2023 年 46 巻 1 号 p. 26-34
2023 年 46 巻 1 号 p. 26-34
Oxaliplatin (OXA) is a usual chemotherapeutic agent applied in the colorectal cancer (CRC) clinical treatment. Interferon-alpha inducible protein 6 (IFI6) has been proved to promote proliferation and suppress apoptosis in several tumor cells, while the impacts of IFI6 on OXA resistance in CRC still need exploration. HCT116 and SW620 cells were used as the parental to obtain OXA-resistant cells. The influence of IFI6 on OXA sensitivity, cell proliferation and apoptosis were evaluated by overexpression or knockdown IFI6 in cells. In this work, we found that the level of IFI6 was significantly enhanced in HCT116/OXA and SW620/OXA cells as compared to the parental cells. Overexpression of IFI6 decreased the sensitivity of HCT116 and SW620 cells to OXA. However, knockdown of IFI6 enhanced the sensitivity of HCT116/OXA and SW620/OXA cells to OXA. And upregulated IFI6 promoted the proliferation and repressed apoptosis in HCT116 cells, while suppressed IFI6 markedly reduced proliferation and increased apoptosis in HCT116/OXA cells. Additionally, IFI6 suppressed the phosphorylation level of p38, and silenced IFI6 enhanced it. The addition of the p38 kinase inhibitor, SB203580, alleviated the decreased cell proliferation and increased apoptosis in HCT116/OXA cells. Suppressed IFI6 enhanced the reactive oxygen species (ROS) level in HCT116/OXA cells, and blockade of ROS with N-acetyl-L-cysteine (NAC) decreased the enhancement level of ROS and the phosphorylation level of the p38, which was induced by IFI6 down-regulation. We, therefore, implied that suppressed IFI6 reverses OXA-resistance of CRC cells via promoting the ROS-induced p38 mitogen-activated protein kinase (MAPK) signaling pathway.
The incidence of colorectal cancer (CRC) has continued to rise in recent years and CRC is considered to be one of the leading causes of cancer-related death.1–3) In order to improve the survival rate of patients, some treatment strategies are widely used in clinical practice, such as surgical resection, radiotherapy and systemic chemotherapy.4) However, the survival rate of CRC patients remains low, with half of the patients surviving less than 5 years.5,6) Chemotherapy is generally considered as the most effective treatment for patients with advanced CRC. Oxaliplatin (OXA) belongs to the third-generation platinum anticancer drug.7) As a bifunctional alkylating agent, OXA could covalent binds to DNA therefore form platinum-DNA adducts to repress the replication and transcription of DNA.8,9) OXA can bind to DNA nucleobases and interact with DNA in CRC cells, thereby triggering apoptotic related pathways leading to the damage of DNA.9,10) But the frequent application of OXA has led to drug resistance of tumor cells, which has brought great limitations to the treatment of CRC.11–14) To date, drug resistance remains one of the barriers of CRC patient’s treatment, which leads to the poor outcomes in patients.15,16) Therefore, finding new strategies to overcome OXA resistance is of great significance to change the low survival rate of CRC patients.
Interferon alpha inducible protein 6 (IFI6, also known as G1P3), an interferon-stimulating factor, could participate and regulate various tumors progression.17,18) Besides that, IFI6 has been reported could enhance the cell invasion and migration abilities in breast cancer19) and inhibit cell apoptosis in gastric cancer.20) In fact, IFI6 is considered as a factor that can promote proliferation and anti-apoptosis in previous reported studies.19,21,22) Importantly, both in vitro and in vivo results have displayed that chemotherapeutic drugs often activate the apoptosis pathway, thereby promoting their cytotoxicity and inhibiting tumor growth.23) Therefore, we wondered that if IFI6 was involved in regulating chemoresistance of CRC cells. Reactive oxygen species (ROS) is a chemical reactive oxygen-containing molecules, is produced as a natural by-product of cell metabolism.24) Studies have shown that the control of ROS production is involved in many aspects including carcinogenesis, metabolic reprogramming, aggressive cancer phenotypes, and drug resistance development.25) Therefore, increased ROS in cancer cells may provide a unique opportunity to eliminate cancer cells via elevating ROS to highly toxic levels intracellularly, thereby, activating various ROS-induced cell death pathways, or inhibiting cancer cell resistance to chemotherapy.26,27) Studies have shown that ROS can phosphorylate p38, activate the p38 mitogen-activated protein kinase (MAPK) signaling pathway, then participate in the regulation of tumor occurrence and development.28,29) More importantly, study has confirmed that activation of p38 was shown to be a key proapoptotic mediator of OXA-induced cell death.30)
Accordingly, in this work, we intend to explore the essential part of IFI6 in regulating OXA resistance of CRC cell lines and explored the underlying mechanism involved in IFI6-regulated OXA resistance.
The human CRC cell lines HCT116 (RRID: CVCL_0291) and SW620 (RRID: CVCL_0547) were obtained from the iCELL (China). HCT116 cell was cultured in McCOY’s 5A medium (BI, Israel) containing 10% fetal bovine serum (FBS, Tianhang Biotech, China) at 37 °C in 5% CO2. SW620 was cultured in L15 medium (Procell, China) containing 10% FBS at 37 °C. The OXA-resistant cell lines HCT116/OXA and SW620/OXA were obtained by putting parental HCT116 and SW620 cells to a mildly enhancing concentration of OXA and then stayed in the medium added with OXA (Aladdin, China), separately.
Cell TransfectionPlasmids pcDNA3.1 (G114479, YouBio, China) was used to induce the overexpression of IFI6 (NM_002038). Short hairpin RNA (shRNA) targeting IFI6 and corresponding NC were constructed by pRNAH1.1 plasmid vectors (GeneralBiol, China). The sequences specific targeting the IFI6 is as follow: shIFI6-1: 5′-GCTGGTCTGCGATCCTGAA-3′. shIFI6-2: 5′-GGTGGAGGCAGGTAAGAAA-3′.
When cells reached at 90% of confluence, shRNAs targeting IFI6 and ectopic expression of IFI6 were transfected into CRC cells by the mediation of Lipofectamine 3000 reagent (Invitrogen, U.S.A.) following the manufacturer’s protocol. All experiments were performed at 48 h post transfection. After transfection, cells were treated with 1/2 IC50 concentration of OXA. In some sections, SB203580 (5 µM, Aladdin) or N-acetyl-L-cysteine (NAC) (3 mM, Aladdin) was added and incubated for further analysis.
Chemosensitivity AssayCells were seeded in a 96-well plate at a density of 6 × 103 cells/well overnight and then exposed to various concentrations of OXA for 48 h. In the last section, cells were seeded in a 96-well plate at a density of 3 × 103 cells/well for transfection and then treated with SB203580. Cell Counting Kit-8 detection kit (CCK-8, Solarbio, China) was used for the evaluation of cell viability in this work. Simply, after added 10 µL CCK-8 solutions, the cells were incubated at 37 °C for 2 h before detection. The result was measured by a 800TS microplate reader (BIOTEK, U.S.A.) at 450 nm. Based on the cell viability of CRC cells, the IC50 concentration of OXA was acquired by calculation.
Cell ApoptosisCell apoptosis was measured by Apoptosis Detection Kit (Biosharp, China) according to the manufacturer’s instruction. Cells were centrifuged and re-suspended in binding buffer before test. Then, 5 µL AnnexinV-fluorescein isothiocyanate (FITC) was added and incubated for 10 min in the dark. Finally, 10 µL propidium iodide (PI) was added and followed by analyzing on flow cytometry (Aceabio, U.S.A.). Images were obtained by an inverted phase contrast microscope (OLYMPUS, Japan).
Real-Time Quantitative (q) PCRRNA was isolated using the TRIpure (BioTeke, China). The purity and concentration of the extracted RNA were detected by Nano 2000 (Thermo, U.S.A.). The corresponding cDNA was obtained by reverse transcription with BeyoRT II M-MLV (Beyotime, Shanghai, China). The expression level was ascertained through qRT-PCR by using 2 × Taq PCR MasterMix and SYBR Green (Solarbio) reagents and quantified with the Exicycler™ 96 apparatus (BIONEER, Korea). β-Actin was selected as internal reference. PCR primers were designed by Nanjing GenScript Co, Ltd. (China). IFI6 primers: forward 5′-GGGGTGGAGGCAGGTAAGA-3′ and reverse 5′-CCCATTCAGGATCGCAGA-3′.
Western BlottingCells were lysed with radio immunoprecipitation assay (RIPA) buffer and phenylmethylsulfonyl fluoride (PMSF) (Beyotime), separated by 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) (Beyotime), and transferred into a polyvinylidene fluoride (PVDF) membrane (Thermo Fisher Scientific, U.S.A.). Protein quantification was finished by Bicinchoninic acid (BCA) assay kit (Beyotime). Bovine serum albumin (BSA, 5%, Biosharp) was used as the blocking buffer. The membrane was then incubated with primary antibodies overnight at 4 °C and incubated with secondary antibody (1 : 10000, goat anti-rabbit, Proteintech, China) for 40 min. Finally, the bands were detected by ECL (7 Sea Biotech, China) reagent. β-Actin was used as a control. Images were collected and analyzed by gel imaging system (Liuyi, China) and Gel-Pro-Analyzer software. The cleaved caspase-3 (Cat#9661, RRID:AB_2341188), cleaved caspase-7 (Cat#8438, RRID:AB_11178377), cleaved poly(ADP-ribose)polymerase (PARP) (Cat#5625, RRID:AB_10699459), p-p38Thr182/Tyr182 (Cat#4511, RRID:AB_2139682) and p38 (Cat#8690, RRID:AB_10999090) antibodies were obtained from CST (U.S.A.) at a dilution of 1 : 1000. IFI6 antibody was provided by Affinity (Cat#DF10115, RRID:AB_2840695, China) at a dilution of 1 : 500.
Colony Formation AssayCells were seeded into plates about two weeks before detection. Colonies were fixed with 4% paraformaldehyde (Aladdin) and stained with Wright-Giemsa Stain solution (Leagene, China) for 5 min at room temperature. Colonies including more than 50 cells were counted and the mean colony numbers were calculated. Finally, the colonies were photographed and counted with an inverted microscope (OLYMPUS).
ROS DetectionROS level was tested by flow cytometry after staining with the fluorescent probe, 2′,7′-dichlorodihydro-fluorescein diacetate (DCFH-DA, Aladdin). DCFH-DA is deacetylated in cells by esterase to a non-fluorescent compound, DCFH, which remains trapped within the cell and is cleaved and oxidized by ROS in the presence of endogenous peroxidases to a highly fluorescent compound, DCF. Cells were cultured with DCFH-DA (1 : 1000) for 20–30 min at 37 °C. After washing three times, samples were observed by a microscope (OLYMPUS) followed by flow cytometry.
Statistical AnalysisIn this work, Data were expressed as means ± standard deviation (S.D.) and analyzed by the GraphPad 8.0 software (U.S.A.). Student’s t-test or the one-way ANOVA were used for comparisons between two or more experimental groups, respectively. p ≤ 0.05 was considered as the statistical significance.
To obtain OXA-resistant cell lines, we exposed two CRC cell lines, HCT116 and SW620, to different concentrations of OXA. After screening, OXA-resistant CRC cell lines were obtained and named HCT116/OXA and SW620/OXA.
In Figs. 1A and C, the HCT116/OXA and SW620/OXA cell lines exhibited higher resistance to a gradually enhanced concentration of OXA than HCT116 and SW620 cells, as detected by CCK-8 assay. After calculation, we found that the IC50 value in HCT116/OXA cells was significantly higher than that in HCT116 cells (Fig. 1B, p < 0.01), which means the successful acquisition of OXA resistance in HCT116/OXA cells. Similarly, in Fig. 1D, SW620/OXA cells also have a higher IC50 than SW620 cells. To be specific, the IC50 values of HCT116, HCT116/OXA, SW620 and SW620/OXA cells were 7.986 ± 0.563 µM (IC50-1), 137.493 ± 10.585 µΜ (IC50-2), 5.932 ± 0.222 µM (IC50-3) and 105.705 ± 5.528 µM (IC50-4), respectively. We further measured the cell apoptosis by flow cytometric analysis in these cell lines. In order to further verified the effect of OXA on CRC cell lines apoptosis, HCT116 and HCT116/OXA cells were treated with 1/2 IC50-1 (4 µM) concentration OXA, SW620 and SW620/OXA cells were treated with 1/2 IC50-3 (3 µM) concentration OXA, for 48 h before detection. In Figs. 1E and F, we found that OXA resistant cells HCT116/OXA and SW620/OXA had a clearly lower cell apoptosis rate when compared with their parental cells.
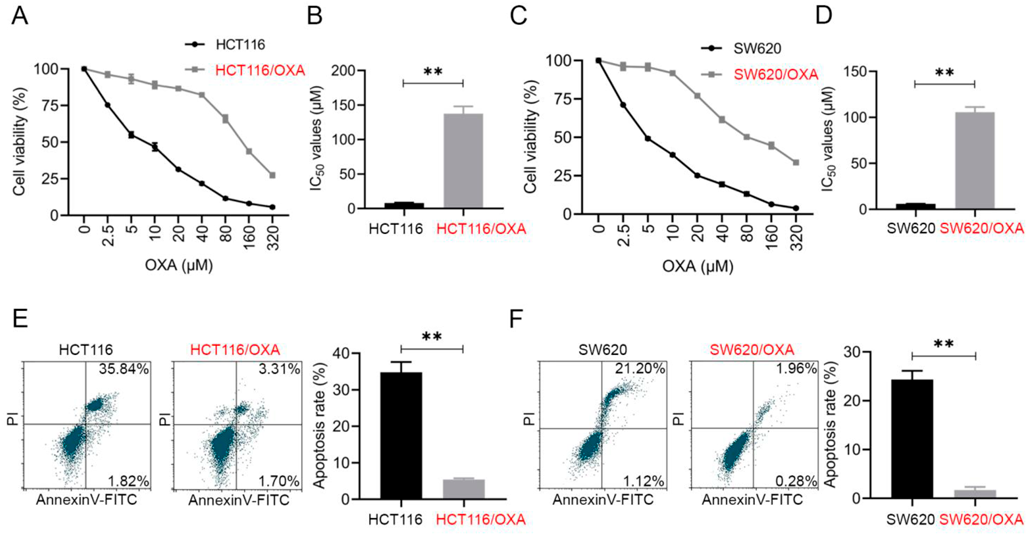
(A) CCK-8 assay was performed to evaluate the cell viability of different concentrations of OXA (0–320 µM) to HCT116 and HCT116/OXA cells. (B) IC50 values of OXA to HCT116 and HCT116/OXA cells. (C) CCK-8 assay was performed to evaluate the cell viability of different concentrations of OXA (0–320 µM) to SW620 and SW620/OXA cells. (D) IC50 values of OXA to SW620 and SW620/OXA cells. (E, F) Cell apoptosis in HCT116, HCT116/OXA, SW620 and SW620/OXA cells was tested by flow cytometric analysis. HCT116 and HCT116/OXA cells were treated with 4 µM OXA, SW620 and SW620/OXA cells were treated with 3 µM OXA. Data are expressed as mean ± standard deviation (S.D.) **, p < 0.01.
In order to figure out the role of IFI6 in OXA-resistant CRC cells, the expression of IFI6 in these four cell lines, HCT116, HCT116/OXA, SW620 and SW620/OXA were measured by qRT-PCR and Western blotting. Compared with their parental cells, HCT116/OXA and SW620/OXA cell lines exhibited an obvious enhancement in the mRNA and protein expression levels of IFI6 (Figs. 2A, B).
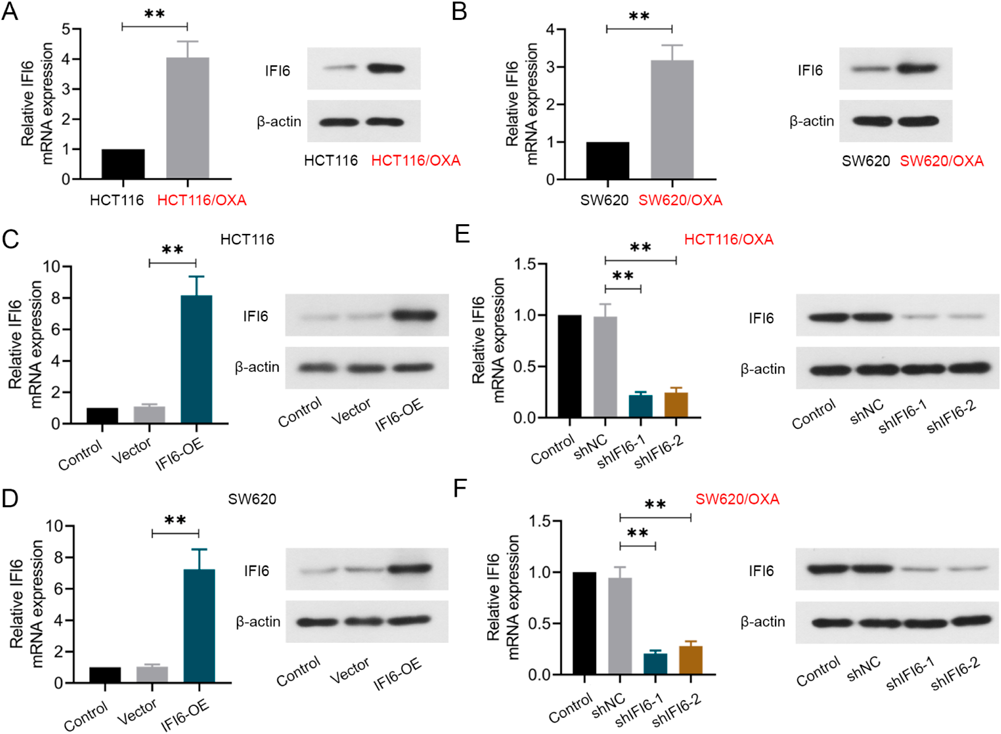
The mRNA and protein levels of IFI6 in HCT116 and HCT116/OXA (A), SW620 and SW620/OXA (B) cell lines were measured by qRT-PCR and Western blotting. The results of qRT-PCR and Western blotting confirmed the efficiency of IFI6 overexpression in HCT116 (C) and SW620 (D) cells, and IFI6 downregulation in HCT116/OXA (E) and SW620/OXA (F) cells. Data are expressed as mean ± S.D. **, p < 0.01.
Accordingly, to further figure out the important role of IFI6 in OXA sensitivity, we overexpressed IFI6 in HCT116 and SW620 cell lines, knockdown IFI6 in HCT116/OXA and SW620/OXA cell lines. The efficiency of up- and down-regulation were confirmed by qRT-PCR and Western blotting in Figs. 2C–F.
Repressed IFI6 Enhances the Chemosensitivity of CRC Cells to OXAAs shown in Figs. 3A and B, upregulated IFI6 decreased the sensitivity of HCT116 and SW620 cells in different concentrations of OXA, as proved by the enhanced IC50 value of OXA. Oppositely, downregulated IFI6 increased the sensitivity of HCT116/OXA and SW620/OXA cells to OXA, as evidenced by the repressed cell viability and the IC50 value of OXA cell lines (Figs. 3C, D). These findings implied that silenced IFI6 enhanced the chemosensitivity of CRC cells toward OXA.

The effects of IFI6 overexpression on the IC50 values of HCT116 (A) and SW620 (B) cell lines were measured by CCK-8 assay. The effects of IFI6 inhibition on the IC50 values of HCT116/OXA (C) and SW620/OXA (D) cell lines were measured by CCK-8 assay. Data are expressed as mean ± S.D. **, p < 0.01.
Additionally, we measured the cell proliferation and apoptosis of HCT116 and HCT116/OXA cells under IFI6 up- or down-regulation. After transfection, cells were pretreated with 4 µM OXA. In Fig. 4A, IFI6-OE transfected HCT116 cells showed an increased colony formation rate than vector transfected cells. IFI6-shRNA transfected HCT116/OXA cells displayed a decreased colony formation rate compare with the shNC transfected cells (Fig. 4B). In addition, the cell apoptosis was detected in Figs. 4C and D. It was clear to see that IFI6 upregulation suppressed cell apoptosis in HCT116 cells, while IFI6 downregulation greatly enhanced cell apoptosis in HCT116/OXA cells. The protein expression level of cleaved caspase-3, cleaved PARP and cleaved caspase-7 was measured by Western blotting. In Fig. 4E, IFI6 overexpression showed a decreased level of cleaved caspase-3, cleaved PARP and cleaved caspase-7 in HCT116 cells. IFI6-shRNA transfected HCT116/OXA cells displayed an increased level of three of them (Fig. 4F). These findings reflected that IFI6 promotes the cell proliferation and anti-apoptosis in CRC cells.
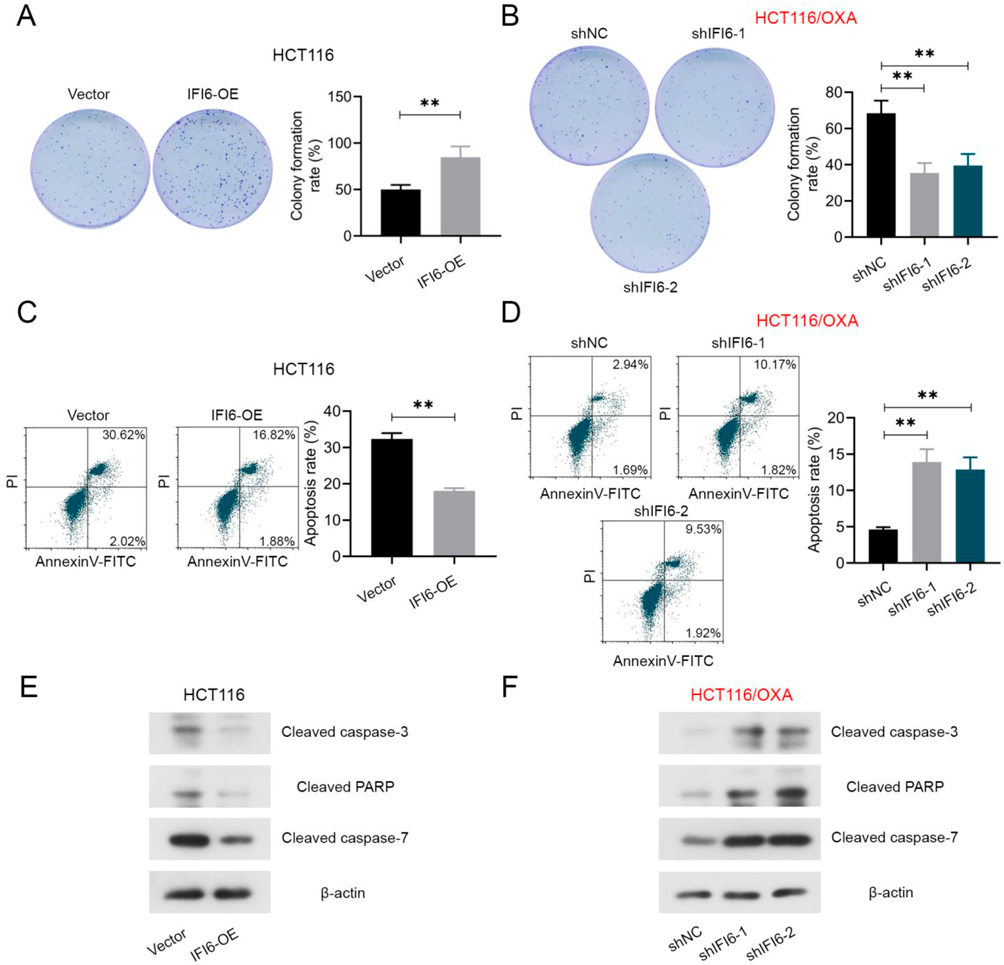
The colony formation rate of IFI6 upregulated HCT116 cells (A) and downregulated HCT116/OXA cells (B). Cell apoptosis in IFI6 upregulated HCT116 cells (C) and downregulated HCT116/OXA cells (D) was detected by flow cytometry. The protein expression level of cleaved caspase-3, cleaved PARP and cleaved caspase-7 was measured by Western blotting in IFI6 upregulated HCT116 cells (E) and downregulated HCT116/OXA cells (F). All the cells were pretreated with 4 µM OXA. Data are expressed as mean ± S.D. **, p < 0.01.
Some researchers have concluded the critical role of p38 signal in cancer cell death31,32) and they also figured out that many chemotherapeutic agents require p38 activity for the induction of apoptosis.33) Activation of p38 was shown to be a key proapoptotic mediator of OXA-induced cell death.30) Accordingly, we explored the effect of IFI6 regulation on the activation of the p38MAPK signaling pathway in CRC cells. All the cells were pretreated with 4 µM OXA. Figures 5A and B exhibited that overexpression of IFI6 inhibited the level of p-p38 in HCT116 cells, while inhibition of IFI6 enhanced the level of p-p38 in OXA-resistant HCT116/OXA cells. As for total p38, there was no obvious difference. To further confirm the role of the p38MAPK signaling pathway in IFI6-regulated OXA sensitivity, the p38 inhibitor SB203580 was used in this section in HCT116/OXA cells. The addition of SB203580 abolished the changes in the p-p38 protein level in IFI6 silenced HCT116/OXA cells (Fig. 5C). And the results of CCK-8 and flow cytometric analyses revealed that the addition of p38 inhibitor reversed the decreased cell viability (Fig. 5D) and enhanced cell apoptosis (Fig. 5E) in IFI6 suppressed HCT116/OXA cells.
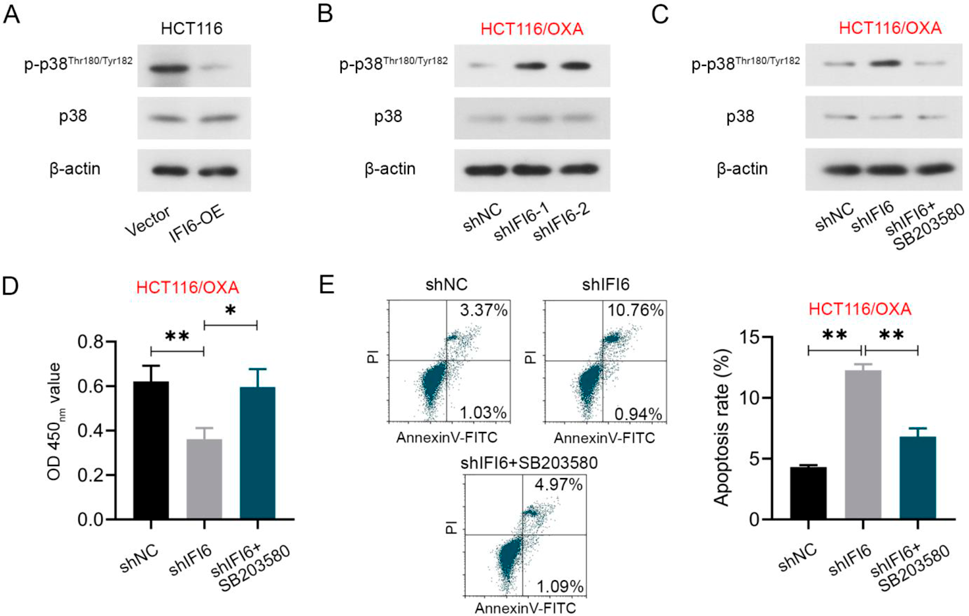
The p38 and p-p38 levels after IFI6 upregulating in HCT116 cells (A) and silencing in OXA-resistant HCT116/OXA cells (B) were determined by Western blotting. (C) The Western blotting detection of p38 and p-p38 proteins in IFI6 suppressed HCT116/OXA cells after p38 kinase inhibitor SB203580 treatment. The cell viability (D) and apoptosis (E) in SB203580 treated IFI6 inhibited HCT116/OXA cells. All the cells were pretreated with 4 µM OXA. Data are expressed as mean ± S.D. *, p < 0.05. **, p < 0.01.
Studies have shown that ROS can activate the p38MAPK signaling pathway, and then participate in the regulation of cancer occurrence and development. Downregulation of IFI6 could inhibit ESCC progression through ROS accumulation.21) To determine whether IFI6 activated p38MAPK signaling pathway was elicited by ROS, we measured the ROS level in HCT116/OXA cells (treated with 4 µM OXA) and found that IFI6 knockdown displayed an enhanced ROS level in cells (Figs. 6A, B).

(A–B) The cellular ROS in IFI6 suppressed HCT116/OXA cells was evaluated by fluorescence microscopy and flow cytometry. (C) After treated with N-acetyl cysteine (NAC), ROS level in IFI6 suppressed HCT116/OXA cells were evaluated by flow cytometry using DCFH-DA probe. (D) The Western blotting detection of p38 and p-p38 proteins in IFI6 suppressed HCT116/OXA cells after NAC treatment. All the cells were pretreated with 4 µM OXA. Data are expressed as mean ± S.D. **, p < 0.01.
Based on this, we utilized the ROS inhibitor NAC in this section for confirmation. In Fig. 6C, IFI6 inhibition exhibited an elevated ROS level in cells, while the addition of NAC reversed this enhancement in HCT116/OXA cells. We further detected the protein level of p38 and p-p38 in the cells added with NAC by Western blotting. Downregulation of IFI6 presented an enhanced p-p38 level in cells, while the addition of NAC reversed this level (Fig. 6D). Besides that, schematic illustration of the above mentioned mechanism of IFI6 in OXA resistance CRC cells was shown in Fig. 7. These findings implied that IFI6 suppression promoted the ROS level in HCT116/OXA cells, thereby activating the p38MAPK signaling pathway.

CRC caused a great attention of variety countries of researchers in worldwide. Although great efforts have been made in drug development across many therapeutic areas, the progress was not satisfied. Chemotherapeutic resistance is still an essential reason of tumor relapse and leads to treatment cessation. OXA is the third generation of platinum based drugs after cisplatin and carboplatin, and it is widely used as the optimal treatment of digestive system cancers (including gastric cancer and CRC).34–36) Nevertheless, approximately fifty to seventy percent of CRC patients developed innate or acquired drug resistance to chemotherapy.37) Accordingly, it is of great urgent to overcome the drug resistance in OXA-based chemotherapy. In this study, we found that IFI6 was significantly upregulated in OXA-resistance CRC cell lines. Upregulation of IFI6 displayed a reduced OXA sensitivity; however, knockdown of IFI6 markedly increased the sensitivity of CRC cells to OXA. Additionally, IFI6 affected the phosphorylation level of p38 in MAPK signaling pathway. Furthermore, suppressed IFI6 showed an enhanced ROS level, and blockade of ROS reversed IFI6 downregulation caused cell apoptosis and the phosphorylation level of p38 MAPK signaling pathway. Therefore, we implied that suppressed IFI6 reverses OXA-resistance of CRC cells by activating ROS-induced p38MAPK signaling pathway.
IFI6 is a mitochondrial targeting protein that acted as an Interferon-stimulated gene takes an essential part in cell apoptosis regulation.38) In Venugopalan’s research, IFI6 can antagonize TRAIL-induced apoptosis in human myeloma cells by inhibiting the release of cytochrome c from mitochondria and delaying the apoptotic process initiated and transduced by the tumor necrosis factor (TNF)-related apoptosis-inducing ligand/caspase 8 pathway.18) The author also demonstrated that coordinated action of multiple pathways elicited by IFI6-induced mitochondrial ROS augment F-actin-containing migratory structures to promote breast cancer cell migration and invasion.19) Besides that, Liu’s study implied the carcinogenesis of IFI6 in esophageal squamous cell carcinoma and revealed that knockdown of IFI6 suppressed proliferation and induced apoptosis by increasing ROS accumulation.21) These findings confirmed the important role of IFI6 in cell apoptosis and other malignant phenotype of different tumors. Based on this, we wondered the biological role of IFI6 in CRC cells, which was still unknown at so far. In the experimental preparation, we found that IFI6 showed an increased level in the OXA-resistant CRC cells. Accordingly, we overexpressed IFI6 in CRC cell lines, knockdown IFI6 in OXA-resistance CRC cell lines. We also detected the cell proliferation and apoptosis in HCT116 and HCT116/OXA cells. The results indicated that OXA resistance caused cell apoptosis inhibition was abated by IFI6 inhibition, which means that inhibited IFI6 could improve the chemosensitivity of HCT116/OXA cells to OXA.
Recent studies have documented that ROS can induce cell apoptosis by activation of the MAPK pathway.28,39) The subgroups of the MAPK pathway such as c-Jun N-terminal kinase (JNK) and p38 can be activated through sequential phosphorylation of MAPK.28) A study demonstrated that p38 MAPK inhibition can be a targeted approach to overcome resistance in head and neck squamous cell carcinoma cells thereby escalating the effectiveness of chemotherapy in carcinoma cells.40) Li et al. found that p38 signal was involved in OXA-induced cell death in gastric cancer.41) In Liu’s study, the protein expression level of p-p38 and activated caspase 3 increased gradually in an OXA concentration dependent manner.42) These studies suggested that p38MAPK signaling pathway is involved in OXA-induced cell death. The effect of p38 inhibitor on apoptosis induced by OXA was also confirmed in previously reported articles. In Chocry’s research, the addition of SB203580 in the OXA treated CRC cells reversed the enhanced cleaved PRAP level when compared with the control.43) The similar results were also obtained in Chiu’s and Liu’s papers.42,44) Accordingly, in this work, we directly used p38 inhibitor to verify the effect of IFI6 knockdown on P38 signaling activation and cell apoptosis. The results reflected that IFI6 inhibition enhanced the level of ROS and promoted the level of p-p38. Furthermore, the addition of SB203580 reversed the suppression of cell viability caused by knockdown of IFI6. Based on the previous studies, we suspected that in IFI6-suppressed CRC cells, IFI6 knockdown induced ROS level enhancement may act as an upstream mediator of p38-MAPK signaling. Similarly, non-small cell lung cancer cells are resistant to cisplatin through the p38 pathway.45) Knocking out p38MAPK gene or using p38 pathway inhibitors can reverse drug resistance of tumor cells and promote tumor cell apoptosis, which is consistent with our results.
To determine whether elevated ROS level affects p38MAPK pathway activation, the ROS inhibitor NAC was introduced in this section. It has been shown that NAC could inhibit ROS accumulation, thus inhibiting MAPK pathway activation.28,46) Likewise, our results showed that the addition of NAC decreased the cell viability and phosphorylation level of p38, inhibited the p38MAPK pathway. These results suggest that ROS plays a key role in the IFI6-mediated p38MAPK pathway, and the enhancement of ROS may be the main mechanism for inducing apoptosis through the IFI6-mediated p38MAPK pathway.
Besides OXA, irinotecan47) and 5-fluorouracil (5-FU)48) were also used as the common chemotherapy drugs in CRC treatment. And the resistance to irinotecan and 5-FU chemotherapy has been shown to be involved in MAPK signaling pathways49) and the recent studies identified that p38MAPK as a mediator of resistance to various agents in CRC patients. A study implied that targeting the p38MAPK pathway inhibits irinotecan resistance in CRC.50) Similarly, Yang et al. showed that the suppression of the p38MAPK pathway could sensitize the human CRC cells to 5-FU treatment.51) Considering the effect of IFI6 on p38MAPK pathway, our further study may focus on the above mentioned drugs to further validate the important role of IFI6 in drug resistant CRC cells.
In conclusion, IFI6 inhibition exhibits a novel strategy for decreasing OXA-resistance of CRC cells, and this may mediated by the suppression of the p38MAPK signaling pathway. Moreover, IFI6 may act as a potential diagnostic and prognostic marker for overcoming OXA resistance in CRC treatment.
Conception and design: Chen Huang, Shipeng Zhao; Administrative support: Shipeng Zhao; Provision of study materials or patients: Chen Huang, Tao Zhou, Lihua Ma; Collection and assembly of data: Chen Huang, Tao Zhou, Lihua Ma; Data analysis and interpretation: Chen Huang, Tao Zhou, Lihua Ma; Manuscript writing: All authors; Final approval of manuscript: All authors.
The authors declare no conflict of interest.