2023 年 46 巻 1 号 p. 67-73
2023 年 46 巻 1 号 p. 67-73
Osteosarcoma (OS), one of the bone tumors, occurs mainly during childhood and adolescence and has an incidence rate of 5%. Cinnamtannin B-1 (CTB-1) is a natural trimeric proanthocyanidin compound found in plants Cinnamomum zeylanicum and Laurus nobilis. Previously, several articles have demonstrated that CTB-1 exerts a certain effect on melanoma and cervical cancer. However, their role in OS remains unclear. In this study, CTB-1 was found to inhibit the proliferation of OS cancer cells, with the dose of CTB-1 positively correlated to the survival rate of HOS and MG-63 cells. Recently, microRNAs (miRNAs) were also reported to play an important role in tumor proliferation. Hence, we performed the miRNA sequencing analysis after CTB-1 treatment to identify miRNA levels in HOS cells and found that the expression of miR-1281 was significantly upregulated. According to the functional analysis, CTB-1 inhibited the growth and migration of OS by upregulating the expression of miR-1281. Additionally, miR-1281 acted as a sponge for Peptidylprolyl Isomerase F (PPIF), inhibiting its expression levels. The rescue experiments revealed that CTB-1 delayed the development of OS by regulating the miR-1281/PPIF pathway. Hence, our findings suggested that CTB-1 inhibited the cell growth, invasion, and migration of OS by upregulating miR-1281 and inhibiting PPIF expression, thereby providing a possible target drug for OS treatment.
Osteosarcoma (OS) is a cancer of the primary bone, which usually occurs during childhood and adolescence.1,2) Currently, the 5-year survival rate of OS patients is low, at around 60%. Additionally, a large proportion of survivors undergo amputation, which seriously affects their QOL.3) To date, the main treatments for OS remain radiotherapy, surgery, and neoadjuvant chemotherapy.4) However, the primary OS cells may still metastasize to adjacent tissues and organs, causing treatment failure, whose mechanism remains unclear.5) Therefore, it becomes important to study OS to explore the underlying mechanisms and its possible treatments.
Agents extracted from plants are increasingly being used in drug discovery and development.6,7) Cinnamtannin B-1 (CTB-1) is an A-type proanthocyanidin, initially isolated from the bark of cinnamon. It exhibits strong anti-tumor activity both in vivo and in vitro. CTB-1 is involved in many cellular processes, including cell proliferation, migration, and apoptosis.8) One study reported that CTB-1 inhibited cell proliferation, migration, and invasion of non-small cell lung cancer, leading to cancer progression.9) Moreover, CTB-1 was useful in the treatment of colon cancer, where it inhibited disease development through the regulation of the p53 pathway.10) However, the involvement of CTB-1 in OS proliferation and metastasis remains unclear. Therefore, our study aimed to investigate the biological functions and potential underlying mechanisms of CTB-1 in OS.
Human osteosarcoma cells (HOS, 143B, MG63, and U2OS) and healthy osteoblasts (hFOB 1.19) were obtained from China Center for Type Culture Collection (Shanghai, China). The cells were cultured at 37 °C in 5% CO2 conditions in Roswell Park Memorial Institute (RPMI) –1640 medium (Invitrogen, Carlsbad, CA, U.S.A.) containing 10% fetal bovine serum (FBS, Hyclone, Logan, UT, U.S.A.). Additionally, miR-1281 mimics, miR-1281 inhibitor, and the corresponding negative control (miR-NC) were obtained from GenePharma (Shanghai, China). For cell transfection, Lipofectamine® 2000 was obtained from Invitrogen. Transfection was performed as per the manufacturer’s protocol.
Quantitative Real-Time PCR (qRT-PCR)Total RNA in cells was extracted using TRIzol (Invitrogen) reagent, while the cDNA was synthesized using Verso cDNA synthesis kit (Thermo Fisher Scientific, CA, U.S.A.). The expression levels of peptidylprolyl isomerase factor F (PPIF) were measured using the SYBR Premix EX Taq II (TaKaRa, Dalian, China), while the expression of miR-1281 was detected using the TaqMan microRNA assay (Applied Biosystems; Thermo Fisher Scientific), where U6 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used as the microRNA (miRNA) and mRNA controls, respectively. The gene levels were calculated using the 2−ΔΔCt approach. The primers used are listed in Table 1.
| Name of primer | Sequence(5′→3′) |
|---|---|
| PPIF-F | TGGTGACACAGGCCACAGAC |
| PPIF-R | CCGGAGCACAGGAGCTTACA |
| miR-1281-F | ACACTCCAGCTGGGTCGCCTCCTCC |
| miR-1281-R | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGGGGAGAGG |
| GAPDH-F | GGTGAAGGTCGGAGTCAACG |
| GAPDH-R | CAAAGTTGTCATGGATGHACC |
| U6-F | CTCGCTTCGGCAGCAGCACATATA |
| U6-R | AAATATGGAACGCTTCACGA |
The Cell Counting Kit-8 (CCK-8, Dojindo, Kumamoto, Japan) assay was performed by culturing the transfected osteosarcoma cells (2 × 103/well) into a 96-well plate. After 48 h of incubation, CCK-8 solution (10 µL) was added to all the wells, followed by further incubation for 2 h. Finally, the absorbances (optical density (OD)) were measured at 450 nm.
5-Ethynil-2′-deoxyuridine (EdU) AssayWe utilized the Click-iT® EdU imaging kit (Invitrogen) to assess OS cancer multiplication capacity. HOS and MG63 cells were vaccinated on 96-well plates at 1 × 104/well. EdU was included to the medium after CTB-1 treatment for 2 h. After washing with phosphate-buffered saline (PBS) and fixing with 4% formaldehyde, the cells were incubated with the Click-iT reaction cocktail in the dark. Nuclei were counterstained with 4′-6-diamidino-2-phenylindole (DAPI) (Beyotime, Shanghai, China) and observed under a confocal microscope (ZIESS, Oberkochen, Germany).
Transwell AssayCell invasion and migration were detected using the Transwell assay. The Transwell chambers (Corning Inc., Corning, NY, U.S.A.) were placed into a 24-well plate. After transfection, 2 × 105 cells were pelleted and suspended in RPMI-1640 medium without serum (300 µL). The obtained cell suspension was added to the top chamber while RPMI-1640 medium (600 µL) supplemented with 10% fetal bovine serum (FBS) was added to the bottom chamber. After 48 h, the medium was removed, cells were washed with PBS and fixed with 4% paraformaldehyde (PFA) for 30 min. The cells were then stained with 0.5% crystal violet for 10–15 min and the cell migration was detected under a microscope at a magnification of 200×.
Western BlottingTotal protein was extracted using the Radio immunoprecipitation assay (RIPA) lysis buffer (Beyotime), while protein content was measured using the BCA kit (Beyotime). The obtained protein aliquots were separated using Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE, 10%), followed by protein transfer onto polyvinylidene fluoride (PVDF) membranes (Bio-Rad Laboratories, Hercules, CA, U.S.A.). Next, the membranes were blocked in 5% defatted milk under ambient/room temperature (around 25 °C) for 2 h, followed by overnight incubation with primary antibody at 4 °C, including anti-Ki67 (1 : 2000; Abcam, Cambridge, U.K.), anti-GAPDH (1 : 10000; Abcam), and anti-PPIF (1 : 1000; Proteintech, Wuhan, China) antibodies. Next, the membranes were incubated with a corresponding horse radish peroxidase (HRP)-conjugated secondary antibody (1 : 10000; Abcam) at room temperature for 2 h. Finally, the bands were measured using an ECL substrate while the images were obtained using the CLINX ChemiScope 3300 Imager.
Dual-Luciferase Reporter AssayThe PPIF sequence containing the putative binding site of miR-1281 was inserted into the pmirGLO vector for synthesis of luciferase reporter vector PPIF-wild type (PPIF-WT). The core region for miRNA binding on 3′UTR was mutated using a site-directed mutagenesis kit (Thermo Fisher Scientific) to construct control plasmid PPIF-MUT. The HOS and MG63 cells were co-transfected with PPIF-WT (1 µg/mL) or PPIF-MUT (1 µg/mL), and miR-1281 mimic (50 nM) or miR-NC (50 nM). Transfection was performed using lipofectamine 2000 (Invitrogen, Shanghai, China) and as per the manufacturer’s protocol. Finally, the luciferase activity of each group was measured after 24 h using the Dual-Luciferase® Reporter Assay System (Promega, WI, U.S.A.).
Xenograft Mouse ModelThis assay was approved by the Animal Experiment Ethics Committee of Jiangsu University (UJS-IACUC-AP-132). The HOS cells (1 × 107) were administered to BALB/c nu/nu female mice (5-week-old) via subcutaneous injection into their flanks (n = 6 mice each). CBT-1 was given as intragastric administration at a dose of 20 mg/per kg body weight. Later, tumors were measured in two dimensions (length and width) with a digital caliper every three days. The tumor volume (mm3) was determined using the formula V = W2 × L/2, where W and L indicated the shortest and longest diameters in mm, respectively. The tumor weight was measured after 21 d.
Statistical AnalysisData from at least three independent experiments were presented as mean ± standard deviation (S.D.). Statistical analysis was performed using GraphPad Prism software (version 6.01; GraphPad Software, Inc., San Diego, CA, U.S.A.). Additionally, student’s t-test and one-way ANOVA were used to evaluate the difference between two and more groups, respectively. A value of p < 0.05 indicated statistical significance.
The effect of CTB-1 on the anti-proliferation of OS was confirmed by treating the OS cell lines (143B, HOS, U2OS, and MG63) and osteoblast cells (hFOB1.19) with CTB-1 for 48 h. CCK-8 assay was used to plot the growth curve and measure the effect of CTB-1 on cell proliferation. Compared to the untreated group, the OS cells treated with different concentrations of CTB-1 showed a decrease in their survival rate (Fig. 1A). Contrastingly, at a certain range of concentration, osteoblast cell lines were less affected. These results indicated that CTB-1 inhibited the proliferation of OS cells but interfered only slightly with the growth of normal osteoblasts. Additionally, the role of CTB-1 in OS cell proliferation was further verified by EdU analysis and Ki67 test (an indicator of cell proliferation). Figure 1B shows a significantly decreased EdU positivity rate in HOS and MG63 cells after CTB-1 treatment. Consistent with these results, Western blotting also determined the abundance of Ki67 protein, suggesting that CTB-1 inhibited the growth of HOS and MG63 cells at 48 h (Fig. 1C). Overall, our results confirmed that CTB-1 inhibited the proliferation of OS cells.
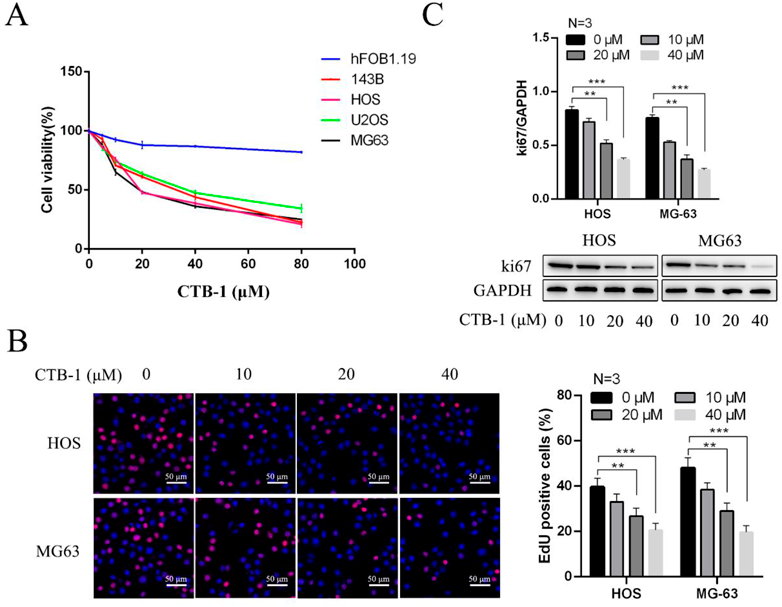
(A) OS cell lines (143B, HOS, U2OS and MG63) and normal human osteoblast cells (hFOB1.19) were treated with CTB-1 for 48 h, and cell proliferation was determined using CCK-8 assays. (B) The ratio of EdU-positive cells to HOS and MG63 cells following 48 h of CTB-1 treatment was visualized by EdU staining. (C) WB assays shown that the expression levels of ki67 in HOS and MG-63 cells were decreased after treated with CTB-1. Data are indicated by mean ± S.D. of three trials. ** p < 0.01, *** p < 0.001. The scale bar = 50 µm.
Evidence suggests that miRNAs play a key role in tumor development.11) Hence, we hypothesized that CTB-1 inhibits HOS cell proliferation by regulating miRNA expression. To prove this, we performed miRNA sequencing analysis and determined miRNA levels in HOS cells after the CTB-1 treatment. As a result, compared to the control group, changes were observed in various miRNA expressions after CTB-1 treatment (Fig. 2A). Furthermore, we performed qRT-PCR analysis to verify the differential expression of miR-1281 and found that miR-1281 was significantly upregulated compared to the control, which was consistent with the results of miRNA sequencing analysis (Fig. 2B). These results indicated that CTB-1 treatment led to abnormal expression of miRNAs in HOS cells.

(A) Differentially expressed miRNAs identified in HOS cells after the treatment with CTB-1 (20 µM) for 48 h. The heatmaps showing a linear color code, with green color indicating the lowest and red indicating the highest expression. (B) The miR-1281 expression was quantiffed using qRT-PCR analysis. Data are indicated by mean ± S.D. of three trials. ** p < 0.01, *** p < 0.001.
To confirm whether the abnormal expression of miR-1281 induced by CTB-1 also regulated the proliferation, migration, and invasion of the OS cells, we transfected the HOS and MG-63 cells with miR-1281 inhibitor or inhibitor NC and then exposed them to CTB-1 for 48 h. Subsequently, the cell viability was measured using the CCK-8 assay, while the proliferation rate was detected using the EdU assay. Figures 3A and B show that CTB-1 significantly reduced the viability and proliferation of OS cells. However, the miR-1281 inhibitor showed a significant reversal of these effects compared to the inhibitor NC. Moreover, the inhibition of migratory and invasive activity induced by CTB-1 was rescued by the knockdown of miR-1281 in HOS and MG-63 cells (Figs. 3C, D). Overall, our results indicated that the inhibition of cell proliferation, migration, and invasion induced by CTB-1 could be rescued by the knockdown of miR-1281 in OS cells.

Cells were transfected with miR-1281 inhibitor at a final concentration of 50 nM and treated with CBT-1 at a concentration of 20 µM for 48 h. (A) The viability of HOS and MG63 cells was observed by CCK-8 assay. (B) The proliferation of HOS and MG63 cells was observed by EdU assay. (C) The migration of HOS and MG63 cells was observed by transwell assay (Scar bar = 100 µm). (D) The invasion of HOS and MG63 cells was observed by transwell assay (Scar bar = 100 µm). Data are indicated by mean ± S.D. of three trials. * p < 0.05, ** p < 0.01, # p < 0.05.
A previous study has demonstrated that PPIF is a target gene of miR-1281.12) Additionally, we verified the potential of miR-1281 to target mRNAs using TargetScan7, where PPIF was identified as the target. Subsequently, the MUT and WT-PPIF luciferase reporters were obtained (Fig. 4A). According to the dual-luciferase reporter assay (Fig. 4B), miR-1281 led to a decrease in the luciferase activity of PPIF-WT in the HOS and MG-63 cells compared to that of the PPIF-MUT. Besides, the miR-1281 mimics suppressed the PPIF levels (Figs. 4C, D), which were previously overexpressed in the OS cell lines. To better determine the role of CTB-1 in regulating the PPIF levels, further related experiments were conducted. CTB-1 treatment resulted in a decline in the PPIF levels (Figs. 4E, F). Moreover, the suppression of miR-1281 further abolished the levels of PPIF, suggesting that the CTB-1 promoted PPIF levels by upregulating the miR-1281 expression in OS.
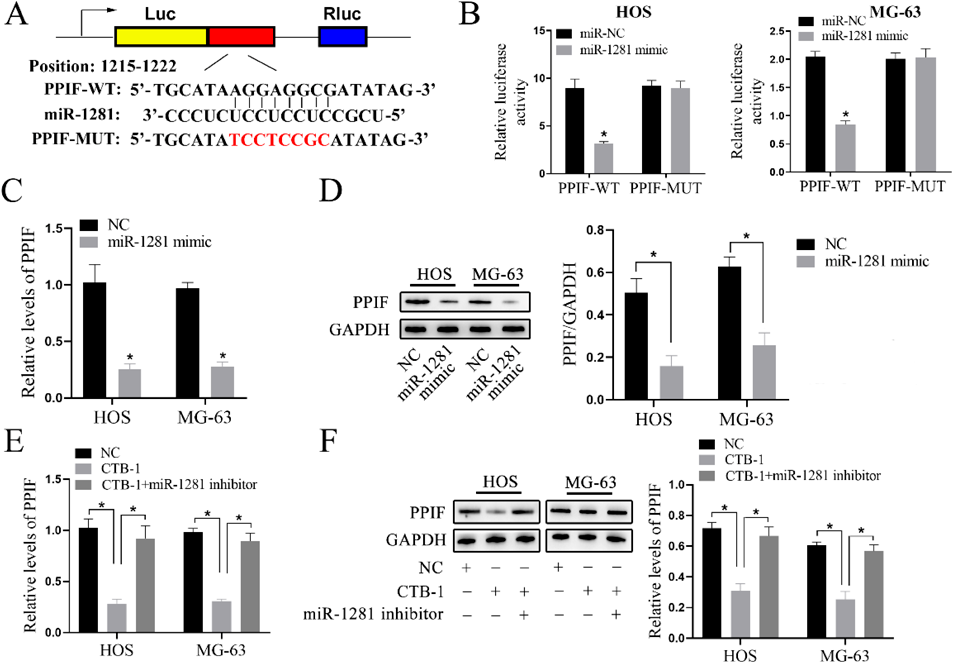
Cells were transfected with miR-1281 mimic and inhibitor at a final concentration of 50 nM and treated with CBT-1 at a concentration of 20 µM for 48 h. (A) Potential binding sites between miR-1281 and PPIF. (B) Binding effect of miR-1281 and PPIF was observed by dual-luciferase reporter assay. (C, D) The mRNA and protein levels of PPIF were inhibited by miR-1281 mimics in HOS and MG-63 cells. (E, F) CTB-1 inhibited the PPIF levels upon the stimulation of miR-1281. Data are indicated by mean ± S.D. of three trials. * p < 0.05.
We used xenograft assays to analyze the underlying mechanism of CTB-1 function in vivo. CTB-1 treatment resulted in a reduction of tumor weight and volume (Figs. 5A–C), with CTB-1 group samples showing decreased PPIF expression and elevated miR-1281 expression (Figs. 5D, E). Therefore, our findings also verified the role of CTB-1 in inhibiting the proliferation of OS in vivo.
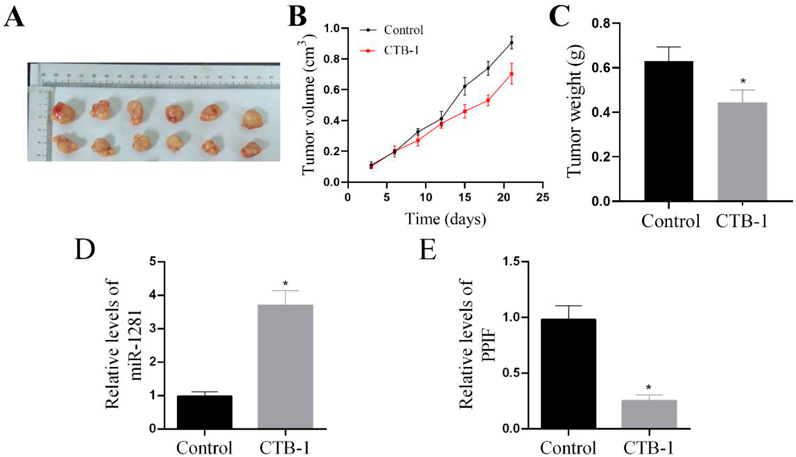
(A, B) CTB-1 decreased tumor volumes. N = 6 for each group. (C) Tumor weights were measured after 21 d post injection. N = 6 for each group. (D, E) Relative expression levels of miR-1281 and PPIF in tumor tissues were analyzed by qRT-PCR. Data are indicated by mean ± S.D. of six animal models. * p < 0.05.
Osteosarcoma affects 1–3 children per million every year worldwide.13,14) Although significant efforts are underway to identify novel treatment methods for OS, the overall survival rate of OS patients has not shown a significant increase in recent years. Also, its pathogenesis remains insufficient.15) Proanthocyanidin, a central component in cinnamon, is essentially known for its antioxidant action.16) CTB-1, a naturally occurring proanthocyanidin, is isolated from cinnamon and a few other plants.17) CTB-1 is a valuable antioxidant consisting of three flavan-3-ol monomeric units and has been reported to have protective effects on ROS in many cell types.18–21) It also exerted strong antioxidative properties in type 2 diabetes, mediating some beneficial effects.22) Since the anti-tumor effect of CTB-1 in OS has not been studied yet, we aimed to explore the inhibition of CTB-1 on the proliferation, migration, and invasion of OS.
For the first time, we also identified that CTB-1 increased the miR-1281 expression in OS. Moreover, the CTB-1-induced enhancement of migration and invasive activity was rescued by the knockdown of miR-1281 in HOS and MG-63 cells. To better determine its effect on OS, we also carried out various functional experiments. According to the CCK-8 assay, miR-1281 reduced the proliferation of HOS and MG-63 cell lines, while the miR-1281 over-expression mitigated the cell metastasis of OS. These findings indicated that CBT-1 could exhibit anti-tumor activities by modulating miR-1281. However, the exact mechanism by which it increases miR-1281 expression has not been fully understood yet. Since p53 and nuclear factor (NF)-kappa B were reported as downstream signaling molecules modulated by CBT-1,9,10,23) playing established roles in p53 and NF-kappa B-involved inflammatory pathway for regulating the miRNA network,24,25) we believed that CBT-1 might modulate miR-1281 through these mediators. Subsequently, we also analyzed the downstream targets of the miR-1281 using bioinformatics analysis. TargetScan7 was used to predict PPIF as the target of miR-1281. Additionally, we conducted a luciferase reporter assay to validate their association and found that while PPIF inhibited miR-1281 in the HOS and MG-63 cell lines, CTB-1 promoted it. PPIF, also called cyclophilin D, plays an important role in mitochondrial protein folding machinery. Several studies have shown that exogenous stimuli-induced PPIF suppressed cell apoptosis via interaction with BCL-2.26,27) Based on the analysis of its gene co-expression network, the overexpression of PPIF was found to be related to the dismal survival of endometrial cancer.28) Meanwhile, many studies have also highlighted that p53 is the key factor mediating PPIF-induced cell growth arrest and non-apoptotic cell death.29,30) However, the effects of PPIF or miR-1281 on the growth of osteosarcoma have not been established. We transfected the HOS and MG-63 cells with miR-1281 mimic, mimic NC, si-NC or si-PPIF and then cultured for 48 h. Subsequently, the cell viability was measured using the CCK-8 assay. Supplementary Fig. S1 shows that miR-1281 mimic and si-PPIF significantly reduced the viability of OS cells compared to the mimic NC or si-NC respectively. Furthermore, we found that the down-regulation of PPIF induced by CBT-1 was associated with suppression of tumor growth, highlighting the role of PPIF in cell proliferation.
Overall, for the first time, our study revealed that CTB-1 acted on the proliferation behavior of OS cells via the miR-1281/PPIF pathway. Our findings can help to understand the underlying mechanism of CTB-1 in OS treatment and provide a basis for novel OS treatment drugs.
This work was supported by the Clinical Medical Science and Technology Development Fund of Jiangsu University [Grant No. JLY2021135].
The authors declare no conflict of interest.
This article contains supplementary materials.