2023 年 46 巻 1 号 p. 95-101
2023 年 46 巻 1 号 p. 95-101
To prevent denosumab-induced hypocalcemia in patients with renal dysfunction, combination therapy with 1α,25-dihydroxy-vitamin D3 (active vitamin D) is recommended. We previously developed a risk prediction model for hypocalcemia in patients with cholecalciferol/calcium (natural vitamin D). However, the prescription status and the risk factors of patients with active vitamin D have not been identified, so we designed this retrospective observational study using a large practice database covering June 2013 to May 2020 to analyze prescription status and risk factors. Patients were classified according to vitamin D type. After that, factors associated with development of hypocalcemia in patients with active vitamin D were explored. Univariate analysis was conducted to compare patient backgrounds between the hypocalcemia and non-hypocalcemia groups. Receiver operating characteristic analysis was conducted to evaluate the predictive potential of the extracted factors. Of the 33442 patients who received denosumab, 22347 and 3560 patients were co-administered natural and active vitamin D, respectively. Patients with active vitamin D had significantly lower renal function (estimated glomerular filtration rate (eGFR) median: 74.0 vs. 69.7 mL/min/1.73 m2), but some patients (23.6%) with sufficient renal function (eGFR ≥90) were also receiving active vitamin D. Of the 3560 patients with active vitamin D, non-hypocalcemia (n = 166) and hypocalcemia (n = 17) groups who met the study criteria were analyzed. Renal function was lower in the hypocalcemia group, and alkaline phosphatase gave the best discrimination. High aspartate aminotransferase (AST), renal dysfunction, high alkaline phosphatase (ALP), and low hemoglobin may be significant factors in risk prediction for hypocalcemia in patients with active vitamin D.
Denosumab is a monoclonal antibody directed against receptor activator for nuclear factor-κB ligand (RANKL), and is used in the treatment of bone metastases of solid tumors, though severe hypocalcemia is a clinical problem. In order to prevent hypocalcemia, it is recommended to combine denosumab with cholecalciferol (vitamin D3)/calcium (natural vitamin D). In patients with renal dysfunction, 1α,25-dihydroxy-vitamin D3 (active vitamin D) is recommended instead of natural vitamin D because of their impaired ability to activate vitamin D. However, the actual status of prescription of the two types of vitamin D in combination with denosumab remains unclear. Furthermore, retrospective studies from a single center have shown that some cases of grade ≥2 hypocalcemia associated with denosumab administration may occur even under vitamin D supplementation.1,2) Our recent retrospective study using a large database confirmed this.3) Therefore, identifying risk factors for hypocalcemia in such cases is still an important clinical issue.
Several risk factors including renal dysfunction have so far been identified1,2,4,5) although the type of vitamin D and whether or not combined administration was conducted differed from study to study, so the relevance of these factors to risk management in clinical practice is still uncertain. Therefore, we previously developed a risk prediction model for grade ≥2 hypocalcemia in patients with natural vitamin D.3) However, patients with active vitamin D are expected to have different background factors, especially renal function, from those with natural vitamin D. Thus, it may be necessary to develop a different prediction model for patients with active vitamin D.
The aim of this study was to clarify the actual prescription status of vitamin D administered in combination with denosumab in patients with bone metastases and to explore the factors affecting the development of grade ≥2 hypocalcemia in patients administrating denosumab in combination with active vitamin D.
This is a retrospective observational study. A hospital clinical database (Medical Data Vision Co., Ltd., Tokyo, Japan) was analyzed. This database includes electronic health insurance claims and diagnosis procedure combination payment system data from > 400 acute care facilities in Japan, and has a 25% coverage rate of such hospitals.
This study was approved by the ethics committee of the Keio University Faculty of Pharmacy (No. 211014-3).
Study PatientsPatients with bone metastases who received a first dose of 120 mg denosumab (RANMARK) between June 2013 and May 2020 were retrospectively collected.
For the investigation of prescription status, vitamin D prescribed at the first dose of denosumab was investigated and classified into 3 groups (no prescription group, natural vitamin D group, active vitamin D group). Furthermore, patients in the natural and active vitamin D groups for whom estimated glomerular filtration rate (eGFR) data were available were compared.
For the analysis of risk factors, patients with active vitamin D were further targeted based on the availability of calcium and albumin measurements.
Data CollectionFactors reported to increase the risk of hypocalcemia include prostate cancer,5) gastric cancer,1,3) high alkaline phosphatase,1–3,6) low albumin,3) low hemoglobin,2) and concomitant use of a proton pump inhibitor,2,3) while factors that decrease the risk of hypocalcemia include breast cancer,3) osteoporosis,3) high calcium levels2,3) and pretreatment with zoledronic acid.7) Patient background factors that were previously reported and considered clinically pivotal were collected: sex, age, clinical laboratory tests (corrected calcium [mg/dL], albumin [g/dL], sodium [mEq/L], potassium [mEq/L], chlorine [mEq/L], phosphorus [mg/dL], serum creatinine [mg/dL], eGFR [mL/min/1.73 m2], blood urea nitrogen [BUN, mg/dL], aspartate aminotransferase [AST, U/L], alanine aminotransferase [ALT, U/L], alkaline phosphatase [ALP, U/L], hemoglobin [g/dL]), medical history (osteoporosis, prostate cancer, breast cancer, gastric cancer, lung cancer), and drug treatment (proton pump inhibitor (PPI), zoledronic acid). Vitamin D was categorized as follows: natural vitamin D (cholecalciferol/calcium supplement) or active vitamin D (alfacalcidol, eldecalcitol, calcitriol, falecalcitriol). The prescription status of calcium preparations and the type and dose of active vitamin D at the time of the first denosumab dose were also investigated. The codes for diseases and drugs are shown in Supplementary Table 1.
Age and clinical laboratory test data at baseline were collected. Given the possibility that the frequency of monitoring during the observational period may have influenced the detection of hypocalcemia, the frequency of clinical laboratory testing during the 28-d period in both groups was investigated. The frequency of testing was also investigated until the day of onset in the hypocalcemia group. Disease data were collected for diagnoses before the first dose of denosumab. Vitamin D and PPIs were defined as combination therapy if they were administered continuously for 28 d (or until the onset of hypocalcemia) after the first dose of denosumab. Continuous administration was judged on the basis of the date of prescription and the number of administration days. Pretreatment with zoledronic acid was defined as positive if the drug was administered within 60 d before the first dose of denosumab. A 2-d deviation was allowed.
If the albumin level was < 4 g/dL, the calcium level was corrected by using Payne’s formula,8) as follows.
 |
eGFR was calculated using the following formula, proposed by the Japanese Society of Nephrology:
 |
For the investigation of prescription status, vitamin D types concomitantly administered with the first dose of denosumab were investigated for each year. In addition, eGFR was evaluated to compare renal function in the natural and active vitamin D groups. History of osteoporosis diagnosis was also investigated.
In the analysis of risk factors for the development of hypocalcemia in patients receiving the active vitamin D combination, calcium levels were followed for 28 d after the first dose of denosumab. The background factors of patients who developed grade ≥ 2 hypocalcemia (Ca < 8.0 mg/dL) and those who did not (Ca ≥ 8.8 mg/dL) were evaluated.
Definition of HypocalcemiaThe lower limit of the reference range of calcium level was defined as 8.8 mg/dL, and grade ≥ 2 hypocalcemia was defined as < 8.0 mg/dL, based on the Common Terminology Criteria for Adverse Events version 5.0.
Statistical AnalysisTo compare eGFR in the natural and active vitamin D groups, the χ2 test or Mann–Whitney U test was used as appropriate.
To analyze risk factors in patients with active vitamin D, univariate analysis was first conducted to compare background factors in the hypocalcemia and non-hypocalcemia groups. Fisher’s exact test was used for categorical variables and the Mann–Whitney U test for continuous variables. Factors satisfying p < 0.05 were extracted, and receiver operating characteristic (ROC) analysis was conducted. The area under the curve (AUC) and the cut-off value based on the Youden index (sensitivity + specificity − 1) were calculated. Furthermore, the sensitivity and specificity were calculated using the cutoff values. If the number of samples was sufficient, we aimed to develop a prediction model by multivariate logistic regression analysis, taking p < 0.05 as the criterion of statistical significance.
Statistical analyses in this study were conducted with SAS software version 9.4 for Windows (SAS Institute Inc., Cary, NC, U.S.A.). This study follows the reporting guidelines of STROBE for observational studies.
Among the 37634 patients who received denosumab, those whose first administration was before June 2013 and those with multiple myeloma or giant cell tumor of bone were excluded. Data on vitamin D prescriptions for 33442 patients were classified into 3 groups: no prescription group (n = 3939), natural vitamin D group (n = 22347), and active vitamin D group (n = 3560). Prescribing trends were investigated for these 3 groups. Furthermore, eGFR data in the natural and active vitamin D groups were investigated. Among the active vitamin D group, patients (n = 315) with calcium test values before and after the first denosumab dose were classified by uncorrected calcium levels (Ca ≥ 8.0 mg/dL; n = 217, Ca < 8.0 mg/dL; n = 98). Subsequently, we examined whether the corrected calcium level met the criterion for grade ≥ 2 hypocalcemia, taking into account albumin levels (if the albumin level is missing but the uncorrected calcium level is ≥ 8.8 mg/dL, the patient is included in the non-hypocalcemia group). Finally, 166 patients in the non-hypocalcemia group and 17 patients in the hypocalcemia group were identified. Incidence of hypocalcemia in patients with active vitamin D was 9.3% (17/183), which was similar to the incidence of 9.4% (124/1315) in patients with natural vitamin D.3) The flow chart for extraction of study patients is shown in Fig. 1.

A total of 33442 patients were classified according to their prescription status of vitamin D (patients with no vitamin D prescription, n = 3939; with natural vitamin D combination, n = 22347; with active vitamin D combination, n = 3560). Based on calcium and albumin levels in patients with active vitamin D, grade ≥ 2 hypocalcemia group (n = 166) and non-hypocalcemia group (n = 17) were identified. Ca, calcium.
The proportions of vitamin D types per year of first denosumab administration in the 3 groups (no prescription group, natural vitamin D group, and active vitamin D group) is shown in Fig. 2. It was found that the proportion of prescriptions for natural vitamin D has been gradually increasing since June 2013, when natural vitamin D became available in clinical practice, while the proportion of prescriptions for active vitamin D has been decreasing. Some patients were not prescribed any vitamin D in each year.
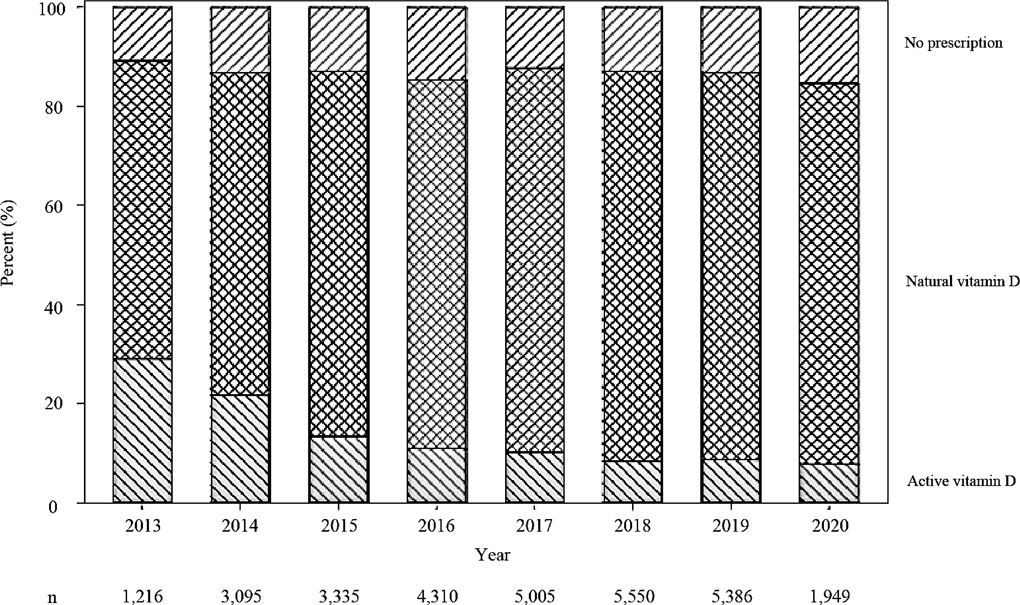
The percentage of vitamin D prescriptions for each year from June 2013 to May 2020 was investigated.
Renal function (eGFR) was investigated in the natural and active vitamin D groups. The results are shown in Table 1. eGFR was significantly lower in the active vitamin D group (eGFR median: 74.0 vs. 69.7 mL/min/1.73 m2). We found that 2.1% of patients with natural vitamin D had extremely low renal function (eGFR < 30 mL/min/1.73 m2) and 23.6% of patients with active vitamin D had high renal function (eGFR ≥90 mL/min/1.73 m2). The number of patients with a diagnosis of osteoporosis prior to first dose of denosumab was 3091 (/22347, 13.8%) in the natural vitamin D group and 1620 (/3560, 45.5%) in the active vitamin D group, and the difference was statistically significant (p < 0.001). eGFR was not significantly different between non-osteoporosis (n = 215) and osteoporosis (n = 141) patients in the active vitamin D group (eGFR median: 70.8 vs. 68.5 mL/min/1.73 m2, p = 0.205).
| Variable | Natural vitamin D combination (n = 2539) | Active vitamin D combination (n = 356) | p-Value |
|---|---|---|---|
| eGFR (mL/min/1.73 m2) | |||
| Median (IQR) | 74.0 (59.5–90.4) | 69.7 (53.1–88.5) | < .001a) |
| 90 ≤ | 645 (25.4) | 84 (23.6) | < .001b) |
| 60 ≤, < 90 | 1242 (48.9) | 145 (40.7) | |
| 30 ≤, < 60 | 599 (23.6) | 99 (27.8) | |
| < 30 | 53 (2.1) | 28 (7.9) |
Values are median (IQR) or n (%). eGFR, estimated glomerular filtration rate; IQR, interquartile range. Of the 22347 patients in the natural vitamin D group and 3560 patients in the active vitamin D group, we extracted patients for whom eGFR data at baseline were available. Among data from 30 d before to 7 d after the first dose of denosumab, the eGFR obtained closest to the date of the first dose was used. a) Mann–Whitney U test. b) χ2 test.
Table 2 shows a comparison of patient background factors between the non-hypocalcemia (n = 166) and hypocalcemia (n = 17) groups. Deficiency rates and the type/amount of active vitamin D at baseline are shown in Supplementary Tables 2 and 3. Some patients in both the non-hypocalcemia (n = 117/166) and hypocalcemia (n = 9/17) groups were prescribed calcium preparations, but the difference was not statistically significant (p = 0.170). The number (median) of clinical laboratory tests was 2 (interquartile range (IQR): 1–3) in the non-hypocalcemia group. In contrast, it was 5 in the hypocalcemia group (IQR: 2–7), although it was only 1 (IQR: 1–2) up to the day of development of hypocalcemia. Statistically significant factors were BUN, AST, ALP, and hemoglobin. ROC analysis was performed for these factors and the results are shown in Fig. 3. Multivariate analysis could not be performed because the sample size was too small.
| Variable | Non-hypocalcemia (n = 166) | Hypocalcemia (n = 17) | p-Value |
|---|---|---|---|
| Female | 66 (39.8) | 5 (29.4) | .448 |
| Age (years) | 73 (66–78) | 72 (65–78) | .624 |
| Clinical laboratory tests | |||
| Corrected calcium (mg/dL) | 9.6 (9.3–9.9) | 9.4 (8.7–9.6) | .058 |
| Albumin (g/dL) | 3.7 (3.4–4.1) | 3.7 (3.2–4.1) | .905 |
| Sodium (mEq/L) | 140 (138–142) | 140 (137–142) | .380 |
| Potassium (mEq/L) | 4.3 (4.0–4.6) | 4.3 (4.0–4.6) | .932 |
| Chloride (mEq/L) | 105 (102–106) | 105 (101–106) | .518 |
| Phosphorus (mg/dL) | 3.6 (3.2–4.0) | 3.7 (3.2–4.0) | .992 |
| Serum creatinine (mg/dL) | 0.80 (0.63–0.99) | 0.88 (0.77–1.33) | .086 |
| eGFR (mL/min/1.73 m2) | 68.0 (50.9–80.1) | 59.0 (40.9–69.9) | .155 |
| BUN (mg/dL) | 16.5 (11.8–20.0) | 21.0 (15.0–23.9) | .017 |
| AST (U/L) | 24.0 (19.0–32.0) | 35.0 (23.5–43.5) | .038 |
| ALT (U/L) | 18.0 (12.5–27.0) | 22.5 (16.0–41.0) | .053 |
| ALP (U/L) | 297 (227–464) | 1032 (430–2453) | < .001 |
| Hemoglobin (g/dL) | 11.75 (10.4–13.2) | 10.2 (9.7–12.4) | .027 |
| Osteoporosis | 65 (39.2) | 5 (29.4) | .602 |
| Cancer | |||
| Prostate cancer | 57 (34.3) | 5 (29.4) | .793 |
| Breast cancer | 31 (18.7) | 1 (5.9) | .314 |
| Gastric cancer | 37 (22.3) | 7 (41.2) | .131 |
| Lung cancer | 70 (42.2) | 3 (17.6) | .068 |
| PPI combinationa) | 46 (27.7) | 6 (35.3) | .574 |
| Pretreatment with zoledronic acidb) | 22 (13.3) | 0 | .231 |
Values are median (IQR) or n (%). ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; IQR, interquartile range; PPI, proton pump inhibitor. a) Continuous 28 d after the first denosumab dose (or until the day of hypocalcemia). b) Within 60 d prior to the first denosumab dose.
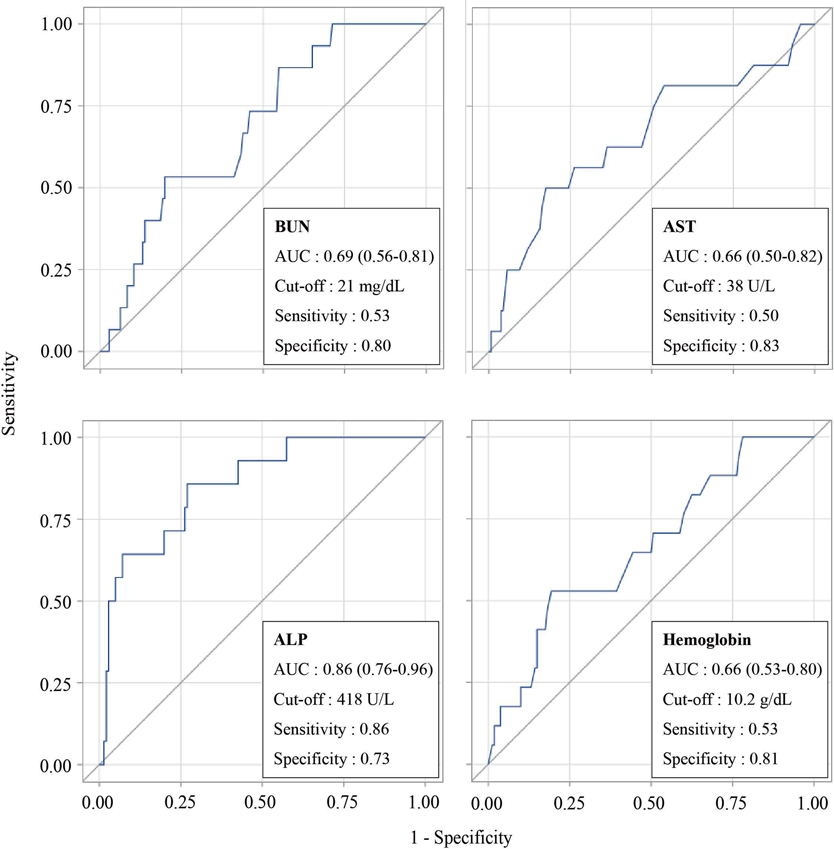
ROC analysis was performed for statistically and clinically pivotal factors (BUN, AST, ALP, hemoglobin). The area under the curve (AUC) and the cut-off value were based on the Youden index (sensitivity + specificity − 1).
This study aimed to clarify changes in prescribing trends over time regarding the type of vitamin D co-administered with denosumab. Furthermore, factors affecting the development of grade ≥ 2 hypocalcemia in patients administrating denosumab with active vitamin D were explored. Although only natural vitamin D has an indication for the prevention of hypocalcemia caused by denosumab and, thus, is used as the first-line drug, the present analysis reveals that some patients were prescribed active vitamin D instead of natural vitamin D. The seven-year data from a large clinical practice database allowed us to compare background factors between patients prescribed active and natural vitamin D, and to analyze factors affecting the development of hypocalcemia in patients with active vitamin D, which has not previously been possible due to small sample sizes. Since the present analysis included multicenter clinical data, it is expected to have high external validity.
Investigation of prescription trends revealed that most patients treated with denosumab were taking it in combination with vitamin D, which was mainly natural vitamin D, although some patients were not prescribed vitamin D. A possible reason for this is that some patients had been judged not to need combination therapy based on their baseline calcium level and/or other background factors. It is also possible that some patients had already been treated with vitamin D at other hospitals, in which case data would not have been available.
The active vitamin D group had significantly lower renal function than the natural vitamin D group, indicating that there is a relationship between renal function and the type of vitamin D prescribed. Actually, the package insert of denosumab states that renal dysfunction increases the risk of hypocalcemia, and it recommends the combination of active vitamin D depending on the degree of renal dysfunction. On the other hand, some patients with high renal function (eGFR ≥ 90 mL/min/1.73 m2, 23.6%) were prescribed active vitamin D and some patients with low renal function (eGFR < 30 mL/min/1.73 m2, 2.1%) were prescribed natural vitamin D. Although the database does not allow detailed evaluation of the clinical judgment regarding individual patients, renal function may not have been taken into consideration in some cases. The present study showed that the incidence of hypocalcemia in the active vitamin D group, which was considered to be at higher risk, was similar to that in the natural vitamin D group. Our results suggest that the choice of vitamin D type based on renal function in clinical practice may contribute to reducing the risk of developing hypocalcemia.
As described above, the natural vitamin D group has higher renal function than the active vitamin D group in general, which is clinically reasonable. On the other hand, active vitamin D is also used in patients with osteoporosis as a treatment.9) Thus, it is possible that some patients with osteoporosis might use active vitamin D regardless of renal function. However, we found no significant difference in renal function between patients with and without osteoporosis in the active vitamin D group, so we considered that analysis of risk factors including patients with osteoporosis was acceptable.
This study suggests that high AST, renal dysfunction, high ALP, and low hemoglobin may be significant factors in risk prediction for hypocalcemia in patients receiving denosumab with active vitamin D. Although it may be necessary to conduct multivariate analysis to predict the risk more accurately, ROC analysis showed that ALP had the best discriminant performance. The finding that high ALP is a risk factor for hypocalcemia is consistent with previous reports.1–3,6) High ALP indicates active bone formation, as long as there are no hepatic or biliary abnormalities.10,11) Furthermore, ALP is more readily available in clinical practice than bone-specific alkaline phosphatase (BSAP), an index reflecting osteogenic activity. Therefore, it is reasonable to apply ALP for risk prediction in clinical practice. It is reported that anemia (low hemoglobin) is a risk factor for electrolyte abnormalities12) including hypocalcemia and is also influenced by nutritional status,13) and the result that low hemoglobin is a risk factor for hypocalcemia is also consistent with a previous report.2) It is therefore reasonable to assume that maintaining hemoglobin in the normal range through nutritional management helps to prevent hypocalcemia. However, unlike our previous study,3) which identified albumin as a factor reflecting nutritional status, this study found no difference in albumin. The reason for this may be that the albumin level was markedly influenced by renal function in the present study patients. Although AST showed a significant difference in this study, there is no previous report suggesting that AST influences the development of hypocalcemia to our knowledge. Since we could not carry out covariate adjustment by means of multivariate analysis, care is needed in interpreting this finding. In addition, BUN is an indicator of renal function, but is also affected by extrarenal factors such as dehydration. However, creatinine and eGFR also tended to be lower in the hypocalcemia group, suggesting that, in contrast to the previous study,3) renal disfunction may be associated with increased risk.
The frequency of laboratory testing during the observation period tended to be higher in the hypocalcemia group than in the non-hypocalcemia group, but the number of tests until the day of onset of hypocalcemia was similar. This suggests that monitoring was more frequent after the onset of hypocalcemia. However, the data are insufficient to establish whether there is a relationship between the frequency of testing and the time at which the onset of hypocalcemia was noted.
Based on the fact that the type of vitamin D prescribed is dependent on patient background factors, the importance of differential risk management in the two populations should be emphasized. In contrast to the present study, for example, renal function was not identified as a risk factor in our previous study of patients with natural vitamin D.3) Based on our previous and present findings, we propose the following approach for risk management of denosumab-induced hypocalcemia in patients receiving combined treatment with vitamin D. i) The type of vitamin D should be decided based on the renal function and calcium level of patients with bone metastases who are to receive denosumab. ii) For patients to be given natural vitamin D, risk determination would be conducted using a risk prediction model which does not include renal function. iii) For patients to be given active vitamin D, risk determination would be based on a comprehensive assessment of factors including renal function. iv) For patients at high risk, reconsideration of vitamin D type (from natural vitamin D to active vitamin D), dose modification of vitamin D, switching to zoledronic acid, which is known to be associated with a lower frequency of hypocalcemia,14,15) and advancing the next hospital visit date to monitor calcium level can be considered.
There are some limitations in this study. First, although the present study was conducted using 7 years of data, which is the largest data set yet examined to our knowledge, multivariate analysis could not be performed due to the small sample size. When further cases have been accumulated, it would be desirable to analyze the risk after more closely matching the patient background factors, including the frequency of laboratory testing, as well as to update the cutoff values by ROC analysis or to develop multivariate models to deal with confounding factors. Second, categorical variables such as medical history and drug treatment could not be adequately considered. We investigated them based on previous reports, but low statistical power meant that we could not properly analyze them. It may become necessary to focus on new factors when the statistical power increases with the accumulation of further cases. Third, more patients in the non-hypocalcemia group were prescribed calcium preparations at the time of the first dose of denosumab in this study. It would be preferable to match the patient backgrounds more closely, even though the difference was not statistically significant. This is also the case for the type and amount of active vitamin D. Fourth, a general limitation of database studies is that the accuracy of diagnosis (i.e., ICD-10 codes) of cancer type and osteoporosis could not be determined.
In conclusion, there were some patients whose renal function may not have been considered in the selection of the type of vitamin D to be given in combination with denosumab. Patients in the active vitamin D group had lower renal function than those in the natural vitamin D group. High AST, renal dysfunction, high ALP, and low hemoglobin may influence the development of hypocalcemia. The risk prediction protocol may need to be different from that of patients with natural vitamin D.
This study was supported by JST SPRING, Grant No. JPMJSP2123.
The authors declare no conflict of interest.
This article contains supplementary materials.