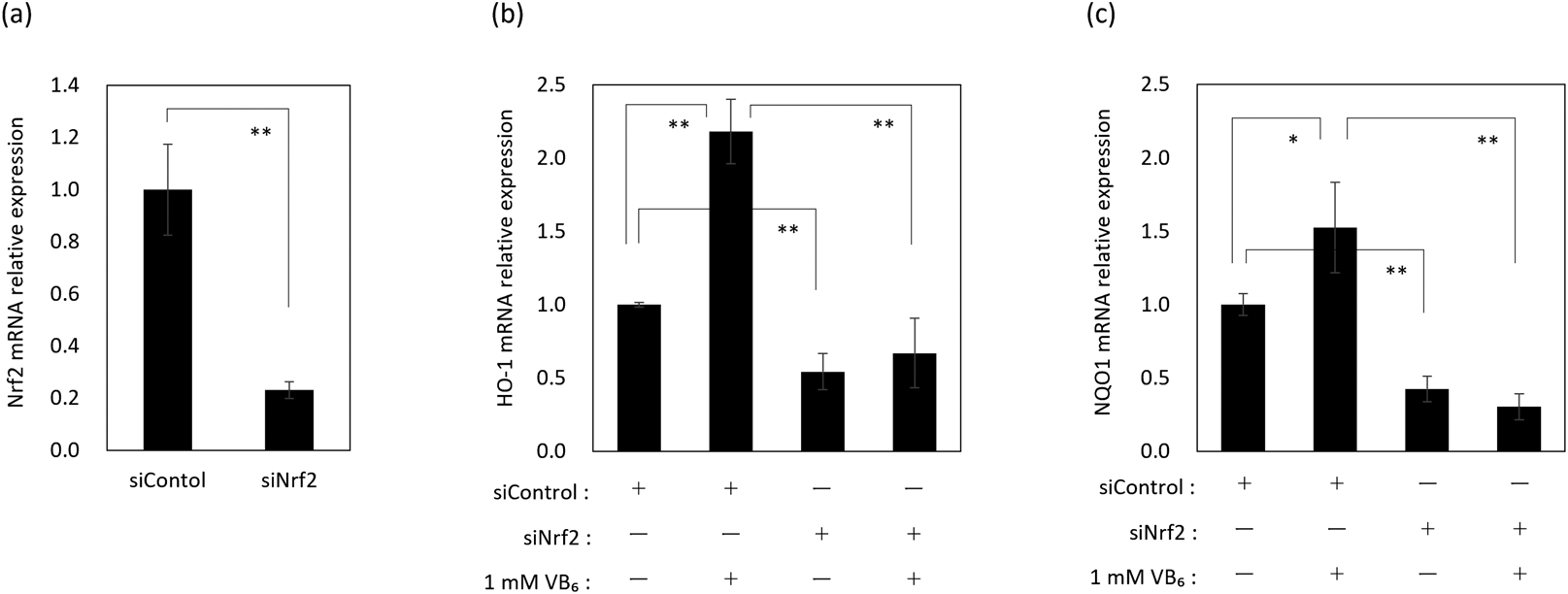
(a) NHEKs were treated with 100 nM siNrf2 or siControl for 24 h. mRNA expression levels of Nrf2 in NHEKs were quantified using real-time PCR. Significance: ** p < 0.01 vs. siControl. (b, c) After treating NHEKs with 100 nM siNrf2 or siControl for 24 h, the cells were further incubated with or without 1 mM VB6 for 24 h. The mRNA expression levels of HO-1 and NQO1 in NHEKs were quantified. Bars indicate means ± S.D. (n = 3). Significance: * p < 0.05, ** p < 0.01.
In order to evaluate the effects of VB6 on the phagocytic ability of keratinocytes in higher oxidative situations, we examined the ratio of FB incorporation into NHEKs. NHEKs in higher oxidative situations without cytotoxicity were prepared by the depletion of GSH due to the inhibition of GSH synthesis with BSO,23) and by irradiation with UVB. Treatment with VB6 caused a significant suppression of the incorporation of FBs in NHEKs elevated by UVB exposure and by BSO treatment (Figs. 2, 3).

NHEKs were incubated with or without VB6 for 24 h, and then were exposed to 20 mJ/cm2 UVB. (a) NHEKs were incubated with FBs for 4 h and their incorporation of FBs was quantified by the measurement of fluorescence intensity. Bars indicate means ± S.D. (n = 4). Significance: ** p < 0.01. (b) Representative fluorescence images observed using a Floid® Cell Imaging Station. Red: FBs, Blue: Hoechst 33342 (nuclei). Scale bar: 100 µm.
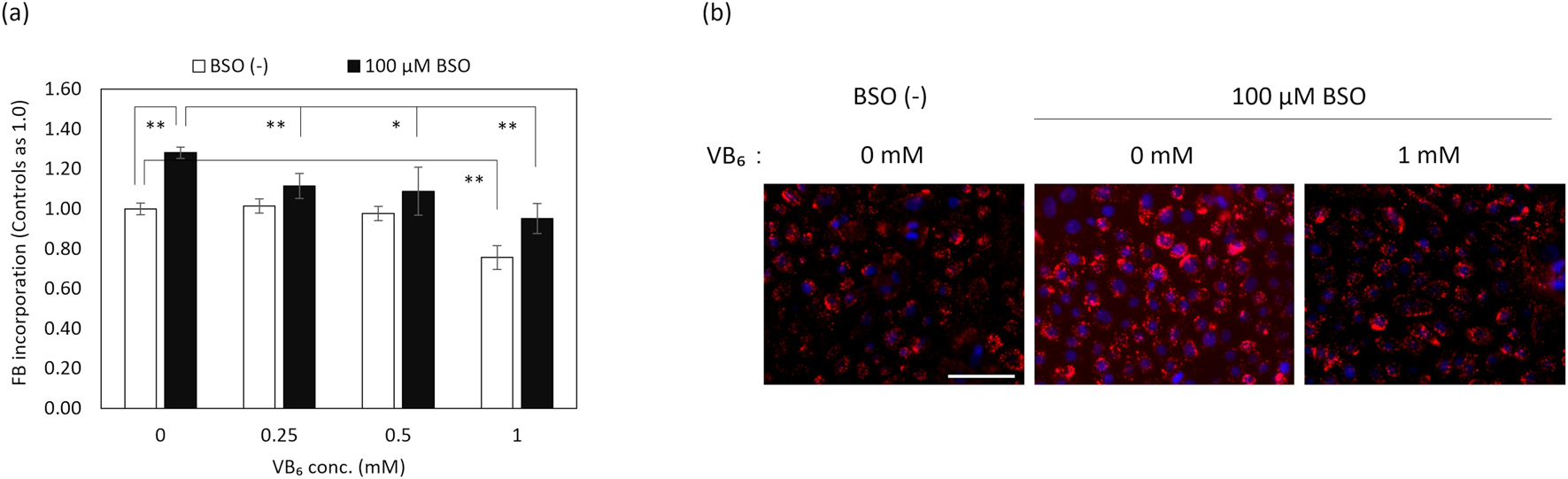
After treatment with 100 µM BSO for 24 h, NHEKs were further incubated with or without VB6 for 24 h. (a) NHEKs were incubated with FBs for 4 h and their incorporation of FBs was quantified by the measurement of fluorescence intensity. Bars indicate means ± S.D. (n = 4). Significance: * p < 0.05, ** p < 0.01. (b) Representative fluorescence images observed using a Floid® Cell Imaging Station. Red: FBs, Blue: Hoechst 33342 (nuclei). Scale bar: 100 µm.
We then examined the involvement of Nrf2 in the reduced FB incorporation into NHEKs caused by VB6 using Nrf2 knockdown. Interestingly, the suppression of FB incorporation caused by VB6 in UVB-irradiated NHEKs was abrogated by the treatment with siNrf2 (Fig. 4).

After treatment with 100 siNrf2 or with siControl for 24 h, NHEKs were further incubated with or without 1 mM VB6 for 24 h. After exposure to 20 mJ/cm2 UVB, NHEKs were allowed to incorporate FBs for 4 h. (a) The incorporation ratio of FBs in NHEKs was quantified by the measurement of fluorescence intensity. Bars indicate means ± S.D. (n = 4). Significance: ** p < 0.01. (b) Representative fluorescence images observed using a Floid® Cell Imaging Station. Red: FBs, Blue: Hoechst 33342 (nuclei). Scale bar: 100 µm.
It has been reported that the epidermis in pigmented areas of solar lentigos due to the high incorporation of MSs shows decreased expression of keratin 10 (K10), involucrin (IVN) and loricrin (LOR), which are differentiation markers of keratinocytes.24,25) This fact suggests that the differentiation of keratinocytes is disrupted by the incorporation of materials like MSs. So, we examined the influence of FB incorporation on the differentiation of NHEKs at 1 or 5 d after FB incorporation.
The results show that the mRNA expression levels of K10, IVN and LOR were down-regulated in NHEKs that had incorporated FBs 1 d earlier (Fig. 5). Additionally, those protein expression levels were confirmed to be decreased at 5 d after FB incorporation (Fig. 6). These results confirmed experimentally that the incorporation of materials such as FBs disrupted the differentiation of keratinocytes at the mRNA and protein expression levels.
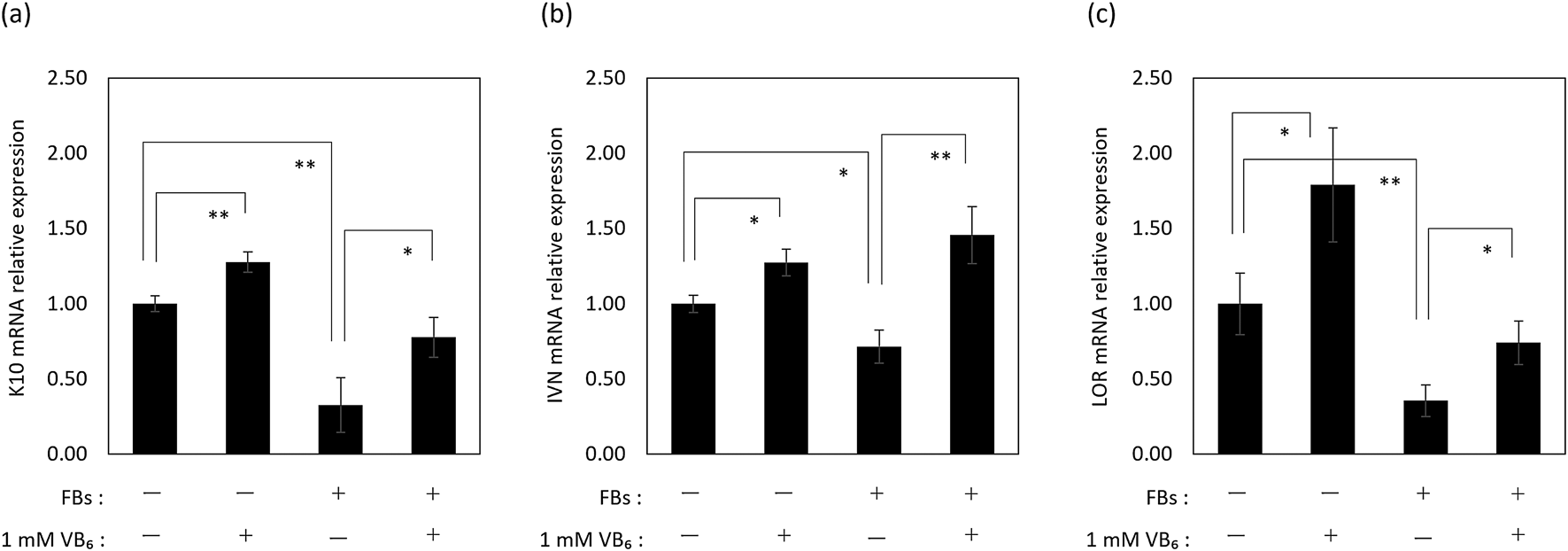
NHEKs were incubated with or without 1 mM VB6 for 24 h, and then were allowed to incorporate FBs for 4 h. After a further 24 h of culture, mRNA expression levels of (a) K10, (b) IVN and (c) LOR in NHEKs were quantified. Bars indicate means ± S.D. (n = 3). Significance: * p < 0.05, ** p < 0.01.
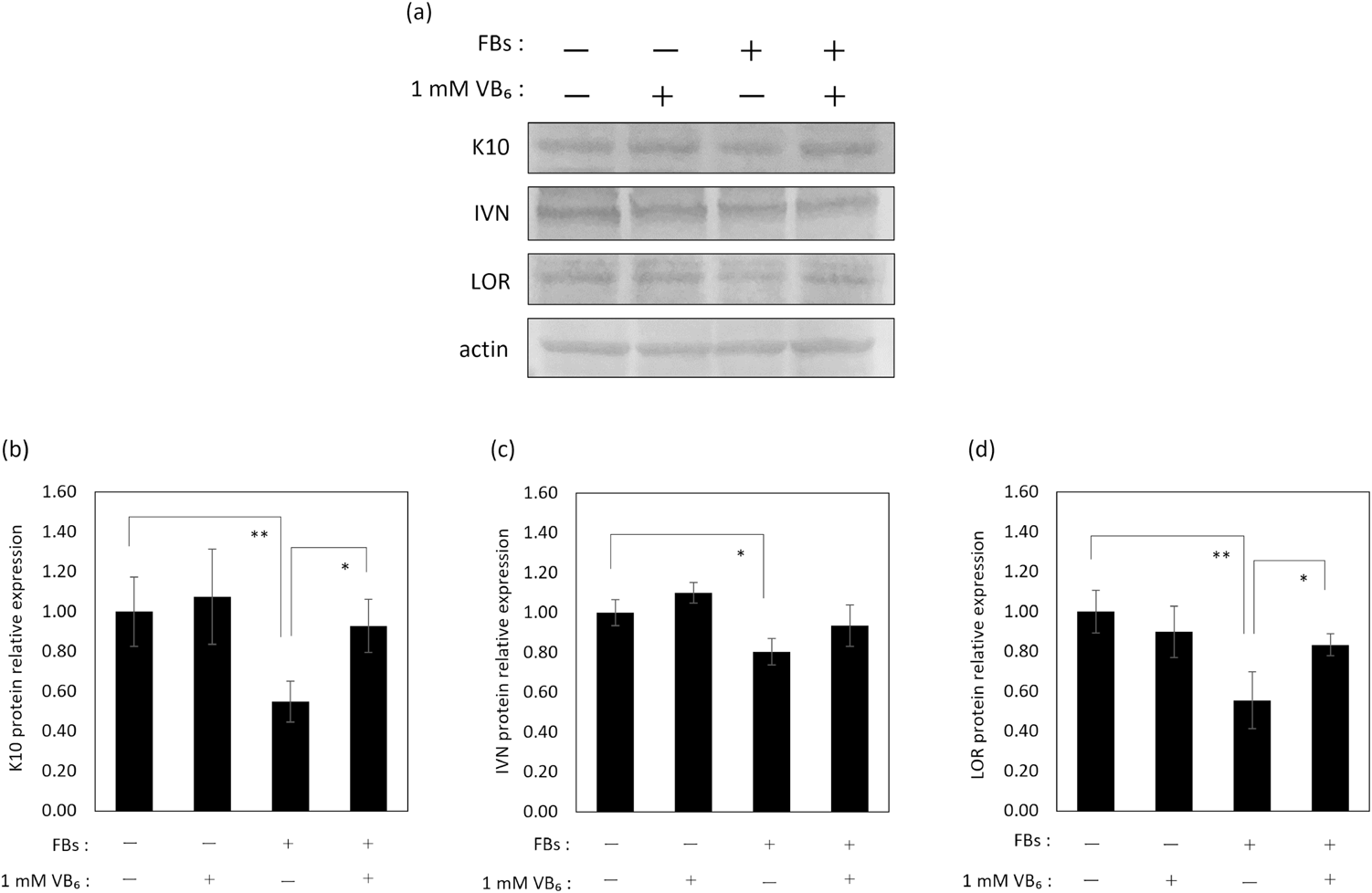
NHEKs were incubated with or without 1 mM VB6 for 24 h, and then were allowed to incorporate FBs for 4 h. After a further 5 d of culture, (a) the protein expression levels of K10, IVN and LOR in NHEKs were detected by Western blotting. (b, c, d) Quantification of those proteins was conducted using Image J. Bars indicate means ± S.D. (n = 3). Significance: * p < 0.05, ** p < 0.01.
Treatment with VB6 significantly restored the mRNA expression level of those differentiation markers at 1 d after FB incorporation (Fig. 5). Further, a significant restoration of K10 and LOR protein expression levels in NHEKs was found at 5 d after FB incorporation (Figs. 6a, b, d). Regarding the protein expression level of IVN, its restoration by VB6 was confirmed (Figs. 6a, c). However, VB6 did not show any influence on the expression of differentiation markers in FB-unincorporated NHEKs (Fig. 6).
Pigmented spots like solar lentigos are a type of skin hyperpigmentation that appears at small limited areas and their appearance is accelerated by chronic sun exposure.12,13,24) However, the reasons for the appearance of hyperpigmentation in limited areas is still unknown. To date, it has been well demonstrated that ROS is a major trigger of UV-induced skin pigmentation. ROS generated in keratinocytes enhances melanin synthesis in MSs by melanocytes through the binding of melanocyte activating substances such as α-melanocyte-stimulating hormone, stem cell factor and endothelin-1 (ET-1) secreted from keratinocytes that bind to specific receptors on melanocytes (melanocortin 1 receptor/c-kit/ET-BR).13,26) Mature MSs containing abundant melanin are transported from the perinuclear area of melanocytes to the tips of dendrites and finally they are incorporated into neighboring keratinocytes by phagocytosis.27–30) Thus, skin pigmentation results not only due to increases of melanin synthesis but also due to the diffusion of MSs by phagocytosis into keratinocytes. In a previous study, we reported that external or internal oxidative stresses caused by hydrogen peroxide or by the depletion of glutathione by BSO enhanced the phagocytic ability of keratinocytes.16) Thus, the sum of these results suggests that a higher oxidative environment in keratinocytes is one of the key events that initiates UV-induced pigmentation.
On the other hand, although previous studies reported that VB6 has the potential to improve skin problems due to its antioxidant property,19,20) the anti-aging effects of VB6 in the skin has not been fully demonstrated. Here, to demonstrate the potential of VB6 to prevent skin pigmentation, we examined the effects of VB6 on the incorporation of MSs into keratinocytes by phagocytosis in a higher oxidative environment due to UVB irradiation.
We first confirmed the antioxidant profile of VB6, focusing on the expression of several antioxidant enzymes, HO-1 and NQO1, that are encoded downstream of Nrf2. VB6 enhanced the mRNA expression levels of HO-1 and NQO1 via Nrf2 as well as γ-GCS, as previously we reported20) (Fig. 1). These results confirmed that VB6 in fact enhances the anti-oxidative defense in keratinocytes via the activation of Nrf2.
UVB and GSH depletion by BSO increased intracellular ROS levels in NHEKs (data not shown). Next, we found that VB6 suppressed the incorporation of FBs into NHEKs under higher oxidative situations caused by UVB or GSH depletion (Figs. 2–4). Additionally, the suppression of VB6 on the elevated incorporation of FBs was canceled by the knockdown of Nrf2 (Fig. 4), which suggested that VB6 suppresses FB incorporation elevated by UVB in NHEKs through the amelioration of intracellular oxidative stress via Nrf2.
In this study, we used FBs instead of MSs to evaluate the incorporation of MSs into keratinocytes by phagocytosis. Because keratinocytes which have incorporated FBs show similar behaviors to keratinocytes that have incorporated MSs, FBs are often used instead of MSs in studies of MS incorporation into keratinocytes.16,17,31–34) Furthermore, the use of FBs instead of MSs makes it possible to evaluate phagocytosis quantitatively and accurately.16,17,31–34) Summarizing these facts, we deduced that VB6 suppresses the incorporation of MSs into keratinocytes in higher oxidative situations.
Considering the fate of MSs incorporated into keratinocytes, MSs are exfoliated from the skin surface through the desquamation of corneocytes, which are the end-products of the differentiation of keratinocytes.18) It is known that the characteristics of the epidermis in pigmented areas are different from non-pigmented epidermis at peripheral regions. One notable characteristic is that the rates of proliferation and of differentiation of keratinocytes in pigmented areas are slower than keratinocytes in non-pigmented peripheral areas.24,25,35) In fact, MSs incorporated into keratinocytes in the basal layer do not express Ki67, which is a proliferation marker,36) and additionally pigmented epidermis shows a lower expression of differentiation marker proteins.24,25) These facts suggest that the incorporation of MSs is one cause that disturbs epidermal homeostasis, which is an imbalance of supplying progenitor keratinocytes and differentiated keratinocytes by decreased proliferation and differentiation, resulting in a long-term retention of MSs in the epidermis by suppressing the exfoliation of corneocytes from the surface. In this study, we found that NHEKs that incorporated FBs showed lower expression levels of K10, IVN and LOR, which are early, middle and late markers of differentiation, respectively, compared with NHEKs without FBs (Figs. 5, 6). Thus, we consider that the differentiation of keratinocytes is suppressed by incorporating materials like MSs and FBs. Meanwhile, pretreatment with VB6 restored the decreases of mRNA and protein expression levels of those differentiation markers in NHEKs incorporated with FBs, despite the fact that VB6 didn’t affect the protein expression levels of those differentiation markers in NHEKs without FBs (Figs. 5, 6). These results indicate that VB6 specifically improves the decreased differentiation of NHEKs caused by the incorporation of FBs. Currently, the mechanism involved in the suppression of differentiation marker proteins by the incorporation of FBs or MSs is unclear. It is interesting that VB6 improved the disruption of differentiation caused by the incorporation of FBs or MSs. The mechanism by which VB6 improves the differentiation of keratinocytes, including the mechanism where differentiation is disturbed by the incorporation of materials into keratinocytes requires future study.
Taken together, our study demonstrated that VB6 suppresses the activation of the phagocytic behavior of keratinocytes in higher oxidative situations by increasing the expression levels of antioxidant enzymes via the activation of Nrf2. Additionally, VB6 improved the decreased differentiation of keratinocytes caused by the incorporation of FBs. These experimental results support the possibility that VB6 will prevent the appearance of pigmented spots due to increasing levels of intracellular antioxidants and improving the differentiation of keratinocytes caused by the incorporation of MSs. We thus conclude that VB6 has the potential to be an anti-skin pigmentation substance.
This study was funded by a research Grant provided by the SSP Co., Ltd. Koichi Hiyama, Atsushi Sawamura and Ichiro Kawase are employees of SSP Co., Ltd. All authors do not have shares of SSP Co., Ltd.