2023 Volume 46 Issue 2 Pages 343-347
2023 Volume 46 Issue 2 Pages 343-347
Owing to their unique physicochemical properties and diverse biological effects, ultrafine bubbles (UFBs) have recently been expected to be utilized for industrial and biological purposes. Thus, this study investigated the biological safety of UFBs in water for living beings in drinking the water with a view to future use in health sciences. In this study, we used H2-filled UFBs (NanoGAS®) that can hold hydrogen in the aqueous phase for a long time. Mice were randomly assigned to one of three groups: those receiving NanoGAS® water, reverse osmosis water, or natural mineral water, and they ingested it ad libitum for one month or three months. As a result, subchronic drinking of NanoGAS® water does not affect either the common blood biochemical parameters or the health of the organs and mucosal membranes. Our results, for the first time, scientifically demonstrated the biological safety of H2-filled UFBs water for subchronic oral consumption.
Ultrafine bubbles (UFBs) are gas-filled bubbles with diameters less than 1 µm. It has been reported that they are negatively charged and their zeta potential do not vary over time.1,2) UFBs can enclose a variety of gases, including O2, O3, and H2, and can withstand prolonged immersion in water. In addition to their unique physicochemical properties, UFBs water contains only water and gas, therefore, its use in medicine and industry is gaining interest as a future material suitable for Sustainable Development Goals. Recently, their utility in various industrial fields such as cleaning and plant cultivation has been recognized,3,4) and they are actually being utilized.5) We have also found H2- or O3-filled UFBs water had strong bactericidal activity.6) In another clinical application, oxygen nanobubbles improved hypoxia, a characteristic of solid tumors, and it has been reported to improve the therapeutic efficiency of chemotherapy, radiation therapy and photodynamic therapy in cancer treatment.7)
When considering the potential future use of water with UFBs in health sciences, it is crucial to confirm the safety of UFBs for consumption by living beings. Water with UFBs is generally considered biocompatible; however, this aspect has not been scientifically proven. Therefore, before determining the clinical utility of UFBs, we investigated the biological safety of orally administered water containing UFBs. In this study, we used H2-filled UFBs (NanoGAS®) that hold hydrogen in the aqueous phase for a long period of time.8) To the best of our knowledge, the biological safety, and physiological effects of H2-filled UFBs water in oral intake for a long period of time have not been reported. Thus, we studied the histopathology of various organs and physiological levels of common biochemical parameters in the blood of normal mice after a maximum of 3 months oral intake. The 3-months study is considered as a subchronic oral stoxicity study by OECD.9,10) Since oxygen nanobubble water and hydrogen-rich water have been reported to promote the growth in mice3) and rats,11) respectively, we also observed the amount of food and water intake in this study. If the biological safety of UFBs water is established, the possibility of using UFBs water as a new strategy for delivering H2 to the living body via oral administration becomes viable.
H2-filled UFBs (NanoGAS®) were produced using the rotary shear method.12) NanoGAS® and its raw material water, reverse osmosis (RO) water was provided by Shinbiosis Corp. (Osaka, Japan). The average size and number of NanoGAS® were analyzed by Nanoparticle tracking analysis using NanoSight NS300 (Malvern Panalytical Ltd., Tokyo, Japan) and determined to be 124.3 ± 6.8 nm and 1.6 ± 0.5 × 107/mL (mean ± standard deviation), respectively.6)
They were stored at room temperature until further use. Natural mineral (NM) water as a control was purchased from SUNTORY Holdings Ltd. (Tokyo, Japan). Self-test glucose meter (One Touch Ultra Vue®) and self-test glucose kit (LFS Quick Sensor®) were purchased from LifeScan IP Holdings, LLC (Tokyo, Japan). Pentobarbital sodium (Somnopentyl®) was purchased from Kyoritsu Pharmaceutical Co., Ltd. (Tokyo, Japan). Twenty percent formalin liquid was purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan) and diluted to 10% solution with Milli-Q® water.
Animal StudiesThis study was conducted at Kobe Gakuin University in accordance with the regulations of the Committee on Ethics in the Care and Use of Laboratory Animals. Male ddY mice (6-weeks old) weighting about 30 g were purchased from Japan SLC Inc. (Shizuoka, Japan). All mice were housed in rooms maintained at 23 ± 1 °C and 55 ± 5% relative humidity under a 12 h light/dark cycle and were allowed free access to water and food.
In Vivo Study of Water IngestionBlood glucose levels (BGLs) are shown as a percentage of the BGL measured one day before the experiment (Day 0) after overnight fasting. Similarly, body weight was measured on Day 0. Based on these measurements, mice were randomly assigned to one of three groups: those receiving NanoGAS® water, RO water, or NM water (one or three months). In each case, the mice were given free access to the test water.
Mice in all groups had their body weight and amount of drinking and feeding measured once a week. Mice in 1-month and 3-months groups had their BGL measured on day 30 and weekly after overnight fasting, respectively. On the last day of each test period, mice in the 3-months group were anesthetized with Somnopentyl® (100 mg/kg) and 1 mL of blood was collected from the caudal vena cava with a heparinized syringe. Plasma was separated by centrifugation at 1200 × g for 10 min at 4 °C and stored at −80 °C until further analysis. The organs (stomach, pancreas, liver, small intestine (jejunum, ileum), and left kidney) were excised and fixed in 10% neutral buffered formalin for histopathological evaluation.
Evaluation of Biochemical Parameters in BloodPlasma samples for biochemical parameter evaluation were blinded and outsourced to Oriental Yeast Co., Ltd. (Tokyo, Japan). Various plasma biochemical measurements were performed using 7180 Clinical Analyzer (Hitachi High-Tech Corporation, Japan).
Histopathological EvaluationThe histopathological evaluation was conducted to assess the cytotoxicity of NanoGAS® water following 3 months of ingestion of each test water. The digestive and metabolic organs were randomly selected from each group. The histopathological evaluation was blinded and outsourced to Applied Medical Research Laboratory Co., Ltd. (Osaka, Japan). The organs were embedded in paraffin wax and sectioned at a thickness of 3 µm using a microtome for standard histology analysis. Staining was done with hematoxylin–eosin (H&E) and slides were imaged with the cellSens Standard Software system (Olympus, Tokyo, Japan).
Statistical AnalysisAll the operations were carried out at least in triplicate (n ≥ 3), except for one datapoint showing water intake amount at week 1 in the 1-month intake of the NW water group (n = 2). The data values, where n ≥ 3, were expressed as the mean ± standard error of the mean (S.E.M.). The significance of differences in mean values was assessed using Student’s unpaired t-test. For multiple comparisons with the control group, ANOVA with Tukey’s, Games–Howell, or Kruskal–Wallis test was used. IBM SPSS Statistics Version 26, 27 (IBM Corp., Armonk, NY, U.S.A.) was used for statistical analysis. A p-value <0.05 was considered statistically significant in this study.
Blood biochemical parameters, such as protein and low molecular weight nitrogen compounds, electrolytes and trace metals, enzymes, lipid-related substances, bile pigment, and carbohydrate, were analyzed following 1 month and 3 months ingestion of each test water. The results of the blood biochemical tests are summarized in Table 1. As shown in Table 1, these parameters did not change over the test period, nor did they differ between groups. These results clearly demonstrate that subchronic drinking of NanoGAS® water does not affect common blood biochemical parameters.
| Category | Parameter | Group | |||||
|---|---|---|---|---|---|---|---|
| NM water | RO water | NanoGAS® water | |||||
| 1 month (n = 3) | 3 months (n = 7) | 1 month (n = 3) | 3 months (n = 5) | 1 month (n = 4) | 3 months (n = 6) | ||
| Protein and low molecular weight nitrogen compounds | TP (g/dL) | 4.8 ± 0.2 | 5.0 ± 0.1 | 5.0 ± 0.1 | 4.9 ± 0.1 | 5.3 ± 0.3 | 5.0 ± 0.1 |
| ALB (g/dL) | 2.97 ± 0.15 | 2.98 ± 0.07 | 3.10 ± 0.06 | 2.94 ± 0.05 | 3.28± 0.18 | 3.03 ± 0.09 | |
| BUN (mg/dL) | 29.4 ± 4.2 | 18.2 ± 1.5 | 27.9 ± 8.5 | 25.6 ± 4.2 | 27.0 ± 3.5 | 27.2 ± 2.4 | |
| CRE (mg/dL) | 0.15 ± 0.01 | 0.15 ± 0.01 | 0.15 ± 0.03 | 0.18 ± 0.03 | 0.15 ± 0.01 | 0.19 ± 0.02 | |
| Electrolytes and trace metals | Na (mEq/L) | 156.3 ± 0.3 | 155.2 ± 0.6 | 155.0 ± 0.6 | 153.9 ± 0.5 | 156.3 ± 0.9 | 154.7 ± 0.6 |
| K (mEq/L) | 3.8 ± 0.7 | 3.4 ± 0.3 | 4.3 ± 0.7 | 3.7 ± 0.4 | 4.0 ± 0.5 | 4.7 ± 1.3 | |
| Cl (mEq/L) | 118.0 ± 1.2 | 116.2 ± 1.2 | 111.7 ± 2.8 | 114.0 ± 0.8 | 113.3 ± 2.5 | 114.2 ± 1.9 | |
| Ca (mg/dL) | 8.7 ± 0.1 | 8.6 ± 0.1 | 9.4 ± 0.1 | 8.6 ± 0.1 | 9.0 ± 0.2 | 8.8 ± 0.3 | |
| IP (mg/dL) | 10.9 ± 0.6 | 8.9 ± 1.0 | 12.3 ± 1.8 | 10.5 ± 0.8 | 12.9 ± 1.6 | 11.8 ± 2.3 | |
| Enzymes | AST (IU/L) | 189.3 ± 30.3 | 90.0 ± 22.4 | 229.3 ± 89.8 | 80.9 ± 7.3 | 229.8 ±85.1 | 77.8 ± 15.2 |
| ALT (IU/L) | 43.3 ± 5.8 | 25.4 ± 3.0 | 49.7 ± 15.7 | 26.0 ± 2.1 | 40.8 ± 6.0 | 23.8 ± 3.1 | |
| LDH (IU/L) | 1271.7 ± 189.5 | 523.8 ± 113.7 | 1988.3 ± 1033.1 | 528.7 ± 87.4 | 2167.0 ± 938.2 | 483.0 ± 126.4 | |
| AMY (IU/L) | 3382.0 ± 703.4 | 3860.4 ± 770.1 | 3405.0 ± 409.9 | 3062.3 ± 410.2 | 3071.8 ± 411.6 | 2848.2 ± 220.9 | |
| CK (IU/L) | 2236.3 ± 1027.5 | 601.4 ± 205.8 | 4595.7 ± 643.3 | 491.9 ± 90.1 | 3402.8 ± 956.8 | 674.0 ± 282.0 | |
| Lipid-related substances | Γ-GT (IU/L) | 6> | 6> | 3> | 3> | 6> | 6> |
| T-CHO (mg/dL) | 123.0 ± 19.9 | 141.8 ± 17.7 | 144.7 ± 6.4 | 144.9 ± 4.9 | 164.8 ± 19.4 | 147.5 ± 10.6 | |
| TG (mg/dL) | 12.3 ± 0.9 | 35.2 ± 8.9 | 30.7 ± 11.6 | 20.0 ± 3.4 | 15.8 ± 2.3 | 30.7 ± 5.6 | |
| LDL-C (mg/dL)※ | 54.2 ± 8.8 | 56.2 ± 8.2 | 58.2 ± 5.0 | 57.6 ± 1.7 | 71.1 ± 9.0 | 59.0 ± 4.7 | |
| HDL-C (mg/dL) | 66.3 ± 11.3 | 78.6 ± 8.1 | 80.3 ± 5.5 | 83.3 ± 3.5 | 90.5 ± 10.7 | 82.3 ± 5.1 | |
| Bile pigment | T-BIL (mg/dL) | 0.11 ± 0.03 | 0.09 ± 0.01 | 0.12 ± 0.02 | 0.08 ± 0.01 | 0.10 ± 0.01 | 0.08 ± 0.01 |
| Carbohydrate | GLU (mg/dL) | 147.3 ± 11.1 | 132.6 ± 6.8 | 151.0 ± 4.0 | 120.3 ± 9.8 | 173.8 ± 11.3 | 121.0 ± 11.8 |
Abbreviations: TP, total protein; ALB, albumin; BUN, blood urea nitrogen; CRE, creatinine; AST, asparate aminotransferase; ALT, alanine aminotransferase; LDH, lactic acid dehydrogenase; AMY, amylase; CK, creatine kinase; γ-GT, γ-glutamyl transpeptidase; T-CHO, total cholesterol; TG, triglyceride; LDL-C, low density lipoprotein cholesterol (※calculated value); HDL-C, high density lipoprotein cholesterol; T-BIL, total bilirubin; GLU, glucose. Data indicate the mean ± S.E.M. values.
Figure 1 shows the results of organ pathological examinations performed on randomly selected individuals from NanoGAS® water intake 3-months group. The photomicrographs in Fig. 1 show that there is no apparent histological damage to the tissue (a–c) or mucosal membranes (d–f) caused by the ingestion of NanoGAS® water for 3 months. The histopathological results, which were evaluated under blinded observation at specialized institutions, showed no observable change in the organ tissues or mucosal membranes. These data demonstrate that subchronic oral ingestion of NanoGAS® water does not induce cytotoxicity and has no effect on the health of organs and mucosal membranes.
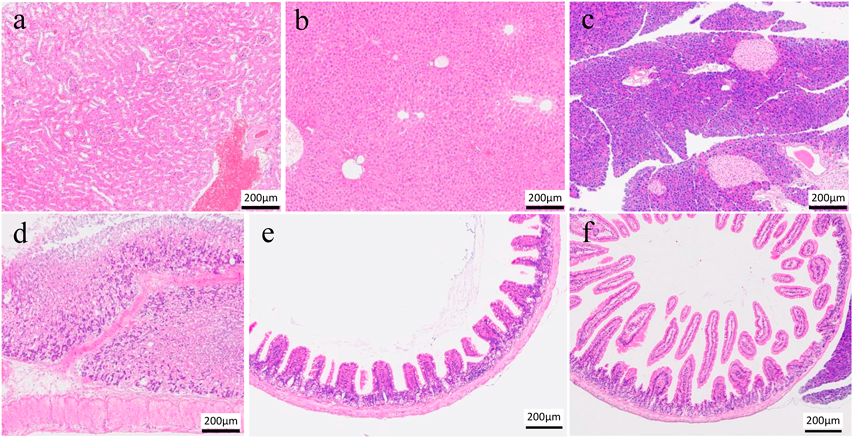
(a) Left Kidney, (b) Liver, (c) Pancreas, (d) Stomach, (e) Small intestine (Jejunum) and (f) Small Intestine (Ileum). The bar indicates 200 µm. Tissues were stained with hematoxylin and eosin after fixation in 10% formalin neutral buffer solution.
Changes in BGLs, body weight, and average food and water intake of mice in 3 groups of the 1 month and 3 months are shown in Figs. 2 and 3, respectively. No significant difference in any parameter among the three groups in the 1 month and 3 months was noted. Although individual variation was high, the amount of food intake of mice in the 3 months NanoGAS® water group tended to be higher than other groups during weeks 7 to 12 (Fig. 3D). Therefore, the total amount of food intake in the first half (weeks 1–6) and the second half (weeks 7–12) of the study period was calculated separately and compared (Fig. 4). In the second half of the study period, the total amount of food intake was slightly increased in the NanoGAS® water group compared to that of the NM water group. It has been reported that nanobubble increase food intake and promote growth of mice by delivering oxygen effective for vertebrate growth.3) Hydrogen is known to have hypermetabolic effects,13,14) therefore, it might relate to increase food intake. However, there are no direct reports of hydrogen promoting growth; therefore, detailed research is needed to estimate the potential biological effects of H2-filled UFBs water.
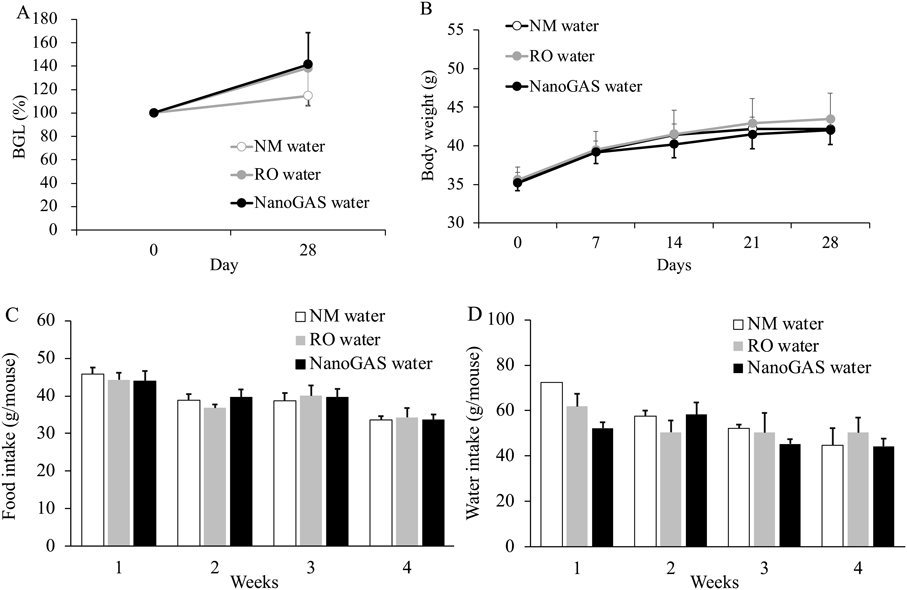
(A) BGLs, (B) Body weight, (C) Food intake, and (D) Water intake. Data indicate the mean ± S.E.M. values (N = 3–4). Data of drinking water of NM water on week 1 was N = 2.
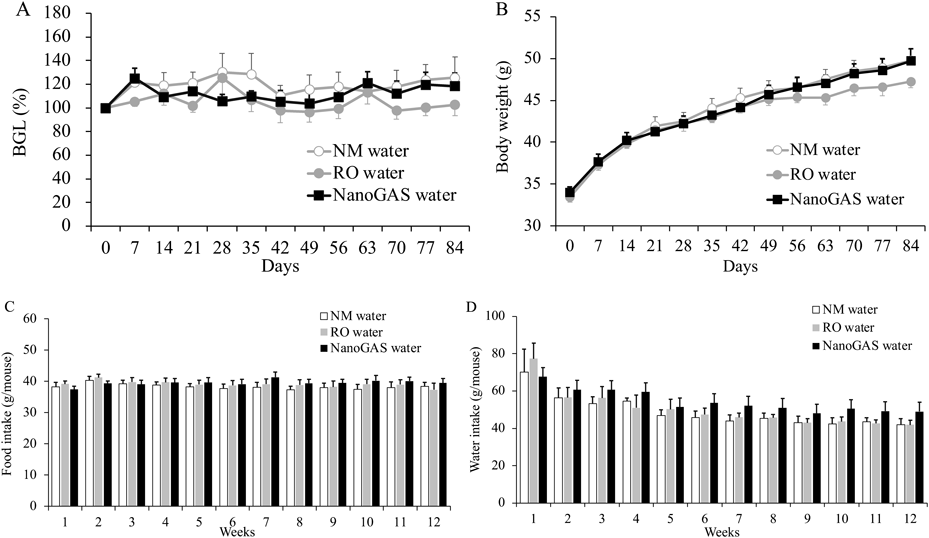
(A) BGLs, (B) Body weight, (C) Food intake (N = 6–7), and (D) Water intake (N = 3–7). Data indicate the mean ± S.E.M. values.

(A) Cumulative food intake in the first half of study period (Weeks 1–6) and (B) Cumulative food intake in the latter half of study period (Weeks 7–12). Data indicate the mean ± S.E.M. values (N = 6–7).
As hydrogen supply method for the living body, oral intake of hydrogen-rich water is currently the most common. The half-life of dissolved hydrogen at saturated solubility, however, is short, so the water must be prepared on demand. Therefore, we assume that water containing UFBs has an advantage, because it can provide a stable supply of hydrogen over a longer timeframe. This study examined the biological safety of H2-filled UFB water in animals in subchronic drinking water with the goal of future application in health sciences. Given an overall evaluation of the findings, no toxic effects of H2-filled UFBs on living bodies were observed, indicating its biological safety for the first time. In addition, H2-filled UFB water is suggested to contribute to appetite enhancement in mice, and further detailed investigation of such physiological effect is expected in the future.
This study was partially supported by a Kobe Gakuin University Research Grant B.
The authors declare no conflict of interest.