2023 年 46 巻 1 号 p. 61-66
2023 年 46 巻 1 号 p. 61-66
Suplatast is a T helper 2 (Th2) cytokine inhibitor. Here, we tested its therapeutic effects using a mouse model of renal interstitial fibrosis caused by unilateral ureteral obstruction (UUO). In this model, suplatast was found to prevent the induced fibrosis in the obstructed kidney when given in the drinking water at 100 mg/kg/d. Mechanistically, suplaplast inhibited the phosphorylation of extracellular signal-regulated kinase (ERK) that was otherwise increased by UUO. Similarly, suplaplast reduced the increased accumulation of KIM-1, transforming growth factor β (TGF-β), type I collagen, interleukin-4 (IL-4), janus kinase (JAK)1 and signal transducer and activator of transcription (STAT)3 mRNA seen in the kidneys of UUO-treated mice. Furthermore, STAT3 phosphorylation, which was stimulated by UUO, was also significantly decreased by suplatast. Collectively, these data show that suplatast reduces UUO-induced renal interstitial fibrosis via mechanisms including a reduction of phosphorylation of ERK and JAK/STAT pathway signaling.
In mice, unilateral ureteral obstruction (UUO) results in interstitial fibrosis in the kidney associated with the occurrence of ischemia, mechanical stretching, hypoxia and oxidative stress.1) Fibrosis causes scarring due to excessive secretion of extracellular matrix (ECM) components by fibroblasts as a result of chronic inflammation.2) Various harmful stimuli, such as those resulting from toxins, pathogens, autoimmune responses, mechanical stresses and macrophage infiltration induce fibrotic cell responses. As we reported earlier, the involvement of inflammatory responses is emphasized by alleviation of fibrosis by specific inhibition of cyclooxygenase (COX) 2.3)
Whereas T helper 1 (Th1) cells are crucial for cellular immunity,4) Th2 cells facilitate the induction and maintenance of chronic inflammatory responses and allergic symptoms by secreting interleukin-4 (IL-4) and IL-5, and recruiting B cells that produce immunoglobulin E (IgE) antibodies.5) An imbalance of Th1 and Th2 activity is implicated in the etiology of numerous different pathologies.6)
Suplatast inhibits Th2 cytokine secretion. Thus far, it is licensed only in Japan. It is active in bronchial asthma, at least in mouse models,7) and prevents bleomycin-induced pulmonary fibrosis.8) Here, we have investigated whether suplatast also reduces fibrosis in a UUO-induced model of renal interstitial fibrosis (RIF) in mice. We report that suplatast ameliorates fibrosis in this model by several mechanisms including a reduction of phosphorylation of extracellular signal-regulated kinase (ERK) and janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway signaling.
Suplatast was sourced from the Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Antibodies specific for ERK1/2 (#9102), phospho-ERK1/2 (Thr202/Tyr204) (#9101), c-Jun N-terminal kinase (JNK) (#9252), phospho-JNK (Thr183/Tyr185) (#4668), p38 mitogen-activated protein kinase (MAPK) (#9212), phospho-p38MAPK (Thr180/Tyr182) (#9211), STAT3 (#12640), and phospho-STAT3 (#9145) (Tyr705), anti-rabbit horseradish peroxidase-conjugated IgG (#7074) were all from Cell Signaling Technology Inc. (Beverly, MA, U.S.A.). Other chemicals and drugs were reagent grade or the highest quality available.
AnimalsFive-week-old male BALB/cCrSlc mice were sourced from Japan SLC Inc. (Hamamatsu, Japan). They were maintained in a 12 h light–dark environment with 3–5 mice in each for at least one week, and then UUO was carried out by surgery, after approval by the Ethics Committee for Animal Experiments. All procedures followed the Guidelines for Animal Experiments of Takasaki University of Health and Welfare and the Japanese Government Animal Protection and Management Law. The number of mice employed in the study was reduced as far as possible consistent with experimental validity, and animal suffering was minimized.
Experimental ProtocolsPerformance of UUO was previously described,9) namely, a midline abdominal incision was made in mice under medetomidine hydrochloride 0.3 mg/kg, midazolam 4 mg/kg, and butorphanol 5 mg/kg intraperitoneal anesthesia. The left ureter was exposed and ligated with 4–0 silk. Controls were sham-operated and treated in the same manner but without UUO ligation. Thirteen days after UUO, urine was collected from the mice over 24 h with the animals in metabolic cages. One day later, blood and kidney samples were taken from anesthetized animals, and immediately stored at −80 °C before use. Following UUO, mice received suplatast daily in their drinking water.
Kidney FunctionCommercial kits were used to quantify serum creatinine, blood urea nitrogen (BUN) and urinary glucose, following the manufacturer’s instructions (Wako Pure Chemical Corporation, Osaka, Japan).
Histopathological AnalysisRIF was determined on paraffin-embedded kidney tissue sections (1 µm) by staining with Masson’s trichrome. The affected area in the outer medulla was quantified with the help of Leica Qwin image analysis software (Leica Microsystems, Tokyo, Japan).
Western BlottingPRO-PREP protein extraction solution (iNtRON Biotechnology, Sungnam, Korea) was used to isolate total proteins from kidney homogenates, followed by Western blotting as described earlier.9) Polyvinylidene difluoride (PVDF) membranes were incubated for 2 h with 0.5% skim milk in Tris-buffered saline with Tween 20, after which they were reacted with a 1 : 1000 dilution of antibodies to ERK, phospho-ERK, JNK, phospho-JNK, p38MAPK, phospho-p38MAPK, STAT3 or phospho-STAT3. Finally, membranes were incubated with a 1 : 3000 dilution of an anti-rabbit horseradish peroxidase-conjugated IgG at 25 °C for 2 h and read out using an enhanced chemiluminescence (ECL) assay kit (GE Healthcare UK Ltd., Little Chalfont, U.K.) and a luminescent image analyzer (LAS-3000, FUJIFILM, Tokyo, Japan). Image Gauge (FUJIFILM) was used to quantify band density.
Real-Time RT-PCRTotal RNA was extracted using GenElute Mammalian Total RNA Miniprep Kits (Sigma-Aldrich, St. Louis, MO, U.S.A.), adhering to the manufacturer’s protocol. RT-PCR was carried out with an Mx3000P analyzer (Stratagene, La Jolla, CA, U.S.A.) using SYBR Premix Ex Taq (TaKaRa Bio., Otsu, Shiga, Japan) for cDNA amplification. Primer sequences except JAK1 and STAT3 are presented in Table 1. Expression of JAK1 and STAT3 was determined by the PrimePCR system from Bio-Rad, customized with primers specific for the indicated genes. RT-PCR conditions were 95 °C for 2 min, 30 cycles of 15 s at 95 °C, 30 s at 56 °C and finally 30 s at 72 °C. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used to normalize gene expression levels.
| Gene | Sense | Antisense | References |
|---|---|---|---|
| KIM-1 | TTAAACCAGAGATTCCCACA | TTGGAGGAGTGGAGGTAGAGA | Abraham et al.19) |
| TGF-β | CCGCAACAACGCCATCTATGA | GGGGGTCAGCAGCCGGTTAC | Ouyang et al.20) |
| Col-1 | GAGCGGAGAGTACTGGATCG | TACTCGAACGGGAATCCATC | Fu et al.21) |
| TNF-α | GCATGATCCGCGACGTGGAA | AGATCCATGCCGTTGGCCAG | Ranganathan et al.22) |
| IL-4 | CTTCCAAGGTGCTTCGCATA | CTTATCGATGAATCCAGGCAT | Lee et al.23) |
| IL-5 | GACGAGGCAGTTCCTGGAT | GCATATGGTATCCCTTGCATT | Lee et al.23) |
| GAPDH | TTCACCACCATGGAGAAGGC | GGCATGGACTGTGGTCATGA | Mitazaki et al.24) |
Protein concentrations were determined by a bicinchoninic acid assay (Thermo Fisher Scientific, Rockford, IL, U.S.A.) with bovine serum albumin as the standard.
Data AnalysisResults are expressed as means ± standard error of the mean (S.E.M.), and significance was determined by one-way ANOVA with Dunnett’s test for multiple comparisons. p < 0.05 was taken as significant.
We performed UUO in mice given or not given suplatast. UUO treatment caused significant increases in urinary glucose, BUN and serum creatinine (Fig. 1). Suplatast administered to mice in the drinking water at an average dose of 100 mg/kg/d did not affect urinary glucose, BUN and serum creatinine.
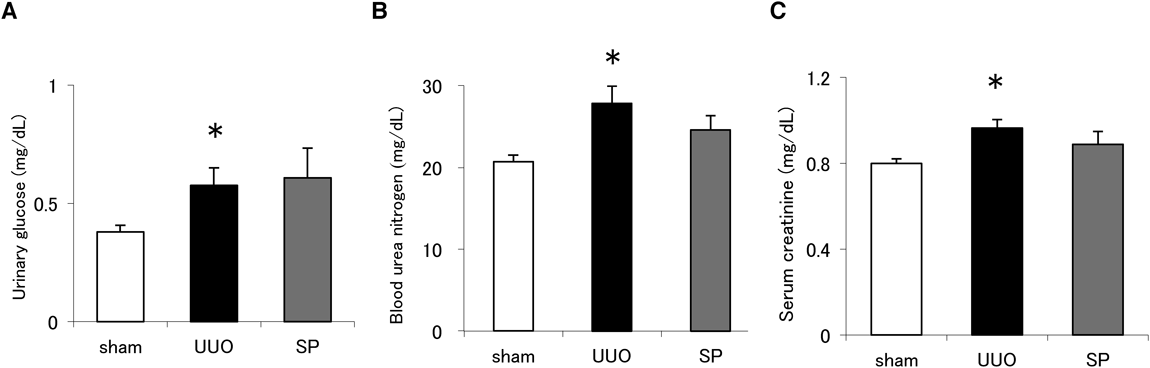
(A) Urinary excretion of glucose, (B) BUN and (C) serum creatinine in sham-operated mice (sham), UUO-treated mice or UUO mice given suplatast (SP) at day 14 after surgery. N = 8–10 per group. Data are means ± S.E.M. *Significantly different from sham at p < 0.05.
To investigate any effect of suplatast on UUO-induced RIF, we quantified the fibrotic area by Masson’s trichrome staining 14 d after the UUO procedure. The renal tubules of the mice were partially dilated after UUO, and the estimated fibrotic area was significantly increased. However, the administration of suplatast significantly reduced the affected area (Fig. 2). Suplatast did not affect percentage of fibrotic area in mice without UUO treatment (3.6 ± 0.7%).

Masson’s trichrome staining. Scale bar = 100 μm. Magnification, 200×. (D) Fibrotic are quantification. N = 5 per group. Data are means ± S.E.M. *Significantly different from sham at p < 0.05. †Significantly different from UUO at p < 0.05.
The fibrotic process involves the activation of MAPKs,10) and for this reason we investigated MAPK status in animals receiving UUO with or without suplatast. We noticed a significant increase in ERK and p38 MAPK phosphorylation following UUO (Figs. 3A, B); suplatast significantly reduced the former but not the latter, whereas there were no differences for JNK in any case (Fig. 3C).
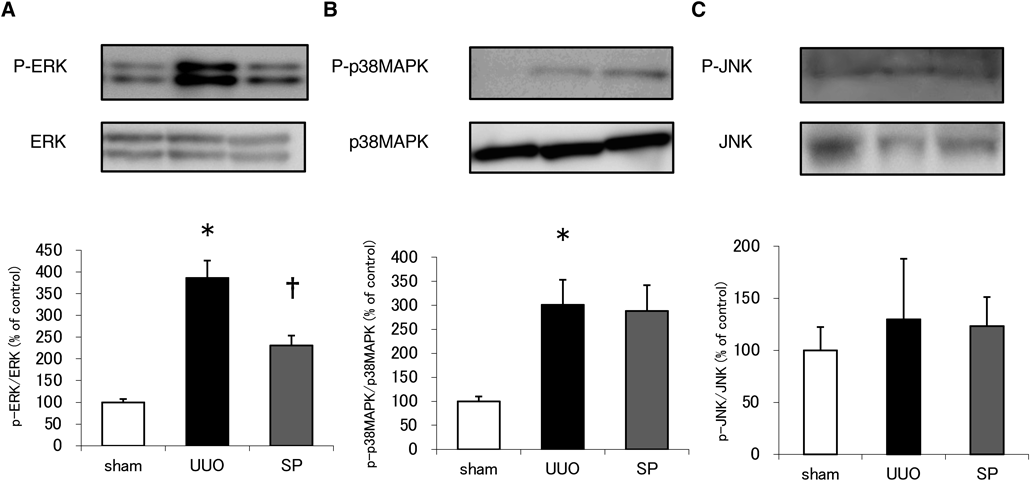
Western blotting for ERK or phospho-ERK (A), p38MAPK or phospho-p38MAPK (B), JNK or phospho-JNK (C). Data are means ± S.E.M. N = 6–8 per group. Phosphorylation normalized to the total levels of ERK, p38MAPK or JNK is presented as a percentage of the sham control. *Significantly different from sham at p < 0.05. †Significantly different from UUO at p < 0.05.
KIM-1 is a general injury marker of inflammation regulation, and transforming growth factor β (TGF-β) is a well-recognized mediator of collagen production involved in fibrogenesis. Hence, we investigated KIM-1 and TGF-β by RT-PCR and found that the accumulation of mRNA for both was increased following UUO. We found that suplatast prevented this to a significant degree (Figs. 4A, B). We also quantified collagen expression in this model and found that suplatast significantly reduced type I collagen mRNA (Fig. 4C). It has been reported that tumor necrosis factor (TNF)-α is also implicated in modulating the division and apoptosis of renal tubular and interstitial cells in obstructive renal injury.11) Here, we found that TNF-α mRNA levels were also increased following UUO, and that this was also reduced by suplatast, although again without achieving statistical significance (Fig. 4D).
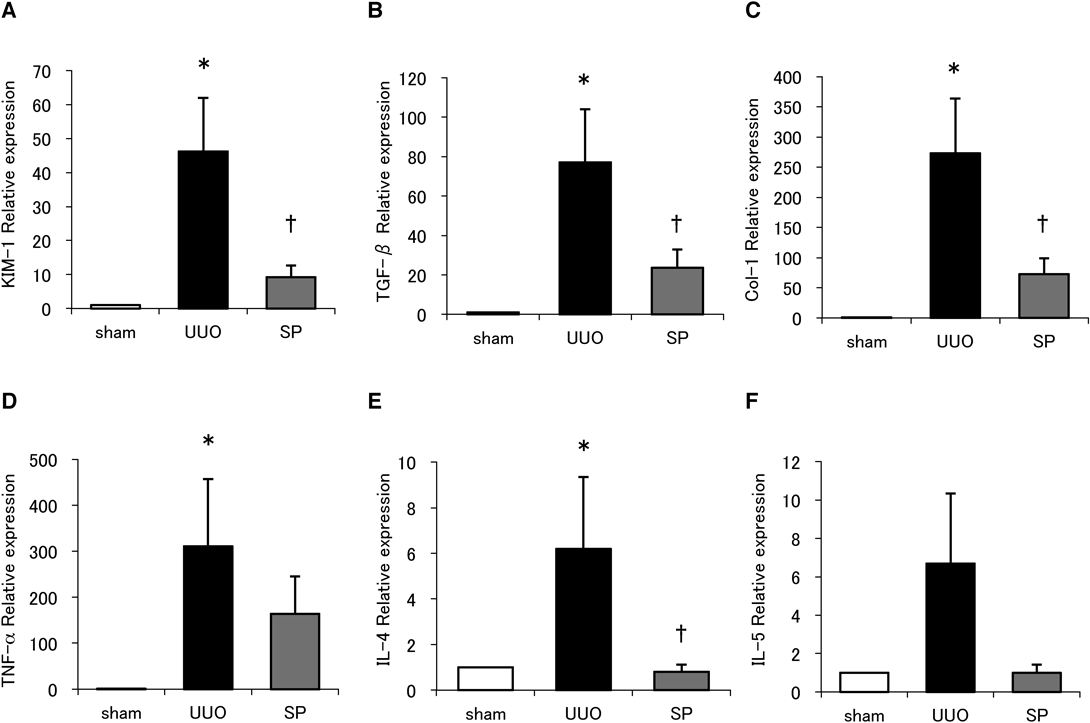
N = 7–10 per group. Data are means ± S.E.M. *Significantly different from sham at p < 0.05. †Significantly different from UUO at p < 0.05.
Because suplatast blocks Th2 cytokine production, we also quantified IL-4 and IL-5 mRNA. We found that both IL-4 and IL 5 were increased after UUO, and that suplatast significantly inhibited IL-4 mRNA accumulation (Fig. 4E), but did not reduce to a significant level in that of IL-5 (Fig. 4F).
JAK/STAT mRNA Expression and STAT PhosphorylationMany reports implicate JAK/STAT signaling in inflammatory and autoimmune diseases. Therefore, we next sought to determine whether JAK/STAT mRNA expression and signaling was altered by UUO in animals treated or not with suplatast. Since Bai et al. reported that Ruxolitinib, a selective inhibitor of JAK 1/2, ameliorated UUO-induced RIF via reducing STAT3 activation, we focused on JAK1 and STAT3.12) We found that the increase of JAK1 and STAT3 mRNA levels following UUO was significantly reduced by suplatast (Figs. 5A, B). Finally, we quantified STAT3 phosphorylation and found that suplatast also significantly inhibited its UUO-induced increase (Fig. 5C).
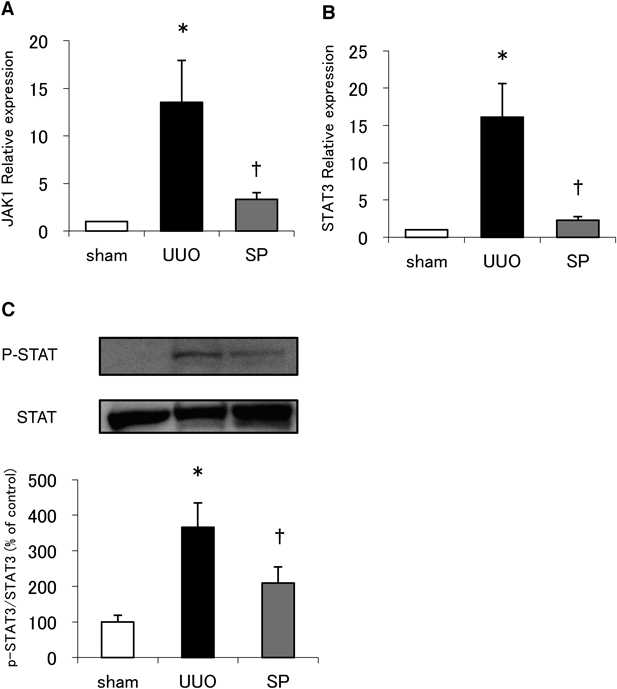
Effect of suplatast treatment on STAT3 phosphorylation in UUO kidneys (C). Western blotting with anti-STAT3 or anti-phospho-STAT3 antibodies. N = 5–8 per group. Data are means ± S.E.M. Phosphorylation was normalized to the total levels of STAT3 and presented as a percentage of the sham control. *Significantly different from sham at p < 0.05. †Significantly different from UUO at p < 0.05.
Here, we report that the Th2 cytokine inhibitor suplatast ameliorated UUO-induced RIF in a mouse model. Furthermore, inhibition of ERK and STAT3 phosphorylation and the expression of mRNA for KIM-1, TGF-β, type I collagen, IL-4, JAK1 and STAT3 was associated with this beneficial effect.
A close association between the occurrence of inflammation and the development of fibrosis has been extensively discussed elsewhere. There is strong evidence for the involvement of T lymphocytes in these processes; their recruitment and activation might represent a crucial early event mediating the beginning of fibrogenesis in the kidney, because typically macrophage influx into damaged kidneys occurs afterwards.13) Moreover, Liu reported that in a mouse model of Th2-vs-Th1 reconstitution, the former developed renal fibrosis more readily than the latter. Consistent with this, we show here that the Th2 cytokine inhibitor, suplatast, reduced RIF in the UUO model. Thus, inhibition of Th2 cytokine production may have beneficial anti-fibrotic effects.
When cytokines bind their receptors, signals are transduced through a common pathway, the JAK/STAT pathway, which can either result in activation or repression of DNA transcription.14) Ruxolitinib, a selective inhibitor of JAK1/2, ameliorated UUO-induced RIF via reducing STAT3 activation.12) In a similar UUO model in rats, treatment with fluorofenidone, a novel pyridone agent, reduced STAT3 activation and prevented renal fibrosis progression.15) In the present study, suplatast significantly reduced ERK and STAT3 phosphorylation after UUO. We previously reported that N-acetyl cysteine, a reactive oxygen species inhibitor, reduces RIF in this model by inhibiting phosphorylation of ERK.9) Xuan et al. reported that ischemic proconditioning activated ERK, induced phosphorylation of STAT3.16) Thus, it is possible that the inhibition of ERK by suplatast reduced the phosphorylation of STAT3. Therefore, we conclude that the JAK/STAT pathway is involved in the development of fibrosis, and that its suppression may be an effective method to reduce fibrosis.
Numerous reports have revealed that suplatast is effective against lung disease, for example, radiation-induced lung injury which is reduced by suplatast via suppression of oxidative stress.17) Suplatast also prevents bleomycin-induced pulmonary fibrosis by inhibiting the production of monocyte chemoattractant protein-1 in alveolar macrophages.8) Yanagihara et al. reported that suplatast has no antagonistic activity on IL-4 function, but blocks IL-4 production by a Th2 clone.18) In the present study, we found that IL-4 mRNA levels were increased after UUO, which was prevented by suplatast. Therefore, it seems that the decrease of IL-4 by suplatast suppresses the JAK/STAT pathway, resulting in the alleviation of RIF. It remains necessary to dissect the detailed mechanisms of action of suplatast in future studies.
To conclude, here we have provided evidence that the Th2 cytokine inhibitor suplatast reduces RIF in a mouse UUO model. Although we have not determined the exact mechanisms employed by suplatast to prevent renal fibrosis, these are likely to include the inhibition of ERK and STAT3 phosphorylation and ameliorated KIM-1, TGF-β, type I collagen, IL-4, JAK1 and STAT3 production. Therefore, outcomes for patients with RIF may be improved by administering Th2 cytokine inhibitors as well as other renal protective medicines.
The authors declare no conflict of interest.