2023 Volume 46 Issue 2 Pages 309-319
2023 Volume 46 Issue 2 Pages 309-319
We examined whether the α1L-adrenoceptor (AR), which shows low affinity (pA2 < 9) for prazosin (an α1-AR antagonist) and high affinity (pA2 ≈ 10) for tamsulosin/silodosin (α1A-AR antagonists), is involved in phenylephrine-induced contractions in the guinea pig (GP) thoracic aorta (TA). Intracellular signaling induced by α1L-AR activation was also examined by focusing on Ca2+ influx pathways. Tension changes of endothelium-denuded TAs were isometrically recorded and mRNA encoding α-ARs/Ca2+ channels and their related molecules were measured using RT-quantitative PCR. Phenylephrine-induced contractions were competitively inhibited by prazosin/tamsulosin, and their pA2 value were calculated to be 8.53/9.74, respectively. These contractions were also inhibited by silodosin concentration-dependently. However, the inhibition was not competitive fashion with the apparent pA2 value being 9.48. In contrast, phenylephrine-induced contractions were not substantially suppressed by L-765314 (an α1B-AR antagonist), BMY 7378 (an α1D-AR antagonist), yohimbine, and idazoxan (α2-AR antagonists). Phenylephrine-induced contractions were markedly inhibited by YM-254890 (a Gq protein inhibitor) or removal of extracellular Ca2+, and partially inhibited by verapamil (a voltage-dependent Ca2+ channel (VDCC) inhibitor). The residual contractions in the presence of verapamil were slightly inhibited by LOE 908 (a receptor-operated Ca2+ channel (ROCC) inhibitor) and strongly inhibited by SKF-96365 (a store-operated Ca2+ channel (SOCC) and ROCC inhibitor). Among the mRNA encoding α-ARs/SOCC-related molecules, α1A-AR (Adra1a)/Orai3, Orai1, and Stim2 were abundant in this tissue. In conclusion, phenylephrine-induced contractions in the GP TA can be triggered by stimulation of Gq protein-coupled α1L-AR, followed by activation of SOCCs and VDCCs.
α1-Adrenoceptors (ARs), representative G protein-coupled receptors,1) are classified into three genetically distinct subtypes (α1A, α1B, and α1D).2) Stimulation of these three α1-AR subtypes with AR agonists has been reported to produce contractions in various smooth muscle tissues3–42) (Table 1). Cell lines overexpressing each α1-AR subtype show high affinity for the α1-AR antagonist prazosin (pKB > 9).43–46) However, in native smooth muscle (SM) tissues, the presence of an α1-AR showing low affinity for prazosin (pA2 < 9) was suggested, and this α1-AR type was proposed to be classified as α1L-AR, as opposed to the α1H-AR that shows high affinity for prazosin (pA2 > 9).43–46) Although many approaches have been used to identify the α1L-AR-encoding gene, it has not yet been found. Currently, α1L-AR is regarded as an α1A-AR-derived phenotype that is modified specifically in SM tissues. The evidence supporting this hypothesis is as follows: 1) α1L-AR shows high affinity for α1A-AR antagonists such as tamsulosin and silodosin (pA2 ≈ 10); 2) while α1L-AR characteristic binding sites can be detected using native SMs, the binding site characteristics change to the α1A-AR type using membrane fractions prepared by homogenization; and 3) in α1A-AR knockout mice, both α1L- and α1A-AR characteristics are not detected, while α1L-AR characteristics remain in α1B- and α1D-AR knockout mice.43–46) α1L-AR is abundant in the lower urinary tract and genital SMs and is substantially present in some vascular SM tissues19,28,32,38,39,47–65) (Table 1).
| α1-AR Subtypes | Species | Smooth muscle tissues | References |
|---|---|---|---|
| α1A-AR | Human | Vas deferens | 3,4) |
| Prostate | 5) | ||
| Corpus cavernosum | 6) | ||
| Marmoset | Urethra | 7) | |
| Dog | Superior tarsal muscle | 8) | |
| Mesenteric artery | 9) | ||
| Pig | Urethra | 10) | |
| Coronary artery | 11) | ||
| Rabbit | Thoracic aorta | 12) | |
| Common iliac artery | 12) | ||
| Bronchus | 13) | ||
| Rat | Vas deferens | 14–17) | |
| Prostate | 18) | ||
| Urinary bladder | 19) | ||
| Urethra | 20) | ||
| Cauda epididymis | 21) | ||
| Caudal artery | 22) | ||
| Renal artery | 22) | ||
| Renal interlobar arteries | 23) | ||
| Hamster | Ureter | 24) | |
| Mouse | Urethra | 7) | |
| α1B-AR | Human | Iliac artery branches | 25) |
| Internal mammary artery | 26) | ||
| Rabbit | Corpus cavernosum | 27) | |
| Thoracic aorta | 12) | ||
| Common iliac artery | 12) | ||
| Cutaneous resistance arteries | 28) | ||
| Guinea pig | Spleen | 29) | |
| Rat | Spleen | 14,15) | |
| Tail artery | 30,31) | ||
| Mesenteric resistance artery | 22) | ||
| Portal vein | 32) | ||
| Mouse | Spleen | 33) | |
| α1D-AR | Rabbit | Renal artery | 34) |
| Iliac artery | 34) | ||
| Rat | Vas deferens | 17) | |
| Thoracic/Abdominal aorta | 22,31,35–38) | ||
| Superior mesenteric artery | 22,39) | ||
| Mesenteric artery | 31,37) | ||
| Pulmonary artery | 37) | ||
| Iliac artery | 22,40) | ||
| Carotid artery | 31) | ||
| Femoral artery | 22) | ||
| Mouse | Vas deferens | 41) | |
| Thoracic aorta | 42) | ||
| α1L-AR | Human | Bladder neck | 47) |
| Prostate | 47) | ||
| Urethra | 47) | ||
| Vas deferens | 48) | ||
| Erectile tissue | 49) | ||
| Iris dilator muscle | 50) | ||
| Dog | Prostate | 51) | |
| Urethra | 52) | ||
| Subcutaneous resistance arteries | 53) | ||
| Pig | Urethra | 54) | |
| Rabbit | Bladder trigone | 55,56) | |
| Urethra | 55,56) | ||
| Prostate | 56,57) | ||
| Mesenteric artery | 56) | ||
| Ear artery | 58) | ||
| Cutaneous resistance arteries | 28) | ||
| Iris dilator muscle | 50) | ||
| Guinea pig | Prostate | 59) | |
| Aorta | 60) | ||
| Nasal mucosa vasculature | 61) | ||
| Rat | Urinary bladder | 19) | |
| Superior mesenteric artery | 39) | ||
| Small mesenteric artery | 62) | ||
| Tail artery | 38) | ||
| Femoral artery | 63) | ||
| Portal vein | 32) | ||
| Mouse | Vas deferens | 64) | |
| Prostate | 64,65) |
Regarding SM contractions mediated through α1H-ARs (α1A, α1B, and α1D), extracellular Ca2+ influx through Ca2+ channels plays a primary role similar to the contractions mediated through other drug receptors, and this is evidenced by the following findings: 1) α1A-AR-mediated, noradrenaline (NA)-induced contractions in rat vas deferens were elicited by Ca2+ influxes through voltage-dependent Ca2+ channels (VDCCs) as well as intracellular Ca2+ release from ryanodine-sensitive stores66); 2) α1A-AR-mediated, phenylephrine-induced contractions in rat tail and iliac arteries involved VDCC-dependent Ca2+ influxes, the activation of which was triggered by cation influxes sensitive to LOE 908, an inhibitor of receptor-operated Ca2+ channels (ROCCs)67); 3) α1B-AR-mediated, phenylephrine-induced contractions in rat spleen involved Ca2+ influxes sensitive to SKF-96365, an inhibitor of ROCC and store-operated Ca2+ channels (SOCCs), and intracellular Ca2+ release68); and 4) α1D-AR-mediated, NA-induced contractions in the rat aorta involved nifedipine-sensitive and SKF-96365-sensitive Ca2+ influxes.69) These findings suggest that VDCC-independent and -dependent Ca2+ entry routes trigger α1H-AR-mediated SM contractions. However, to the best of our knowledge, the dependency on extracellular Ca2+ and its entry routes responsible for α1L-AR-mediated SM contractions have not been studied to date.
In this study, to clarify the extracellular Ca2+ entry routes responsible for α1L-AR-mediated SM contractions, we focused on the guinea pig (GP) thoracic aorta (TA), in which NA is reported to induce α1L-AR-mediated contractions.60) GP TA is a commonly used tissue for pharmacological studies and could be more suitable for studying α1L-AR than resistant arteries and lower urinary tract tissues, in which α1H-AR subtypes may be present in addition to α1L-AR (Table 1). Here, we report that α1L-AR-mediated, phenylephrine-induced contractions in the GP TA are triggered by Ca2+ influxes through both SOCCs and VDCCs. We also suggest that the principal SOCC candidates are ORAI3/ORAI1 and stromal interaction molecule 2 (STIM2) based on their mRNA expression in GP TA.
This study was approved by the Toho University Animal Care and User Committee (Approval Nos. 20-51-444, accredited on May 19, 2020; 21-52-444, accredited on April 21, 2021; 22-53-444, accredited on April 9, 2022). The experiments were conducted in accordance with the guidelines of the Laboratory Animal Center of the Faculty of Pharmaceutical Sciences, Toho University. For this study, we purchased male GPs (age, 5–11 weeks; weight, 287–550 g) from Kyudo, Co., Ltd. (Saga, Japan). The GPs were housed under controlled conditions (21–22 °C, relative air humidity 50 ± 5%) and a fixed 12/12 h light–dark cycle (08:00–20:00), with food and water available ad libitum.
Preparation of GP TAThe GPs were anesthetized using isoflurane inhalation and euthanized by exsanguination via the carotid artery. The isolated TAs were cleaned of connective tissue and fat and cut into ring preparations (approximately 2 mm in length) in normal Tyrode’s solution containing (mM): NaCl, 158.3; KCl, 4.0; CaCl2, 2.0; MgCl2, 1.05; NaH2PO4, 0.42; NaHCO3, 10.0; and D-(+)-glucose, 5.6. The intimal surface of ring preparations was rubbed to remove the endothelium.
Measurement of Tension ChangesThe ring preparations were suspended under an optimal resting tension of 1.0 g using stainless steel hooks (outer diameter, 200 µm) in a 5-mL organ bath (UC-5, UFER Medical Instrument, Kyoto, Japan) containing normal Tyrode’s solution. The resting tension was determined according to methods previously described.60) The solution was maintained at 35.0 ± 1.0 °C (pH = 7.4) and continuously bubbled with a mixture of 95% O2 and 5% CO2. TA tension changes were isometrically recorded using force-displacement transducers (T7-8-240, Orientec, Tokyo, Japan; ULA-10GR, MinebeaMitsumi Inc., Nagano, Japan) connected to amplifiers (AM20AZ, Unipulse Corporation, Tokyo, Japan; AP-621G, Nihon Kohden, Tokyo, Japan) and PowerLab™/LabChart™ software (ADInstruments Pty., Ltd., Bella Vista, NSW, Australia). After equilibrated for 60 min in normal Tyrode’s solution, the TA segments were contracted with phenylephrine (10−5 M). After then, to verify the functional absence of endothelium, the TA segments were challenged with acetylcholine (ACh, 10−5 M). TA preparations hardly affected by ACh were regarded as endothelium-denuded TA preparations. The TA preparations were then contracted four times with phenylephrine (3 × 10−4 M). To prevent any possible effects of endogenous prostaglandins, all experiments were performed in the presence of indomethacin (3 × 10−6 M).
Effects of α-AR Antagonists and Ca2+ Channel Inhibitors on Concentration–Response Curves (CRCs) for PhenylephrineAfter the procedures described in “Measurement of Tension Changes,” to obtain a CRC for the control, phenylephrine was cumulatively added to the bath solution. For some experiments, the vehicle (0.2% ethanol (EtOH) for silodosin; 6 × 10−4, 0.02, and 0.3% dimethyl sulfoxide (DMSO) for L-765314, YM-254890, and LOE 908, respectively), and/or verapamil (a VDCC inhibitor, 10−5 M) was administered 20 min before control CRC determination. After washing out, prazosin (an α1-AR antagonist, 3 × 10−9–3 × 10−8 M), tamsulosin (an α1A-AR antagonist, 3 × 10−10–3 × 10−9 M), silodosin (an α1A-AR antagonist, 10−9–3 × 10−8 M), L-765314 (an α1B-AR antagonist, 3 × 10−8 M), BMY 7378 (an α1D-AR antagonist, 10−8 M), yohimbine (an α2-AR antagonist, 10−8 M), idazoxan (an α2-AR antagonist, 10−8 M), YM-254890 (a Gq protein inhibitor, 10−6 M), verapamil (10−5 M), verapamil (10−5 M) + LOE 908 (3 × 10−5 M), or verapamil (10−5 M) + SKF-96365 (3 × 10−5 M) was applied in the bath medium and incubated for 20 min. The concentrations and incubation time of the antagonists and inhibitors used in this study were sufficient to inhibit the targeted receptors and channels based on previous reports.20,43–46,60,70–75) After then, to obtain the CRC in the presence of drugs, phenylephrine was cumulatively added again.
To examine the effects of the Ca2+-free solution, the bath solution was replaced 20 min before CRC with the following solution (mM): NaCl, 158.3; KCl, 4.0; MgCl2, 1.05; NaH2PO4, 0.42; NaHCO3, 10.0; D-(+)-glucose, 5.6; and ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N'-tetraacetic acid (EGTA), 0.2.
RT-Quantitative (q)PCR of α-AR, SOCC-Related, and ROCC-Related mRNA ExpressionIn the mRNA experiment, the endothelium of the TA preparations was carefully denuded. RT-qPCR was performed according to the methods described in a previous report.76) Briefly, total RNA was extracted from the endothelium-denuded TA preparations by the acid guanidinium thiocyanate-phenol-chloroform method. After treated with deoxyribonuclease (Nippon Gene Co., Ltd., Tokyo, Japan) for 30 min at 37 °C, total RNA pellets were obtained by phenol-chloroform extraction/ethanol precipitation and dissolved in diethyl pyrocarbonate-treated water. We used ReverTra Ace® qPCR RT Master Mix with gDNA Remover (TOYOBO Co., Ltd., Osaka, Japan) to synthesize first-strand cDNA by reverse transcription. RT-qPCR was performed on a 7500 Fast Real-Time PCR System (Applied Biosystems, Waltham, MA, U.S.A.) using THUNDERBIRD® Next SYBR® qPCR Mix (TOYOBO Co., Ltd.). The primers used in this study are shown in Supplementary Table 1. The thermal cycler parameters were set to 95 °C for 2 min, followed by 40 cycles of 95 °C for 15 s, 60 °C for 30 s, and 72 °C for 35 s. Fluorescence intensity was measured at each 72 °C step. The mRNA expression level of each gene was analyzed by Sequence Detection Software (Applied Biosystems) and calculated as a relative value to that of β-actin gene (Actb), which was set to 1. We considered the mRNA expression level as 0 for samples that did not reach the fluorescence intensity threshold after 40 cycles.
DrugsThe following drugs were used in this study: l-phenylephrine hydrochloride, idazoxan hydrochloride, (±)-verapamil hydrochloride, and indomethacin (Sigma-Aldrich Co., St. Louis, MO, U.S.A.); prazosin hydrochloride, silodosin, yohimbine hydrochloride, and YM-254890 (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan); tamsulosin hydrochloride (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan); L-765314 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, U.S.A.); BMY 7378 dihydrochloride (Research Biochemicals Inc., MA, U.S.A.); LOE 908 (Nippon Boehringer Ingelheim Co., Ltd., Hyogo, Japan); SKF-96365 (Cayman Chemical Co., Ann Arbor, MI, U.S.A. or Tokyo Chemical Industry Co., Ltd.); and Ach chloride (Daiichi Sankyo Co., Ltd., Tokyo, Japan).
Silodosin was dissolved in EtOH to prepare stock solutions of 5 × 10−3 M and diluted with EtOH to the desired concentrations. Indomethacin was dissolved in EtOH to prepare a stock solution of 10−2 M. L-765314/YM-254890 and LOE 908 were dissolved in DMSO to prepare stock solutions of 5 × 10−3 M and 10−2 M. All the other drugs were dissolved/diluted with distilled water.
Data and Statistical AnalysesThe extent of phenylephrine-induced contractions was calculated relative to the tension level before the phenylephrine administration (0% contraction) and to the 3 × 10−4 M phenylephrine-induced contractions in the control CRC (100% contraction). The data were plotted as a function of phenylephrine concentration and fitted to the following equation:
 |
where E is % contraction at a given phenylephrine concentration, Emax is the maximum contraction, A is the phenylephrine concentration, nH is the Hill coefficient, and EC50 is the phenylephrine concentration that produces a half-maximal response. Curve fitting was performed using GraphPad Prism™ (GraphPad Software Inc., San Diego, CA, U.S.A.). α-AR antagonist potencies were expressed as pA2 values, which were calculated from a Schild plot analysis.77)
Data are expressed as the mean ± standard error of the mean (S.E.M.) or the mean with 95% confidence interval (95% CI), where n refers to the number of experiments. Statistical analyses were performed by Šidák’s test after two-way ANOVA using GraphPad Prism™. All statistical analyses were conducted with a significance level of α = 0.05 (p < 0.05).
Prazosin (3 × 10−9–3 × 10−8 M) inhibited the phenylephrine-induced contractions and shifted the CRCs for phenylephrine to the right (Fig. 1Aa). The slope of the regression line for the Schild plot of prazosin vs. phenylephrine was 1.06 (95% CI: 0.85–1.27), which was not significantly different from unity (Fig. 1Ab). The pA2 value of prazosin vs. phenylephrine was 8.53 (95% CI: 8.42–8.69).

a: Effects of prazosin (3 × 10−9–3 × 10−8 M, Aa), tamsulosin (3 × 10−10–3 × 10−9 M, Ba), and silodosin (10−9–3 × 10−8 M, Ca) on the concentration-response curves (CRCs) for phenylephrine-induced contractions (Aa: n = 16 for control, n = 6 for 10−8 M prazosin, and n = 5 for 3 × 10−9 M and 3 × 10−8 M prazosin; Ba: n = 18 for control and n = 6 for 3 × 10−10–3 × 10−9 M tamsulosin; Ca: n = 20 for control and n = 5 for 10−9–3 × 10−8 M silodosin). b: Schild plot for prazosin (Ab), tamsulosin (Bb), and silodosin (Cb) vs. phenylephrine (Ab: n = 6 for 10−8 M prazosin and n = 5 for 3 × 10−9 M and 3 × 10−8 M prazosin; Bb: n = 6 for each; Cb: n = 5 for each). Data are presented as the mean ± standard error of the mean (S.E.M.). Slope and pA2 values (b) are presented as the mean with 95% confidence interval (95% CI).
Tamsulosin (3 × 10−10–3 × 10−9 M) inhibited the phenylephrine-induced contractions and shifted the CRCs for phenylephrine to the right (Fig. 1Ba). The slope of the regression line for the Schild plot of tamsulosin vs. phenylephrine was 1.14 (95% CI: 0.88–1.41), which was not significantly different from unity (Fig. 1Bb). The pA2 value of tamsulosin vs. phenylephrine was 9.74 (95% CI: 9.58–9.99).
Silodosin (10−9–3 × 10−8 M) inhibited the phenylephrine-induced contractions and shifted the CRCs for phenylephrine to the right (Fig. 1Ca). However, the slope of the regression line for the Schild plot of silodosin vs. phenylephrine was 0.58 (95% CI: 0.37–0.78), which was significantly less than unity (Fig. 1Cb). The apparent pA2 value of silodosin vs. phenylephrine was 9.48 (95% CI: 9.12–10.19).
Effects of L-765314, BMY 7378, Yohimbine, and Idazoxan on Phenylephrine-Induced ContractionsNeither L-765314 (3 × 10−8 M, Fig. 2A) nor BMY 7378 (10−8 M, Fig. 2B) significantly affected the phenylephrine-induced contractions (vs. DMSO/control).

Data are presented as the mean ± S.E.M. (n = 5 for each). * p < 0.05, ** p < 0.01 vs. control (two-way ANOVA followed by Šidák’s test).
Both yohimbine (10−8 M, Fig. 2C) and idazoxan (10−8 M, Fig. 2D) significantly, but only slightly, inhibited or augmented the phenylephrine-induced contractions (vs. control). We concluded that the effects of yohimbine and idazoxan were negligible.
Comparison of mRNA Expression Levels of α-ARs Assessed by RT-qPCRThe relative mRNA expression levels of α-ARs (α1A-AR (Adra1a), α1B-AR (Adra1b), α1D-AR (Adra1d), α2A-AR (Adra2a), α2B-AR (Adra2b), and α2C-AR (Adra2c)) in endothelium-denuded TA are shown in Fig. 3. The most abundant α-AR mRNA was Adra1a, followed by Adra1d, Adra1b, and Adra2c. In contrast, mRNA expression levels of Adra2a and Adra2b were the lowest.

The expression level of each mRNA is shown relative to the mRNA expression level of Actb, which is set as 1. Data are expressed as the mean ± S.E.M. (n = 12 for Adra1a, Adra1b, and Adra2b; n = 11 for Adra2a and Adra2c; and n = 10 for Adra1d).
YM-254890 (10−6 M) completely inhibited the phenylephrine-induced contractions (Fig. 4).
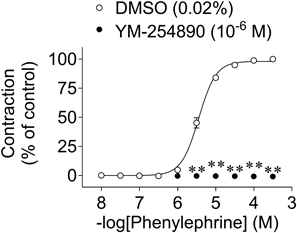
Data are presented as the mean ± S.E.M. (n = 5). ** p < 0.01 vs. DMSO (YM-254890 vehicle) (two-way ANOVA followed by Šidák’s test). DMSO: dimethyl sulfoxide.
The CRCs for phenylephrine-induced contractions in normal Tyrode’s solution and Ca2+-free solution containing 0.2 mM EGTA are shown in Fig. 5A. The phenylephrine-induced contractions were strongly inhibited by the Ca2+-free solution; at 3 × 10−4 M phenylephrine, the contraction was inhibited by approx. 95% (Fig. 5A).
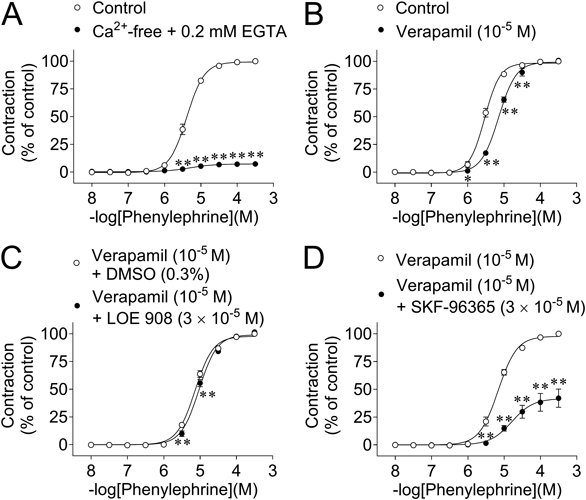
Data are presented as the mean ± S.E.M. (n = 5 for each). * p < 0.05, ** p < 0.01 vs. control/verapamil/verapamil + DMSO (LOE 908 vehicle) (two-way ANOVA followed by Šidák's test).
Verapamil (10−5 M) significantly inhibited the contractions at low concentrations (10−6–3 × 10−5 M) (vs. control) (Fig. 5B). In contrast, the contractions were not affected by verapamil (10−5 M) at higher concentrations (≥10−4 M).
Effects of LOE 908 and SKF-96365 on Phenylephrine-Induced Contractions in the Presence of VerapamilLOE 908 (3 × 10−5 M) slightly but significantly inhibited the CRCs for phenylephrine-induced contractions in the presence of verapamil (10−5 M) (vs. verapamil + DMSO) (Fig. 5C).
SKF-96365 (3 × 10−5 M) strongly inhibited the CRCs for phenylephrine-induced contractions in the presence of verapamil (10−5 M); at 3 × 10−4 M, the contraction was inhibited by approx. 60% (Fig. 5D).
Comparison of mRNA Expression Levels of SOCC-Related Molecules (Orai and Stim) Assessed by RT-qPCRThe relative mRNA expression levels of SOCC-related molecules (Orai (Orai1–3) and Stim (Stim1 and Stim2)) in endothelium-denuded TA are shown in Fig. 6. The most abundant SOCC-related molecule mRNA was Orai3 and Stim2, followed by Orai1. In contrast, the mRNA expression levels of Orai2 and Stim1 were the lowest.

The expression level of each mRNA is shown relative to the mRNA expression level of Actb, which is set as 1. Data are expressed as the mean ± S.E.M. (n = 5 for each).
The relative mRNA expression levels of ROCC-related molecules (TRP channels (Trpc1, Trpc3, Trpc4, Trpc5, Trpc6, Trpc7, Trpm8, and Trpv4)) in endothelium-denuded TA are shown in Supplementary Fig. 1. We tested the presence of TRPC channels which are considered to play a major role in receptor-operated Ca2+ entry in SMs78) and TRPM8/TRPV4 channels which are reported to involve rat aortic SM contractility.79) The most abundant TRP channel mRNA was Trpc6, followed by Trpc1 and Trpc3. The mRNA expression levels of the other TRP channels were very low compared with the Trpc6 mRNA level, in the following order of expression: Trpc5 ≈ Trpv4 > Trpc4 ≈ Trpc7 ≫ Trpm8.
We showed that phenylephrine-induced contractions in GP TA SMs were triggered by the stimulation of Gq protein-coupled α1L-AR, which was followed by Ca2+ influx through SOCCs (ORAI1/3 and STIM2) and VDCCs. GP TA could be more suitable for studying α1L-AR than resistant arteries and lower urinary tract tissues, in which α1H-AR subtypes may be present in addition to α1L-AR (Table 1). Furthermore, its easy-to-handle properties will contribute to the progress of α1L-AR research.
First, we discuss the effect of prazosin on phenylephrine-induced contractions in GP TA. These contractions were competitively suppressed by prazosin, and its pA2 value was calculated to be 8.53 (Fig. 1A). Since this pA2 value was <9, we concluded that the α1-AR responsible for phenylephrine-induced contractions was α1L-AR, as α1H-AR has a pA2 > 9.43–46) Yamamoto and Koike also reported that the α1-AR mediating NA-induced contractions in GP TA was α1L-AR.60)
Second, we discuss the effects of α1A-AR antagonists (tamsulosin/silodosin) on phenylephrine-induced contractions. α1L-AR is defined as α1-AR showing low affinity for prazosin and high affinity for α1A-AR antagonists such as tamsulosin/silodosin (pA2 ≈ 10).43–46) In the present study, the phenylephrine-induced contractions of GP TA were competitively suppressed by tamsulosin, with a pA2 value of 9.74 (Fig. 1B). This value is consistent with the pA2 or pKB values of tamsulosin for α1L-AR: 9.57 (pA2 vs. NA/GP TA)60) and 9.99 (pKB vs. phenylephrine/rabbit prostate).80)
We also tested the effects of silodosin, another specific inhibitor of α1A-AR. Silodosin is reported to show a competitive antagonistic action on α1A-AR/α1L-AR in the following SM tissues based on the regression line slopes of the Schild plots for silodosin vs. the α1-AR agonists being unity: hamster ureter (pA2 = 9.44 vs. phenylephrine), rat tail artery (pA2 = 10.0 vs. NS-49 (an α1A-AR agonist)), dog mesenteric artery (pA2 = 9.9 vs. NS-49), and rabbit ureter (pA2 = 8.71 vs. NA).24,81,82) Although the phenylephrine-induced contractions were suppressed by silodosin in a concentration-dependent manner (Fig. 1Ca), the slope of the regression line for the Schild plot of silodosin vs. phenylephrine was less than unity (0.58 (95% CI: 0.37–0.78)) (Fig. 1Cb), which does not support a competitive antagonist action vs. phenylephrine. Similar results have been reported in human and rabbit prostates, in which α1L-AR mediates NA-/phenylephrine-induced contractions. In both SM tissues, Schild plot analysis was not performed for silodosin-induced inhibition, possibly because of its lack of apparent competitive antagonistic action. In these studies, instead of pA2 values, apparent pKB values were calculated for silodosin (9.45 vs. NA, human prostate; 10.05/9.6 vs. phenylephrine/NA, rabbit prostate).80,83,84) Furthermore, in human prostate tissue, silodosin was suggested to be prone to bind to non-α1-AR sites as well as α1-AR. Namely, the specific binding of [3H]silodosin (500 pM) was shown to be almost equal to the non-specific binding of [3H]silodosin (500 pM).85) Therefore, in GP TA and in prostate tissues, the apparent non-competitive antagonistic action of silodosin vs. phenylephrine might be partly explained by its tendency to bind to non-α1-AR sites. Although competitive antagonism of silodosin vs. phenylephrine was not observed in GP TA, its apparent pA2 value was tentatively calculated to be 9.48, which is consistent with the values for α1A-AR/α1L-AR. Therefore, we conclude that the pharmacological characteristics of α1L-AR, which mediates phenylephrine-induced contractions in GP TA, are almost the same as those in other SM tissues.
Third, we discuss the effects of other α-AR (α1B-, α1D-, and α2-AR) antagonists on the phenylephrine-induced contractions. These contractions were not substantially suppressed by L-765314 (an α1B-AR antagonist, 3 × 10−8 M), BMY 7378 (an α1D-AR antagonist, 10−8 M), yohimbine (an α2-AR antagonist, 10−8 M), and idazoxan (an α2-AR antagonist, 10−8 M) (Fig. 2). Therefore, α1B-AR/α1D-AR/α2-AR are unlikely to be involved in the phenylephrine-induced contractions and this is supported for the following reasons: 1) the concentration of L-765314 used in this study (30 nM) could sufficiently antagonize α1B-AR since its Ki value for α1B-AR is 5.4 nM70); 2) the concentration of BMY 7378 used in this study (10 nM) could sufficiently antagonize α1D-AR since its Ki value for α1D-AR is 0.43 nM20); and 3) the concentrations of yohimbine and idazoxan used in this study (10−8 M, negative logarithm of 8.0) could sufficiently antagonize α2-AR since the pA2 values of yohimbine and idazoxan for α2-AR calculated using rat vas deferens are reported to be 8.24 and 8.70, respectively.71)
The pharmacological characteristics of the α-AR responsible for phenylephrine-induced contractions in GP TA are consistent with those of α1L-AR, which is proposed to be derived from the α1A-AR mRNA (Adra1a).43–46) This is also supported by our present mRNA expression results, showing that Adra1a is the most abundant α-AR (Fig. 3).
Sex differences in α-AR have been reported as follows: 1) Administration of phenylephrine dose-dependently reduced finger blood flow in men, but not in women.86) 2) The number of α-ARs in platelets of women who are hypertensive is 1.5 times higher than in those of women who are normotensive, but the affinity of [3H]dihydroergocryptine for α-AR did not differ between these two groups, or between men and women.87) 3) The binding properties of [3H]prazosin to the rabbit urethra are the same in males and females.88) In this study, we determined that α1L-AR is predominant in GP TA using only male GPs. Further studies using female GPs are required to clarify whether there are sex differences in the function and subtype of α1-ARs in the GP TA.
The pathophysiological role of α1L-AR in GP TA is currently unclear. However, in rat femoral arteries in which α1H-AR and α1L-AR are present, the α1-AR subtypes mediating contraction in the spontaneously hypertensive rat femoral artery were identical with those in the normotensive rat tissue.63) In addition, a study using young adult and aged rat urinary bladder SM suggests that the promotion of contractile responses mediated by α1A-AR, but not α1L-AR, may be the cause of unstable bladder in those who are elderly.19) Therefore, α1L-AR is unlikely to actively be involved in hypertension and unstable bladder in older individuals. Moreover, in human benign prostatic hyperplasia tissue in which α1H-AR and α1L-AR are present, treatment with α1-AR antagonists may not change the conditions of α1-ARs in this tissue.89) Thus, α1-AR antagonists that strongly suppress α1L-AR are needed for the treatment of benign prostatic hyperplasia.
Fourth, we discuss the post-receptor mechanisms of phenylephrine-induced contractions. These contractions were completely suppressed by a Gq-selective inhibitor YM-25489090) (Fig. 4), suggesting that they were mediated by Gq protein activation. In addition, the phenylephrine-induced contractions were potently suppressed by the removal of extracellular Ca2+ (Fig. 5A). This finding indicates that these contractions strongly depend on extracellular Ca2+ influx. One extracellular Ca2+ influx route was indicated to be VDCC-mediated because verapamil (a VDCC inhibitor) partly but significantly suppressed the contractions induced by low concentrations (10−6–3 × 10−5 M) of phenylephrine (vs. control) (Fig. 5B). The concentration of verapamil (10−5 M) used in this study was sufficient to inhibit VDCC because it completely suppressed the binding of [3H]D888 (4.2 nM) to rat cardiac membranes in a previous study.72) Therefore, VDCC plays an important role in the Ca2+ influx pathway when α1L-AR is weakly activated. However, VDCC is not the only Ca2+ influx pathway, and the contribution of VDCC-independent Ca2+ influx is speculated to be substantial because the contractions induced by high concentrations of phenylephrine (≥10−4 M) were not affected by verapamil (10−5 M) (Fig. 5B). VDCC-independent Ca2+ influx is the main pathway when α1L-AR is strongly activated. In support of our results, Tanaka et al. also reported that the contractile responses of GP TA induced by a high concentration (3 × 10−5 M) of NA were not suppressed by various VDCC inhibitors (nicardipine/diltiazem/verapamil).73) In the mouse prostate, a VDCC inhibitor (nifedipine) has been reported to suppress α1L-AR-mediated contractions induced by electrical field stimulation, but the suppression rate differs depending on the frequency of electrical stimulation.91)
To clarify the possible involvement of ROCCs and SOCCs in VDCC-independent Ca2+ influx pathways, the effects of LOE 908 and SKF-96365 on the phenylephrine-induced contractions were investigated. LOE 908 inhibits ROCCs, although it also inhibits VDCCs.92) SKF-96365 is an inhibitor of SOCCs/ROCCs and shows inhibitory effects on VDCCs.92,93) Therefore, in this study, the inhibitory effects of LOE 908 for ROCC were evaluated in the presence of verapamil, and those of SKF-96365 for SOCC were evaluated in the presence of verapamil but in the absence of LOE 908 since the effects of LOE 908 were very limited. Our findings showed that the inhibitory effects of LOE 908 (3 × 10−5 M) vs. phenylephrine were significant, but only slight (Fig. 5C), and those of SKF-96365 (3 × 10−5 M) were very strong (Fig. 5D). Therefore, SOCCs are suggested to represent the principal VDCC-independent Ca2+ influx pathway, although ROCCs may be involved. In GP TA, SKF-96365-inhibitable characteristics have also been reported for NA-induced contractions.73) An important role of SOCCs in α1-AR-mediated contraction has also been reported in non-vascular SM tissues. Specifically, in mouse urethral SM, phenylephrine-induced contractions were strongly attenuated by SOCC inhibitors,94) although it is unclear whether α1A-AR/α1L-AR is predominant in this tissue.7) To the best of our knowledge, our study is the first to show that α1L-AR-mediated SM contraction is triggered by SOCCs/VDCCs through Gq protein activation. However, to clarify whether functional coupling of α1L-AR with SOCCs/VDCCs is conserved among SM type, further studies are required using tissues in which α1L-AR functionally predominates, such as genital and lower urinary tract SMs.
Finally, we discuss the molecular candidates for SOCC/ROCC that are activated by the stimulation of α1L-AR. ORAI channels are molecular candidates for the SOCC, and ORAI channel activation is regulated by STIMs, which are Ca2+ sensors of the endoplasmic reticulum.95) To identify the SOCC/STIM molecular candidates responsible for phenylephrine-induced α1L-AR-mediated contractions, the Orai and Stim mRNA expression levels were assessed by RT-qPCR. Among the tested Orai and Stim mRNA, Orai3 and Stim2 mRNA expression levels were the highest in GP TA, followed by Orai1 (Fig. 6). Therefore, stimulation of α1L-AR in the GP TA is speculated to activate ORAI1 and/or ORAI3 through STIM2. In addition, TRP channels are molecular candidates for the ROCC.96) The relative mRNA expression of the tested TRP channels in GP TA was Trpc6 > Trpc1 > Trpc3 (Supplementary Fig. 1). Therefore, in addition to SOCCs, these TRP channels may function as VDCC-independent Ca2+ influx pathways in α1L-AR-mediated GP TA contractions, although their contributions are very small compared to those of SOCCs/VDCCs. Another role of ROCCs in SM contraction is to trigger the activation of VDCCs.97) Therefore, the verapamil-sensitive component of the phenylephrine-induced α1L-AR-mediated contractions may be generated by being triggered by LOE 908-sensitive ROCCs. By using ROCC inhibitors without VDCC inhibition, this role of ROCCs may be clarified, and this issue should be studied in the future.
The authors would like to thank Dr. Fumiko Yamaki for her expert technical assistance.
The authors declare no conflict of interest.
This article contains supplementary materials.