Abstract
Despite the fact that liver fibrosis is an intractable disease with a poor prognosis, effective therapeutic agents are not available. In this study, we focused on bone morphogenetic factor 7 (BMP7) that inhibits transforming growth factor (TGF)-β signaling, which is involved in liver fibrosis. We prepared an albumin-fused BMP7 (HSA-BMP7) that is retained in the blood and evaluated its inhibitory effect on liver fibrosis. Bile duct ligated mice were used as an acute liver fibrosis model, and carbon tetrachloride-induced liver fibrosis mice were used as a chronic model. All mice were administered HSA-BMP7 once per week. In the mice with bile duct ligation, the administration of HSA-BMP7 significantly suppressed the infiltration of inflammatory cells, the area of fibrosis around the bile duct, and decreased in the level of hydroxyproline as compared with saline administration. The mRNA expression of TGF-β and its downstream fibrosis-associated genes (α-SMA and Col1a2) were also suppressed by the administration of HSA-BMP7. In the carbon tetrachloride-induced liver fibrosis mice, the HSA-BMP7 administration significantly decreased the hepatic fibrosis area and the level of hydroxyproline. Based on these results, it appears that HSA-BMP7 has the potential for serving as a therapeutic agent for the treatment of liver fibrosis.
INTRODUCTION
Chronic liver disease is cause of death of two million people worldwide each year, and there are concerns about an increase in the incidence of this disease. The underlying etiology of chronic liver disease is diverse, and includes chronic viral hepatitis, alcoholic steatohepatitis (ASH), non-alcoholic fatty liver disease (NAFLD), autoimmune diseases and genetic diseases. Liver fibrosis is a common pathology regardless of these causes. In addition, liver fibrosis is related to QOL and prognosis,1,2) and the level of fibrosis is correlated with liver function and is known to be a major risk factor for the development of hepatocellular carcinoma.3)
Liver fibrosis, that disrupts the physiological architecture of the liver, is characterized by the progressive accumulation of extracellular matrix (ECM) containing collagen. Hepatocyte injury and the infiltration of immune cells induce the transdifferentiation of hepatic stellate cells into collagen-producing myofibroblasts.4) In general, fibrotic processes are involved in physiological tissue repair. In the late stage of tissue repair, myofibroblast inactivation, apoptosis and endogenous anti-fibrosis mechanisms inhibit the progression of fibrosis.5,6) It should also be noted that persistent hepatitis causes the persistent activation of myofibroblasts and induces the overproduction of ECM.5,7) Transforming growth factor (TGF-β) mainly contributes to this sustained fibroblast activation and ECM production. TGF-β acts profibrogenically through multiple pathways such as differentiation and induction into myofibroblasts, transformation to mesenchymal cells and the inhibition of ECM degradation. Therefore, therapeutic strategies designed to target TGF-β signaling are promising approaches to the treatment of fibrosis.
Endogenous bone morphogenetic protein 7 (BMP7) suppresses TGF-β signaling.8) Therefore, BMP7 is viewed as a promising drug candidate for the treatment of organ fibrosis. BMP7 is an approximately 15 kDa glycoprotein and is a member of the TGF-β superfamily. When it binds to its receptor on the cell membrane, its downstream signal antagonizes the TGF-β signal. In addition, BMP7 has pleiotropic effects that include anti-inflammatory and anti-apoptotic effects.9) In animal experiments, it has been reported that the frequent and repeated administration of recombinant BMP7 suppressed the progression of fibrosis in the liver,10–13) kidney,14) heart15–17) and small intestine.18) These findings suggest that BMP7 has great potential as a therapeutic drug for the treatment of organ fibrosis, but its poor blood retention and low organ utilization have been issues in its clinical application. This is because when BMP7 is administered intravenously, it is rapidly excreted into the urine by glomerular filtration reflecting its low-molecular-weight. In fact, BMP7 has a very short plasma half-life of less than 30 min in mice, and organ utilization is low.19) Therefore, in order to put BMP7 into practical use for the treatment of organ fibrosis, repeated daily administrations or continuous infusion are essential. To overcome this pharmacokinetic issue associated with BMP7, we succeeded in improving the blood retention of BMP7 and in sustaining its biological activity by introducing albumin fusion technology.20) Albumin fusion technology is a drug delivery system that improves the pharmacokinetics of bioactive peptides or low-molecular-weight proteins.21) By applying this technology, we created a long-acting human serum albumin-fused BMP7 (HSA-BMP7). We then confirmed the anti-fibrotic effect of HSA-BMP7 in the kidney.20)
In this current study, in order to evaluate the usefulness of HSA-BMP7 in liver fibrosis, we investigated the anti-fibrotic effect of HSA-BMP7 using bile duct ligation (BDL)-induced acute liver fibrosis and carbon tetrachloride (CCl4)-induced chronic liver fibrosis model mice.
MATERIALS AND METHODS
Production of Recombinant Proteins Using Pichia pastorisThe recombinant fusion protein (HSA-BMP7) was produced using Pichia pastoris following a previously reported method.20) The fusion protein was purified by chromatography on a Blue Sepharose 6 Fast Flow column (GE Healthcare, Tokyo, Japan) that had been equilibrated with 200 mM sodium acetate buffer (pH 5.5). Using AKTA prime, a 5 mL HiTrap Phenyl HP column (GE Healthcare) was then used for hydrophobic chromatography under the following conditions: Buffer A, 50 mM Tris–HCl +1.5 M ammonium sulfate, pH 7.0; Buffer B, 50 mM Tris–HCl, pH 7.0; gradient, 0–100% (Buffer B) 100 mL; flow rate, 5 mL/min. The purified protein was dialyzed and then lyophilized prior to storage.
BDL-Induced Liver Fibrosis Model MiceSea : ICR mice (4 weeks old, male) were purchased from Japan SLC (Shizuoka, Japan). All animal experiments were conducted using procedures approved by the experimental animal ethics committee at Kumamoto University (Approval Number: A2021-021). The common bile duct was ligated under anesthesia to induce fibrosis. The ventral side of the mouse was opened along the midline, the liver was exposed, intestine was pushed down, and the common bile duct was visually confirmed. After ligation, the intestine and liver were moved back to their original positions, and the peritoneum and skin were sutured. HSA-BMP7 was administered at a dose of 100 nmol/kg through the tail vein immediately after BDL and 7 d after the BDL treatment, and various evaluations were performed 14 d later.
CCl4-Induced Liver Fibrosis Model MiceSea : ICR mice (4 weeks old, male) were purchased from Japan SLC and were used in the experiments reported herein. The dosage was 10 µL/kg body (CCl4 : corn oil = 1 : 9), which was administered intraperitoneally twice per week. HSA-BMP7 was administered at a dose of 400 nmol/kg once per week at 5 and 6 weeks after CCl4 administration.
Quantification of Hydroxyproline in LiverAbout 200 mg of liver tissue was collected from each pathological model and then homogenized and centrifuged at 10000 rpm for 5 min at 4 °C. The resulting precipitate was dissolved with 1 mL of MiliQ, then 500 µL of 0.06M chloramine T was added. After incubation, 500 µL of Ehrlich reagent was added to carry out a reaction (65 °C, 15 min), and the absorbance at 540 nm was measured.
Preparation of Liver Paraffin SectionsThe liver of each pathological model was excised and fixed with 10% phosphate-buffered formalin (4 °C, overnight). The fixed liver was permeated with 100% ethanol, JFC solution, and paraffin for 18, 19, and 42 min, respectively, and then embedded in paraffin. The paraffin-fixed liver was then sliced at a thickness of 4 µm using a cryostat (Leica, CM3000II) and adhered to a slide glass to produce a paraffin section.
Hematoxylin–Eosin (H&E) StainingThe liver sections were deparaffinized by treatment with xylene and ethanol. Staining was then performed by sequentially using iron hematoxylin and eosin solution. Washing with 1% acetic acid was performed when the various staining solutions were changed. After washing, the cells were mounted and images were taken with a microscope (BZ-X700, Keyence, Osaka, Japan).
Masson Trichrome StainingParaffin of the prepared paraffin section of the liver was removed by treatment with xylene and ethanol. The sample was then stained using the first mordant, iron hematoxylin, the second mordant, orange G solution, Masson’s stain solution, 2.5% phosphotungstic acid solution and aniline blue solution. The samples were washed with water or 1% acetic acid was performed between each staining. After dehydration, the cells were mounted and images were obtained with a microscope (BZ-X700, Keyence).
Picrosirius Red Staining and QuantificationAfter removing the paraffin from the prepared paraffin sections using xylene and ethanol, the sections were stained with iron hematoxylin, washed with water, and stained with a Picrosirius red solution. The Picrosirius red staining solution was prepared by dissolving Direct Red 80 in saturated picric acid. Quantification of the areas of fibrosis was performed using Picrosirius red stained images, randomly capturing 10–13 high power fields per mouse and quantifying them (original magnification ×200).
Measurement of mRNA Expression Levels by Quantitative RT-PCRAfter collecting the liver, about 50 mg of tissue was added to RNA iso Plus (TaKaRa Bio Inc., Shiga, Japan), homogenized and centrifuged (13500 rpm, 15 min, 4 °C) and the supernatant was collected. Cells were solubilized by adding RNA iso Plus and incubating for 5 min. Subsequently, chloroform extraction was performed twice, followed by isopropanol precipitation, followed by ethanol precipitation to produce an RNA pellet. The resulting RNA was dissolved in ribonuclease (RNase)-free water, and the purity and content were calculated from the absorbance at 260 and 280 nm. Real-time RT-PCR was followed the protocol of Prime Script® RT reagent Kit (TaKaRa Bio Inc.) and SYBER® Premix ExTaqTM II (TaKaRa Bio Inc.). The sequences of the primers used for PCR are shown in Supplementary Table 1.
Fluorescence ImmunostainingAfter deparaffinization, the sections were thoroughly dried and solubilized with 50 mM Tris/HCI (TB) + 20% Tween (T-TB) (room temperature, 5 min), followed by blocking with Block Ace (room temperature, 15 min). A primary antibody reaction was then performed at 4 °C overnight. After washing with T-TB, a secondary antibody reaction was performed at room temperature for 90 min. After washing, images were obtained with a microscope (BZ-X700, Keyence). The antibodies and dilution concentrations used in the immunostaining are shown in Supplementary Table 2.
Statistical AnalysesThe means for two group datasets were compared by the unpaired t test. The means for more than two groups were compared by one-way ANOVA followed by Tukey’s multiple comparison. Probability values of p < 0.05 or p < 0.01 were considered to be significant.
RESULTS
Anti-fibrotic Effect of HSA-BMP7 on Bile Duct Ligated-Liver Fibrosis ModelWe initially examined the anti-fibrotic effect of HSA-BMP7 on a BDL liver fibrosis model mouse. Since BMP7 itself rapidly disappears from the blood after administration, in the previous study, the administration of BMP7 was performed every 2 d.12) Our recent study demonstrated that the HSA-BMP7 exhibited an approximately eight-fold half-life in mice as compared with BMP7 itself.20) In this study, therefore, HSA-BMP7 was administered via the tail vein once per week (immediately after the BDL treatment and 7 d later) (Fig. 1A). Fourteen days after the BDL treatment, paraffin sections of the liver were prepared and subjected to histomorphological evaluation. From the results for the H&E staining, the infiltration of inflammatory cells around the bile duct and hepatocellular injury observed in the saline-administered group were suppressed by the administration of HSA-BMP7 (Fig. 1B). In addition, Picrosirius red staining and Masson’s trichrome staining were performed to evaluate the fibrotic regions (Fig. 1C). The results showed that a significant stained area around the bile duct was observed in the saline-administered group, but the area was suppressed in the HSA-BMP7-administered group. A quantitative evaluation of Picrosirius red staining also confirmed that the fibrotic area was reduced by the HSA-BMP7 administration (Fig. 1C). Furthermore, from the results of the hydroxyproline quantification, the increase in hydroxyproline levels that was observed in the saline-administered group was significantly suppressed in the HSA-BMP7-administered group (Fig. 1D). The above findings indicate that HSA-BMP7 suppressed the progression of BDL-induced hepatic fibrosis.
In the process of liver fibrosis, hepatic stellate cells are activated and the numbers of collagen-producing myofibroblasts increase. HSA-BMP7 suppressed the level of hydroxyproline, suggesting that the activation of hepatic stellate cells had been suppressed. To confirm this, we performed immunostaining of α-smooth muscle actin (α-SMA), which is a widely used indicator of hepatic stellate cells activation (myofibroblasts). In the saline-administered group, a higher fluorescence was observed around the bile duct similar to the fibrotic region, whereas the fluorescence was attenuated in the HSA-BMP7-administered group (Fig. 1E). These results indicate that myofibroblasts that were increased by BDL were reduced by the HSA-BMP7 administration. Therefore, HSA-BMP7 might prevent the progression of fibrosis by suppressing the activation of hepatic stellate cells.
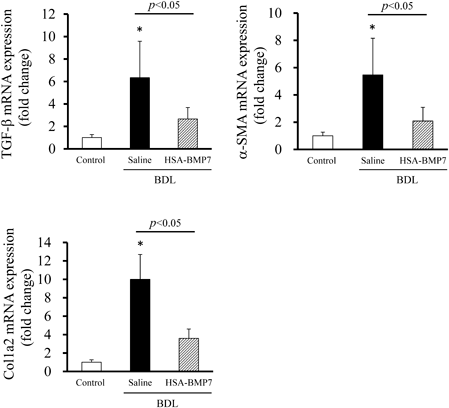
Effect of HSA-BMP7 on TGF-β Signalingα-SMA expression and collagen production are stimulated by TGF-β signaling. BMP7 inhibits the increase in α-SMA expression and the accumulation of collagen. It would therefore be expected that the administration of HSA-BMP7 would suppress these transcriptions. To confirm whether HSA-BMP7 administration suppresses the activation of this pathway, TGF-β, α-SMA and Col1a2 mRNA expression were evaluated. As a result, the expression of TGF-β, α-SMA, and Col1a2, which were elevated by the BDL treatment, were suppressed by the administration of HSA-BMP7 (Fig. 2). These data suggested the possibility that HSA-BMP7 suppressed TGF-β signaling. Therefore, it was indicated that HSA-BMP7 exerted its anti-fibrotic effect in the liver through the suppression of TGF-β signaling.
Anti-fibrotic Effect of HSA-BMP7 on Carbon Tetrachloride-Induced Liver Fibrosis ModelWe next investigated this using CCl4-induced liver injury mice, a chronic hepatitis model. In this study, when a dose of 10 µL/g body (CCl4 : corn oil = 1 : 9) was repeatedly administered twice a week, liver fibrosis was observed at 5 weeks after the administration (data not shown). Therefore, HSA-BMP7 was administered to this model at 5 and 6 weeks (once a week) after the CCl4 treatment (Fig. 3A). Our preliminary study showed that, in CCl4-induced liver fibrosis model, the administration of 100 nmol/kg HSA-BMP7 did not ameliorate liver fibrosis (data not shown). The reason seems to be that HSA-BMP7 was administered after fibrosis was confirmed. Therefore, 400 nmol/kg HSA-BMP7 was administered in this chronic model. Liver fibrosis was evaluated at 7 weeks after the CCl4 administration. Histomorphological evaluation such as H&E staining, Picrosirius red staining and Masson's trichrome staining showed that the cytotoxicity and fibrosis around the blood vessels and sinusoids that were observed in the saline-administered group was suppressed in the HSA-BMP7-administered group (Fig. 3B). In addition, hydroxyproline levels were also significantly decreased in the HSA-BMP7 administered group (Fig. 3C). These findings suggest that HSA-BMP7 suppresses liver fibrosis, even in chronic CCl4-induced liver fibrosis model mice.
DISCUSSION
In this study, we evaluated the efficacy of HSA-BMP7 against liver fibrosis, which, in many cases, determines the prognosis of liver disease, and demonstrated that this fusion protein prevents liver fibrosis in two mice models. To date, it has been shown that BMP7 expression is decreased in fibrotic livers, and that the experimental suppression of BMP7 promotes liver fibrosis.22) Therefore, replenishment therapy using BMP7 may be a useful therapeutic strategy for the treatment of liver fibrosis.
In this study, we used two experimental liver fibrosis models: 1) BDL-induced acute fibrosis and 2) CCl4-induced chronic fibrosis models. This is because the pathology of liver disease can be induced by various factors, and fibrosis progresses from various liver pathologies or pathological stages.23,24) Therefore, to develop an anti-fibrotic agent for the treatment of liver fibrosis, it would be necessary to evaluate the efficacy of this strategy using several liver fibrosis models. In mice with BDL-induced hepatic fibrosis, the administration of HSA-BMP7 suppressed the progression of liver fibrosis. In the liver, the activation of hepatic stellate cells promotes the proliferation of myofibroblasts. Myofibroblasts, which produce ECM, play an important role in liver fibrosis. The expression of α-SMA, an indicator of hepatic stellates cell activation (myofibroblast), was also elevated in the BDL-treated group, but this elevation was suppressed in the HSA-BMP7-treated group. These data suggest that HSA-BMP7 prevents an increase of the production of myofibroblasts. As a result, HSA-BMP7 suppressed the transcription of profibrotic factors (α-SMA, Col1a2) induced by the BDL treatment.
In the CCl4-induced liver fibrosis model, fibrosis is induced by chronic inflammation induced by the repeated administration of CCl4.25) This chronic pathological model is widely used in the pathological analysis of fibrotic liver and for the development of therapeutic agents. As a result, an anti-fibrotic effect of HSA-BMP7 was observed that was similar to that for the BDL-induced fibrosis model. In human liver fibrosis, sinusoidal endothelial cells are transformed into vascular endothelial cells with progression of fibrosis, which impairs the rapid diffusion of solutes between sinusoidal blood and hepatocytes.26,27) In the present CCl4-induced liver fibrosis model, collagen deposition was observed around blood vessels and along sinusoids, and these changes were suppressed by the administration of HSA-BMP7 (Fig. 3B). These findings indicate that HSA-BMP7 also exerts an anti-fibrotic effect, even in CCl4-induced liver fibrosis models.
In this study, HSA-BMP7 was found to be efficacious against two types of liver fibrosis mice model at once-weekly dosing intervals. A similar anti-fibrotic effect of HSA-BMP7 was also reported in a renal fibrosis model.20) Based on these results, we conclude that a once a week dosing schedule for HSA-BMP7 would be a reasonable in mice. However, when human-derived HSA-BMP7 is administered to mice, due to a species difference in the FcRn-mediated albumin recycling mechanism which contributes to the half-life extension of albumin, albumin recycling does not work well.28) Therefore, it appears that the administered HSA-BMP7 might not be present in large amounts in the blood circulation when an administration interval of one week was used. In fact, the blood half-life of HSA-BMP7 in mice is about 8 h.20) We speculate that the sustained action of HSA-BMP7 may be due, not only to the prolonged blood half-life, but also to the sustained pharmacological activity of BMP7. Concerning the mechanism of action of HSA-BMP7, this study suggests that the suppression of TGF-β signaling in hepatic stellate cells may be persistent. BMP7 also acts on hepatocytes to inhibit TGF-β-induced mesenchymal cell transformation.12) In addition, it has been reported that BMP7 promotes the polarization of macrophages and their conversion into anti-inflammatory M2 macrophages29) and to promote liver regeneration. Since these actions of BMP7 are accompanied by changes in cell phenotypes and functions, we speculate that the pharmacological effects of HSA-BMP7 are likely to be persistent. In this study, we were not able to examine the mechanism of action other than TGF-β inhibitory activity, so it will be necessary to examine such points in a future study. In addition, it would be necessary to optimize the dose and administration schedule.
CONCLUSION
HSA-BMP7 was found to be effective against the development of fibrosis in liver fibrotic model mice (Fig. 4). Considering that this blood-retained BMP7 could access various organs through the systemic circulation, it is expected that it could be useful as an anti-fibrotic agent for the treatment of fibrosis in various organs.
Acknowledgments
This study was funded by the Research Foundation for Pharmaceutical Sciences, a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (KAKENHI 15H04758; 16H05114), and the Takeda Science Foundation, Japan
Conflict of Interest
The authors declare no conflict of interest.
Supplementary Materials
This article contains supplementary materials.
REFERENCES
- 1) D’Amico G, Morabito A, D’Amico M, Pasta L, Malizia G, Rebora P, Valsecchi MG. New concepts on the clinical course and stratification of compensated and decompensated cirrhosis. Hepatol. Int., 12 (Suppl. 1), 34–43 (2018).
- 2) Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, Hultcrantz R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology, 61, 1547–1554 (2015).
- 3) Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat. Rev. Dis. Primers, 2, 16018 (2016).
- 4) Elpek GÖ. Cellular and molecular mechanisms in the pathogenesis of liver fibrosis: an update. World J. Gastroenterol., 20, 7260–7276 (2014).
- 5) Aktug Demir N, Kolgelier S, Inkaya AC, Sumer S, Demir LS, Pehlivan FS, Arslan M, Arpaci A. Are bone morphogenetic protein-7 (BMP-7) serum levels correlated with development of hepatic fibrosis? J. Infect. Dev. Ctries., 8, 605–610 (2014).
- 6) Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat. Rev. Immunol., 4, 583–594 (2004).
- 7) Ellis EL, Mann DA. Clinical evidence for the regression of liver fibrosis. J. Hepatol., 56, 1171–1180 (2012).
- 8) Meng XM, Chung AC, Lan HY. Role of the TGF-β/BMP-7/Smad pathways in renal diseases. Clin. Sci. (Lond.), 124, 243–254 (2013).
- 9) Yu Z, Zai-Chun X, Wun-Lun H, Yun-Yun Z. BMP-7 attenuates TGF-β1-induced fibronectin secretion and apoptosis of NRK-52E cells by the suppression of miRNA-21. Oncol. Res., 23, 147–154 (2016).
- 10) Kinoshita K, Iimuro Y, Otogawa K, Saika S, Inagaki Y, Nakajima Y, Kawada N, Fujimoto J, Friedman SL, Ikeda K. Adenovirus-mediated expression of BMP-7 suppresses the development of liver fibrosis in rats. Gut, 56, 706–714 (2007).
- 11) Yang T, Chen SL, Lu XJ, Shen CY, Liu Y, Chen YP. Bone morphogenetic protein 7 suppresses the progression of hepatic fibrosis and regulates the expression of gremlin and transforming growth factor β1. Mol. Med. Rep., 6, 246–252 (2012).
- 12) Zeisberg M, Yang C, Martino M, Duncan MB, Rieder F, Tanjore H, Kalluri R. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J. Biol. Chem., 282, 23337–23347 (2007).
- 13) Zou GL, Zuo S, Lu S, Hu RH, Lu YY, Yang J, Deng KS, Wu YT, Mu M, Zhu JJ, Zeng JZ, Zhang BF, Wu X, Zhao XK, Li HY. Bone morphogenetic protein-7 represses hepatic stellate cell activation and liver fibrosis via regulation of TGF-β/Smad signaling pathway. World J. Gastroenterol., 25, 4222–4234 (2019).
- 14) Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, Kalluri R. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat. Med., 9, 964–968 (2003).
- 15) Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med., 13, 952–961 (2007).
- 16) Merino D, Villar AV, García R, Tramullas M, Ruiz L, Ribas C, Cabezudo S, Nistal JF, Hurlé MA. BMP-7 attenuates left ventricular remodelling under pressure overload and facilitates reverse remodelling and functional recovery. Cardiovasc. Res., 110, 331–345 (2016).
- 17) Elmadbouh I, Singla DK. BMP-7 attenuates inflammation-induced pyroptosis and improves cardiac repair in diabetic cardiomyopathy. Cells, 10, 2640 (2021).
- 18) Flier SN, Tanjore H, Kokkotou EG, Sugimoto H, Zeisberg M, Kalluri R. Identification of epithelial to mesenchymal transition as a novel source of fibroblasts in intestinal fibrosis. J. Biol. Chem., 285, 20202–20212 (2010).
- 19) Vukicevic S, Basic V, Rogic D, Basic N, Shih MS, Shepard A, Jin D, Dattatreyamurty B, Jones W, Dorai H, Ryan S, Griffiths D, Maliakal J, Jelic M, Pastorcic M, Stavljenic A, Sampath TK. Osteogenic protein-1 (bone morphogenetic protein-7) reduces severity of injury after ischemic acute renal failure in rat. J. Clin. Invest., 102, 202–214 (1998).
- 20) Takano M, Toda S, Watanabe H, Fujimura R, Nishida K, Bi J, Minayoshi Y, Miyahisa M, Maeda H, Maruyama T. Engineering of a long-acting bone morphogenetic protein-7 by fusion with albumin for the treatment of renal injury. Pharmaceutics, 14, 1334 (2022).
- 21) Robert S, Gicquel T, Bodin A, Lagente V, Boichot E. Characterization of the MMP/TIMP imbalance and collagen production induced by IL-1β or TNF-α release from human hepatic stellate cells. PLOS ONE, 11, e0153118 (2016).
- 22) Ji D, Li B, Shao Q, Li F, Li Z, Chen G. MiR-22 suppresses BMP7 in the development of cirrhosis. Cell. Physiol. Biochem., 36, 1026–1036 (2015).
- 23) Parola M, Pinzani M. Pathophysiology of organ and tissue fibrosis. Mol. Aspects Med., 65, 1 (2019).
- 24) Correction to lancet gastroenterol hepatol. 2020; 5: 167–228. Lancet Gastroenterol. Hepatol., 5, e2 (2020).
- 25) Dong S, Chen QL, Song YN, Sun Y, Wei B, Li XY, Hu YY, Liu P, Su SB. Mechanisms of CCl4-induced liver fibrosis with combined transcriptomic and proteomic analysis. J. Toxicol. Sci., 41, 561–572 (2016).
- 26) Mori T, Okanoue T, Sawa Y, Hori N, Ohta M, Kagawa K. Defenestration of the sinusoidal endothelial cell in a rat model of cirrhosis. Hepatology, 17, 891–897 (1993).
- 27) DeLeve LD. Liver sinusoidal endothelial cells in hepatic fibrosis. Hepatology, 61, 1740–1746 (2015).
- 28) Andersen JT, Daba MB, Berntzen G, Michaelsen TE, Sandlie I. Cross-species binding analyses of mouse and human neonatal Fc receptor show dramatic differences in immunoglobulin G and albumin binding. J. Biol. Chem., 285, 4826–4836 (2010).
- 29) Pan B, Liu G, Jiang Z, Zheng D. Regulation of renal fibrosis by macrophage polarization. Cell. Physiol. Biochem., 35, 1062–1069 (2015).