Abstract
Vitamin K, a necessary nutritional supplement for human, has been found to exhibit anti-inflammatory activity. In the present study, we investigated the effects of vitamin K family on lipopolysaccharide (LPS) plus nigericin induced pyroptosis and explored the underlying mechanism of its action in THP-1 monocytes. Results showed that vitamin K3 treatment significantly suppressed THP-1 pyroptosis, but not vitamin K1 or K2, as evidenced by increased cell viability, reduced cellular lactate dehydrogenase (LDH) release and improved cell morphology. Vitamin K3 inhibited NLRP3 expression, caspase-1 activation, GSDMD cleavage and interleukin (IL)-1β secretion in pyrophoric THP-1 cells. In addition, vitamin K3 inhibited the pro-inflammatory signaling pathways including nuclear factor-κB (NF-κB) and c-Jun N-terminal kinase (JNK). Vitamin K3 treatment also attenuated tissue damage and reduced serum LDH, IL-1β and IL-6 levels in LPS-induced systemic inflammation of mice. The reduced myeloperoxidase (MPO) activityand F4/80 expression indicated that vitamin K3 effectively reduced the infiltration of neutrophils and macrophages. Moreover, NLRP3 expression in monocytes/macrophages were also decreased in vitamin K3-treatedmice after LPS challenge. These findings suggest that vitamin K3 potently alleviates systemic inflammation and organ injury via inhibition of pyroptosis in monocytes and may serve as a novel therapeutic strategy for patients with inflammatory diseases.
INTRODUCTION
Vitamin K, a necessary nutrient for human, exists different forms. The two natural forms of vitamin K, vitamin K1 and K2, have similar biological function albeit different patterns of absorption, transportation and turnover. Vitamin K3, on the other hand, is not a natural form of vitamin K in food but a catabolite of vitamin K1 after removal of side chain (Fig. 1A). It is thought as a precursor of recycling into vitamin K2.1,2) Vitamin K is best known to support coagulation as a co-factor in carboxylation, which is a necessary type of protein modification for coagulation factors. Moreover, it also participates in bone metabolism, glucose metabolism and tumorigenesis.1,2) Recent studies further found that vitamin K inhibits inflammation.2,3) These discoveries indicate that vitamin K may potentially alleviates a wide variety of diseases associated with inflammation.
Inflammation is a pathophysiological process in immune response. Inflammatory reaction leads to tissue damage through modulating cell composition, structure and function, or even causing cell death.4,5) Immune cells and other tissue cells produce cytokines, chemokines and other inflammatory factors upon stimulation via inflammatory signal pathways such as nuclear factor-κB (NF-κB)6) and mitogen activated protein kinase (MAPK).7) One important downstream target of these pathways is inflammasome, which further processes other inflammatory molecules for their activation.8,9) Ultimately, the activation of inflammasome will initiate pyroptosis, a recently discovered type of programmed cell death with extremely strong pro-inflammatory property.10,11)
Pyroptosis is an inflammation induced programmed cell death predominantly found in monocytes and it further exacerbates inflammation. Upon inflammatory stimulation, NLRP3 dependent inflammasome will be assembled, recruiting and cleaving pro-caspase-1 to its active form. The activated caspase-1 has two major downstream targets, interleukin (IL)-1β and Gasdemin D (GSDMD). IL-1β and GSDMD are both stored in the cytoplasm in their inactive forms and upon cleavage by caspase-1, they gain bioactivity. Activated IL-1β is a strong pro-inflammatory cytokine. It is released from the cells via pores on the plasma membrane formed by activated GSDMD. Membrane GSDMD pores will further compromise the cell integrity, causing swelling and death, termed pyroptosis. IL-1β and other cell content induce strong inflammatory reaction in the microenvironment.10,11) Suppressing pyroptosis is thus considered a potential therapeutic or preventive strategy in inflammation related diseases.
Other studies have already found vitamin K3 regulating programmed cell death.12) In our previous study screening for modulators of pyroptosis, we found vitamin K3 potentially inhibits pyroptosis.13) Here we used a lipopolysaccharide (LPS) plus nigericin pyroptosis cell model and found a specific function of vitamin K3 to rescue pyroptosis in THP-1 cells. This function was possibly mediated by suppressing NF-κB and c-Jun N-terminal kinase (JNK) pathways. Subsequently, the inflammasome mediated pyroptosis pathway was hindered. Our current study revealed a new anti-inflammatory mechanism of vitamin K3, and point out vitamin K3 as a potential intervention target in the nutrition of patients with inflammatory diseases.
MATERIALS AND METHODS
ReagentsLPS (Escherichia coli O111:B4) was purchased from Sigma (St. Louis, MO, U.S.A.). Vitamin K1, vitamin K2 and vitamin K3 were purchased from Solarbio (Beijing, China). Nigericin were obtained from GlpBio (Montclair, CA, U.S.A.). The primary antibodies for NLRP3 (15101), inhibitor of kappaBα (IκBα) (4812), phospho-IκBα (2859), phospho-p65 (3033), p65 (8242), phospho-IKKα/β (2078), IKKα (11930), IKKβ (8943), phospho-JNK (4668), JNK (9252), phospho-extracellular signal-regulated kinase (ERK)1/2 (5726), ERK1/2 (4695), p38(8690), IL-1β (12242), caspase-1 (3866) and GSDMD (97558) were purchased from Cell Signaling Technology (Danvers, MA, U.S.A.). The primary antibodies for phospho-p38 (ab195049) and β-actin (ab5694) were purchased from Abcam (Cambridge, MA, U.S.A.). The immunofluorescence primary antidody for F4/80 (GB113373) and NLRP3 (GB114320) were purchased from Servicebio (Wuhan, China).
Cell Culture and TreatmentThe human acute monocytic leukemia cell line THP-1 was obtained from American Type Culture Collection (Manassas, VA, U.S.A.) and cultured in RPMI 1640 medium supplemented with 10% Fetal Bovine Serum (FBS), 1 mM sodium pyruvate, 25 mM N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid (HEPES) and 1% penicillin–streptomycin solution. The cultures were maintained at 37 °C in an incubator with 5% CO2 and were refreshed with the medium every 2 d. THP-1 cells were treated with different concentrations (0, 6.25, 12.5, 25, 50, 100, and 200 µM) of vitamin K1, K2, and K3 for 24 h for cytotoxicity assay. Pyroptosis was induced according to the previously published procedure.13) Briefly, 1 × 106/mL cells were cultured with RPMI 1640 medium containing 2% FBS overnight and then treated with 1 µg/mL LPS for 3 h, followed by 8 µg/mL Nigericin treatment for 1 h. Vitamin K were added to the cultures 1 h before pyroptosis. The relative lactate dehydrogenase (LDH) release, cell viability and the pyroptosis signaling were then analyzed unless otherwise indicated.
Cell Viability AssayThe cell viability was analyzed with Cell Counting Kit-8 (CCK-8, Dojindo, Shanghai, China). A total of approximately 1 × 105 cells were seeded in 96-well plates with 100 µL medium each well. At the end of drug treatment or induction of pyroptosis, cells were incubated with 10 µL of CCK-8 solution at 37 °C, 5% CO2 for 1 h. The absorbance of 490 nm wavelength light was then measured with the Model 680 Microplate Reader (Bio-Rad, Richmond, CA, U.S.A.).
Relative LDH ReleaseCulture supernatant was collected following pyroptosis induction and the LDH activity was measured with the CytoTox96 Cytotoxicity Assay kit (Promega, Madison, WI, U.S.A.), according to the instructions from the manufacturer. The absorbance of 490 nm wavelength light was measured with the Model 680 Microplate Reader. The relative LDH release was calculated by the formula: (ODsample − ODblank)/(ODcell lysate − ODblank) × 100%.
Calcein AM/Propidium Iodide (PI) StainingThe Calcein AM/PI double staining kit (BestBio, Shanghai, China) was used to observe the ratio of living and dead cells after pyroptosis. The living cells were stained with Calcein AM (green) and the dead cells were stained with PI (red). After the induction of pyroptosis, cells were mixed with 1× assay buffer and were stained with 2 µM calcein AM and 4.5 µM PI per well at 37 °C for 20 min. The images were immediately taken under a fluorescence microscope (Olympus IX51, Shinjuku, Tokyo, Japan).
Western BlotThe cells were collected and lysed with 2× Laemmli sample buffer before boiling for 10 min. The equal amounts of protein were loaded to each lane and separated by electrophoresis on sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) gel. Then the protein was transferred to polyvinylidene difluoride (PVDF) membrane. The membrane was blocked with 5% non-fat milk for 1.5 h at room temperature and incubated at 4 °C overnight with the primary antibodies. After washing with Tris-buffered saline (TBS) supplemented with 0.1% Tween-20 (TBST), the membrane was incubated with HRP-labeled Goat anti-Mouse immunoglobulin G (IgG) or Goat anti-Rabbit IgG secondary antibodies (Thermo Fisher Scientific, Waltham, MA, U.S.A.) at room temperature for 1.5 h. Luminescence was generated after the membranes were exposed to Super Signal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific) and detected by the ChemiDoc XRS system (Bio-Rad).
Measurement of Caspase-1 ActivityThe activity of caspase-1 was detected using the Caspase 1 Activity Assay Kit (Beyotime, Haimen, Jiangsu, China), following the instruction from the manufacturer. Cells were collected with lysis buffer and incubated on ice for 15 min. Cell lysate was centrifuged at 16000 × g for 15 min and then the supernatant was transferred to a new tube. The samples were incubated with acetyl-tyrosine (Tyr)-valine (Val)-alanine (Ala)-aspartic acid (Asp) p-nitroanilide, the substrate of caspase-1, at 37 °C for 2 h. The absorbance of 405 nm wavelength light was measured with the Model 680 Microplate Reader. The activity of caspase-1 was calculated according to the standard curve.
Animals and TreatmentEight-week old, male C57BL/6 mice were housed in an animal room that was maintained at 18–22 °C and 60% humidity with a 12-h light/dark cycle. All the experimental procedures were approved by the Ethics Committee of Nanchang University and were performed in accordance to the guidelines of Animal Use and Care of NIH. Mice were randomly divided into control, LPS-, vitamin K3 (25 mg/kg)- and vitamin K3 (50 mg/kg)-treated groups. Vitamin K3 was administered intraperitoneally 1 h before LPS (20 mg/kg, intraperitoneally) challenge. The control group was injected with saline.
Histology AnalysisMice were sacrificed after 24 h of LPS challenge. Lungs, Kidneys and livers were taken and were fixed in 4% paraformaldehyde. Tissue samples were processed by routine methods and embedded in paraffin wax. Sections of 5 µm thickness were placed onto glass slides and stained with hematoxylin and eosin. Photomicrographs were taken by a light microscope (Olympus BX51).
Detection of IL-1β and IL-6 in the SerumThe levels of IL-1β and IL-6 were measured in the serum of mice, after 24 h of LPS challenge, using the QuantiCyto® Mouse IL-1β enzyme-linked immunosorbent assay (ELISA) kit (Neobioscience, Shenzhen, China) and Mouse IL-6 Uncoated ELISA kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. Absorbance at 450 nm wavelength was measured. The IL-1β and IL-6 levels were determined by interpolation onto absorbance curves generated by recombinant IL-1β and IL-6 protein standard using the Model 680 Microplate Reader.
Myeloperoxidase (MPO) Activity DetectionThe MPO activity of lung tissues were detected by the Myeloperoxidase assay kit (Nanjing Jiancheng, China) according to the manufacturer’s instructions.
ImmunofluorescenceIn the wake of dewaxing, rehydration and antigen recovery, the paraffin-embedded lung sections (4 µm) were permeabilized with 0.3% Triton X-100 and washed 3 times for 15 min each, and blocked with 5% bovine serum albumin (BSA) for an additional 60 min. Sections were incubated with primary antibodies (F4/80 and NLRP3) at 4 °C overnight. Alexa Fluor 488 and Alexa Fluor 594 (Invitrogen) were utilized as secondary antibodies. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI).
Statistical AnalysisAll data were expressed as mean ± standard deviation (S.D.) of at least three independent experiments. Statistical analysis was performed with GraphPad Prism 7.0 software. Differences between the groups were compared using one-way ANOVA analysis followed by a Tukey post hoc test. An unpaired Student’s t-test was used to compare data between two groups. A value of p < 0.05 was considered statistically significant.
RESULTS
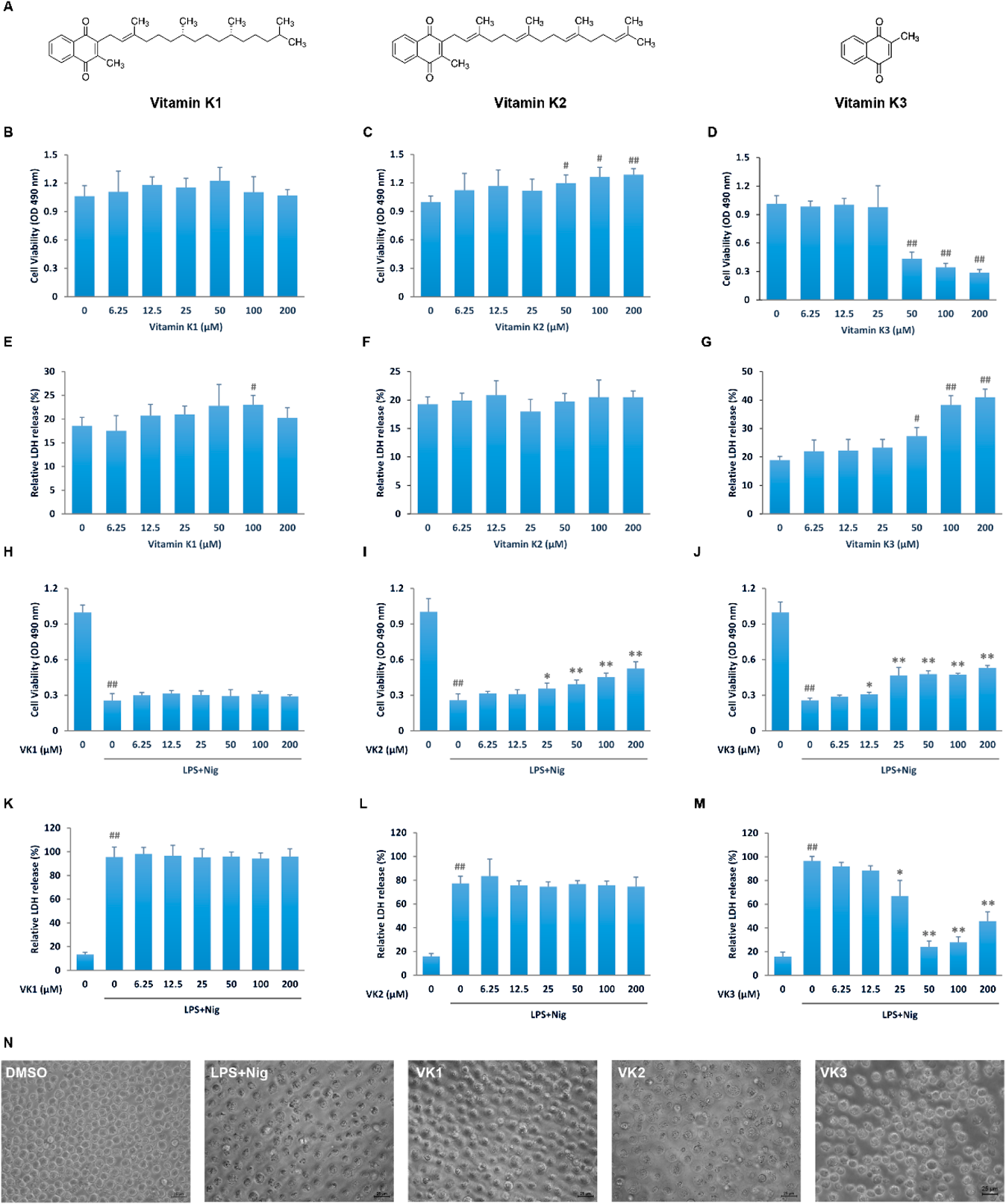
Identification of the Protective Effects of Vitamin K Family on Pyroptosis in THP-1 CellsIn order to analyze the function of vitamin K family on pyroptosis, we established a cell model of pyroptosis in THP-1 cells. After stimulated with 1 μg/mL of LPS for 3 h, the cells were further treated with 0, 2, 4, and 8 μg/mL nigericin for 30 min or 1 h. With the increased concentration of nigericin, cell viability was significantly reduced whereas the relative LDH release was increased (Supplementary Figs. 1A, B). Both 4 μg/mL and 8 μg/mL nigericin treated THP-1 cells showed compromised cell membrane integrity by swelling and fragmentation, which are features of pyroptosis (Supplementary Fig. 1C). NLRP3 expression is significantly increased by nigericin treatment, indicating an activation of inflammasome. More importantly, the activating cleavage of GSDMD also dose dependently elevated by nigericin in both time points (Supplementary Figs. 1D–G). Based on these data, we carried on our further experiments with 8 μg/mL nigericin for 1 h.
Vitamin K1 and K2 did not show any cytotoxicity to THP-1 cells up to the concentration of 200 μM. However, vitamin K3 is cytotoxic to THP-1 cells at 50 μM or above (Figs. 1B–G). In the LPS plus nigericin pyroptotic model, vitamin K1 did not show any effect (Figs. 1H, K). Vitamin K2 dose dependently improved cell viability (Fig. 1I) but did not repress LDH release (Fig. 1L). Interestingly, 25 μM or higher concentration of vitamin K3 pretreatment significantly rescued pyroptosis by increasing cell survival and decreasing LDH release (Figs. 1J, M). Cell swelling and breakdown were also diminished by vitamin K3 compared with pyroptosis model group (Fig. 1N). These results all indicate that vitamin K3, but not vitamin K1 or K2, is a potential inhibitor of pyroptosis in THP-1 cells.
Vitamin K3 Concentration-Dependently Suppresses Pyroptosis in THP-1 CellsAs vitamin K3 at higher than 50 μM exhibits cytotoxicity, we further analyzed its protective function at lower doses (5–30 μM). Vitamin K3 enhanced cell viability and suppressed LDH release in a concentration dependent manner with the pyroptosis induction (Figs. 2A, B), suggesting a protective function at these low concentrations. In addition, 20 and 30 μM vitamin K3 also drastically increased Calcein AM stained (living) while decreased PI stained (dead) cells (Fig. 2C). Low doses of vitamin K3 may thus rescue LPS plus nigericin induced pyroptosis in THP-1 cells.
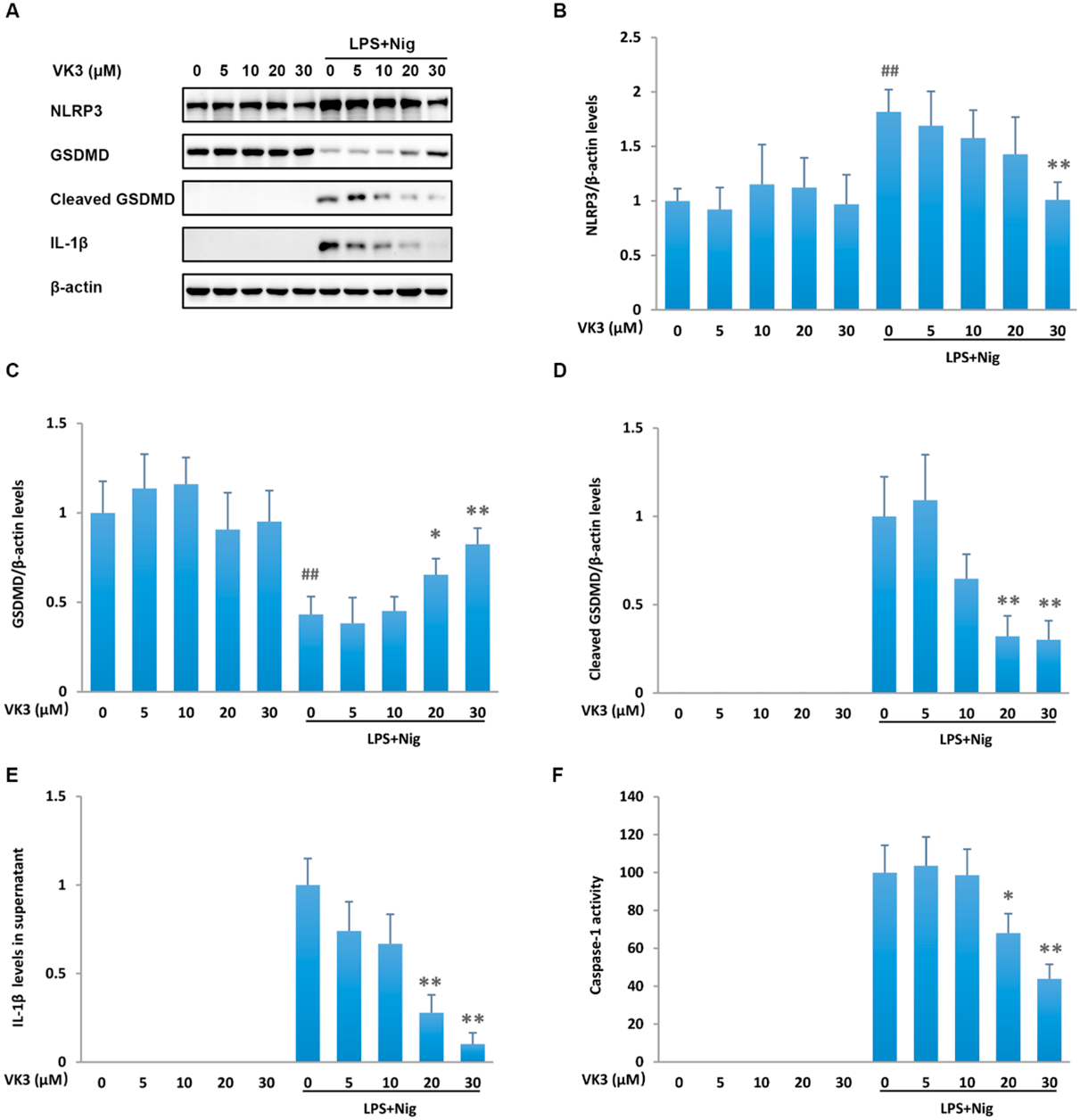
Vitamin K3 Suppresses NLRP3/Caspase-1 Pyroptotic PathwayTo understand the mechanism by which vitamin K3 rescues pyroptosis, we examined the signaling pathways of pyroptosis. LPS plus nigericin stimulation drastically upregulated pyroptosis signal events, including NLRP3 expression, GSDMD cleavage, caspase-1 activation, as well as IL-1β secretion. Vitamin K3 did not modulate the pyroptotic pathway molecules in resting THP-1 cells. However, it dose dependently down-regulated the protein expression of NLRP3 (Figs. 3A, B), suppressed the cleavage of GSDMD (Figs. 3A, C, D), and inhibited the activation of caspase-1 (Fig. 3F). The secretion of IL-1β was also reduced by vitamin K3 treatment in a dose dependent manner (Figs. 3A, E). The suppression of NLRP3/Caspase-1 pyroptotic pathway may be in the center of the rescuing function of vitamin K3 on pyroptosis.
Vitamin K3 Modulates NF-κB and JNK Signaling Pathway in Pyroptotic THP-1 CellsPrevious reports suggest that vitamin K3 inhibits inflammation through modulating the NF-κB and MAPK pathways. Since these pathways direct the activation of inflammasome, a critical pyroptotic signal component, we went on to analyze if vitamin K3 regulates these pathways in pyroptotic THP-1 cells. LPS drastically increased the phosphorylation of p65, IκBα and JNK, as well as the degradation of IκBα. Preincubation with vitamin K3 dose dependently reversed these changes. Phosphorylation of ERK1/2 and p38 after LPS stimulation was not modulated by vitamin K3 (Fig. 4). These results suggest that vitamin K3 inhibits NF-κB and JNK pathways, which may be responsible for its suppressive effect on NLRP3 expression and inflammasome activity.
Vitamin K3 Suppresses Systemic Inflammation and NLRP3 Activation in LPS-Challenged MiceWe further established LPS-induced systemic inflammation model to verify the anti-inflammatory effect of vitamin K3 in mice. LPS caused severe inflammatory infiltration and tissue damage in lungs, kidneys and livers. Vitamin K3 at 25 and 50 mg/kg significantly improved histological changes in LPS-challenged mice (Fig. 5A). The elevated LDH activity, IL-1β and IL-6 levels in serum were observed after LPS exposure, whereas 50 mg/kg vitamin K3 suppressed LDH release and both doses of vitamin K3 reduced IL-1β and IL-6 production (Figs. 5B–D). The decreased MPO activity detected in vitamin K3-treated mice indicated that vitamin K3 could reduce the infiltration of neutrophils (Fig. 5E). The F4/80 staining, as a major macrophage marker showed lower infiltration of macrophages after vitamin K3 treatemetn (Fig. 5F). Moreover, the double immunofluorescence staining showed that vitamin K3 significantly decreased the NLRP3 expression in F4/80+ cells. These results suggest that vitamin K3 prevents systemic inflammation and organ injury probably via inhibition of NLRP3 activation in monocytes/macrophages.
DISCUSSION
Inflammation is an important pathological event in a vast variety of diseases.4) It can be exacerbated by pyroptosis, a recently discovered type of programmed cell death.10,11) Although many studies have pointed out a critical role of pyroptosis in many diseases, possible ways to inhibit pyroptosis is still elusive. In our present study we found vitamin K3 significantly rescued LPS plus nigericin induced pyroptosis in THP-1 cells, probably though suppressing the NF-κB and JNK pathways. Our results suggest that vitamin K3 may serve as a nutritional supplement alleviating inflammatory diseases.
Vitamin K is a fat-soluble vitamin best known for its function in coagulation. It is mostly comprised of two forms, vitamin K1, also known as phylloquinone or phytonadione, and vitamin K2, a series of menaquinones (MKs). Vitamin K1 an K2 both support the post-translational modification of vitamin K dependent proteins by acting as cofactors of γ-glutamyl carboxylase, an enzyme converting glutamic acid into calcium binding γ-carboxyglutamic acid residues. Such reaction is essential for the activity of many coagulation factors, and it claims the major intra-hepatic function of vitamin K1 and K2.1,3) Extrahepatically, many proteins related to mineralization also require this post-translational modification and rely on vitamin K1 and K2 as cofactors. Vitamin K1 and K2 regulate bone homeostasis and cardiovascular pathogenesis in such manner.1,3) Vitamin K1 and K2 both have 2-methyl-1,4-naphtoquinone double ring but differ in their lipophilic side chain. The common backbone, 2-methyl-1,4-naphtoquinone double ring, is designated as vitamin K3, also called menadione.1,3) Paradoxically, vitamin K3 is not a common component of food and mostly considered as an intermediate metabolite in the transition from vitamin K1 to K2. Vitamin K3 has minimal typical function of vitamin K1 or K2 as mentioned above.1) Instead, it is better documented for its function in regulating reactive oxygen species and apoptosis.14,15) In the present study, we found vitamin K3 significantly alleviated pyroptosis, while vitamin K1 and K2 did not. Our discovery adds a new specific function of vitamin K3 as a pyroptosis inhibitor. It is worthwhile to further discuss using vitamin K3 as supplement for patients with inflammatory diseases associated with pyroptosis.
Pyroptosis is a recently discovered pathway of programmed cell death, which is triggered by inflammation and enhances inflammation.10,11) Apart of its role as cofactor of γ-glutamyl carboxylase, vitamin K also exerts anti-inflammation activity independent of γ-glutamyl carboxylase.1,3) Unlike the function as a cofactor of γ-glutamyl carboxylase, which is dependent on the lipophilic side chains, the anti-inflammatory function of vitamin K is dependent on the naphthoquinone ring, which is the backbone structure shared by all members of vitamin K.1) Suppressing inflammation is thus a feature shared by vitamin K1, K2, and K3. Vitamin K represses inflammation mainly though blocking the NF-κB signal pathway.1,3) By reducing the phosphorylation of IκB kinases, endogenous inhibitors of NF-κB transcription factor IκBs would not phosphorylate and degrade, and therefore vitamin K suppress the downstream transcription of inflammatory factors. In addition, vitamin K3 also suppresses MAPK pathways, which are important signals for inflammation.16) These anti-inflammatory actions of vitamin K have been confirmed in vitro and in vivo in LPS treatment models.1,3) Consistently, we also found vitamin K3 inhibits NF-κB and JNK pathways after the treatment with LPS. The present LPS plus nigericin induced pyroptosis model includes LPS as the major stimulant to initiate the inflammatory signal. Vitamin K3 may thus suppress the signal at the very beginning of the signal cascade of pyroptosis.
NF-κB is the major upstream signal for pyroptosis via the upregulation of inflammasome component NLRP3.17) JNK, on the other hand facilitates the activation of caspase-1.9) Without sufficient NLRP3 and active caspase-1 inflammasome will be compromised, so will the cleavage activation of IL-1β and GSDMD, the ultimate downstream executors of pyroptosis pathway. We found a drastic upregulation of the pyroptosis pathway in the LPS plus nigericin treatment, and vitamin K3 dose dependently suppressed NLRP3 expression, caspase-1 activation, GSDMD cleavage, and IL-1 secretion, which corroborates with the finding that vitamin K3 suppress overall inflammasome activation by suppressing NF-κB and JNK pathways. Moreover, NLRP3 is transcriptionally regulated by NF-κB. Previous study has shown that both signaling molecules MyD88 and TRIF of the NF-κB signaling pathway regulated the induction of NLRP3 in response to TLR ligands.18) And the previous study has demonstrated that pyroptosis in macrophage was decreased after using the inhibitor of NF-κB phosphorylation.19) In addition, JNK inhibitor was reported to significantly attenuate surgery-induced cognitive impairments through inhibiting pyroptosis, inflammatory responses, and reducing immunoreactivity of NLRP3 and gasdermin D N terminus in hippocampal microglia.20) However, our study cannot explain why vitamin K1 and K2 fail to alleviate pyroptosis, which are also proved to inhibit LPS induced inflammation with their naphthoquinone rings.1,3) Given the prevalence of vitamin K1 and K2 in food, it is worthwhile to investigate in the future the mechanism why vitamin K1 and K2 do not ameliorate pyroptosis.
CONCLUSION
In summary, we discovered an anti-pyroptotic function specific to vitamin K3. It is probably mediated by its suppressive effect on NF-κB and JNK pathways. Our present study points out that vitamin K3 may be used as a nutritional supplement for patients with inflammatory diseases contributed and exacerbated by pyroptosis.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (Grant 82070080, 82270094 and 81860020 to YQ, 81873659 to HBX, and 81960008 to YL), Jiangxi Provincial Department of Science and Technology, China (20192ACBL20034 and 20202BABL206021 to ZXL), and National Institute of Health Grant (AI138116 to MF). The financial support provided by China Scholarship Council (201906825031 to YQ) is also acknowledged.
Conflict of Interest
The authors declare no conflict of interest.
Supplementary Materials
This article contains supplementary materials.
REFERENCES
- 1) Simes DC, Viegas CSB, Araujo N, Marreiros C. Vitamin K as a diet supplement with impact in human health: current evidence in age-related diseases. Nutrients, 12, 138 (2020).
- 2) Shearer MJ, Fu X, Booth SL. Vitamin K nutrition, metabolism, and requirements: current concepts and future research. Adv. Nutr., 3, 182–195 (2012).
- 3) Shioi A, Morioka T, Shoji T, Emoto M. The inhibitory roles of vitamin k in progression of vascular calcification. Nutrients, 12, 583 (2020).
- 4) Rock KL, Latz E, Ontiveros F, Kono H. The sterile inflammatory response. Annu. Rev. Immunol., 28, 321–342 (2010).
- 5) Kondylis V, Kumari S, Vlantis K, Pasparakis M. The interplay of IKK, NF-kappaB and RIPK1 signaling in the regulation of cell death, tissue homeostasis and inflammation. Immunol. Rev., 277, 113–127 (2017).
- 6) Mitchell JP, Carmody RJ. NF-kappaB and the transcriptional control of inflammation. Int. Rev. Cell Mol. Biol., 335, 41–84 (2018).
- 7) Thalhamer T, McGrath MA, Harnett MM. MAPKs and their relevance to arthritis and inflammation. Rheumatology (Oxford), 47, 409–414 (2008).
- 8) Song N, Li T. Regulation of NLRP3 inflammasome by phosphorylation. Front. Immunol., 9, 2305 (2018).
- 9) Chen X, Guo X, Ge Q, Zhao Y, Mu H, Zhang J. ER stress activates the NLRP3 inflammasome: a novel mechanism of atherosclerosis. Oxid. Med. Cell. Longev., 2019, 3462530 (2019).
- 10) Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev., 277, 61–75 (2017).
- 11) Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol. Rev., 243, 206–214 (2011).
- 12) Bonilla-Porras AR, Jimenez-Del-Rio M, Velez-Pardo C. Vitamin K3 and vitamin C alone or in combination induced apoptosis in leukemia cells by a similar oxidative stress signalling mechanism. Cancer Cell Int., 11, 19 (2011).
- 13) Zhou Z, Li X, Qian Y, Liu C, Huang X, Fu M. Heat shock protein 90 inhibitors suppress pyroptosis in THP-1 cells. Biochem. J., 477, 3923–3934 (2020).
- 14) Badave KD, Khan AA, Rane SY. Anticancer vitamin K3 analogs: a review. Anticancer. Agents Med. Chem., 16, 1017–1030 (2016).
- 15) Ivanova D, Zhelev Z, Getsov P, Nikolova B, Aoki I, Higashi T, Bakalova R. Vitamin K: Redox-modulation, prevention of mitochondrial dysfunction and anticancer effect. Redox biology, 16, 352–358 (2018).
- 16) Checker R, Sharma D, Sandur SK, Khan NM, Patwardhan RS, Kohli V, Sainis KB. Vitamin K3 suppressed inflammatory and immune responses in a redox-dependent manner. Free Radic. Res., 45, 975–985 (2011).
- 17) Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol., 183, 787–791 (2009).
- 18) Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int. J. Mol. Sci., 20, 3328 (2019).
- 19) Li J, Liu J, Yu Y, Liu Y, Guan X. NF-kappaB/ABCA1 pathway aggravates ox-LDL-induced cell pyroptosis by activation of NLRP3 inflammasomes in THP-1-derived macrophages. Mol. Biol. Rep., 49, 6161–6171 (2022).
- 20) He J, Liu T, Li Y, Mi X, Han D, Yang N, Chen L, Li Y, Hong J, Kuang C, Yuan Y, Cao Y, Han Y, Shi C, Li Z, Guo X. JNK inhibition alleviates delayed neurocognitive recovery after surgery by limiting microglia pyroptosis. Int. Immunopharmacol., 99, 107962 (2021).