2023 年 46 巻 2 号 p. 359-363
2023 年 46 巻 2 号 p. 359-363
Amyloid β (Aβ) plays a key role in the pathology of Alzheimer’s disease (AD) and is toxic owing to its ability to aggregate into oligomers and fibrils. Aβ has high aggregative ability and potent toxicity due to the “toxic turn” at positions 22 and 23. Furthermore, APP knock-in mice producing E22P-Aβ with the toxic turn exhibited AD-related phenotypes such as cognitive impairment, Aβ plaque accumulation, and tau hyperphosphorylation. In these mice, it is suggested that the activation of neuroinflammation and dysregulation of hypoxia-inducible factor (HIF) expression in the hippocampus contribute to the pathogenesis of AD-related phenotype. However, it remains unclear which cells are responsible for the dysregulation of HIF expression and the neuroinflammation which was induced by E22P-Aβ with the toxic turn. Here, we investigated the effects of chronic treatment with E22P-Aβ42 and lipopolysaccharides (LPS) on the inflammatory response in BV-2 microglia. Chronic treatment with E22P-Aβ42 and LPS increased nitric oxide production and the expression of interleukin-6 (IL-6), whereas it reduced the expression of HIF-1α and HIF-3α in BV-2 microglia. The reduction of HIF-1α caused by E22P-Aβ42 and LPS was milder than that caused by LPS. Furthermore, chronic treatment with E22P-Aβ42 and LPS increased the nuclear translocation of nuclear factor-kappaB (NF-κB). E22P-Aβ42 could enhance the inflammatory response of microglia with abnormal HIF signaling and contribute to the progression of AD pathology.
Much evidence suggests that amyloid β (Aβ) is toxic by aggregating in order to form oligomers and fibrils and is involved in the onset of Alzheimer’s disease (AD). Irie and colleagues identified the toxic conformer of Aβ with a turn at positions 22 and 23. E22P-Aβ42 with a propensity to adopt the toxic conformer has high aggregative ability and potent cytotoxicity in vitro.1–4)APPNL-P-F/NL-P-F mice (NL-P-F mice) with the overproducing E22P-Aβ42 showed glial activation and dysregulation of hypoxia-inducible factor (HIF) expression in the pathogenesis and development of AD-like phenotypes.5,6) NL-P-F mice also exhibited increased HIF-1α protein and decreased HIF-3α mRNA expression level in hippocampus.6) HIF-3α forms heterodimers with HIF-1α but has no transcriptional activity and represses downstream gene expression in the HIF-1α pathway.7) This suggests that increased gene transcriptional activity of HIF-1α pathway via decreased HIF-3α expression level is involved in the phenotypic changes of AD in NL-P-F mice.6) Microglia play a central role in neuroinflammation in AD.8) Therefore, an activation of inflammatory responses in microglia may be a potential target for E22P-Aβ42. Acute treatment of Aβ or lipopolysaccharides (LPS) activates immune cells, for example, microglia, and increases the inflammatory response.9–11) However, immune tolerance to the stimuli is rendered, which changes the inflammatory cytokine production, migration ability, and other properties in microglia in the chronic condition of inflammation.10,11) Notably, these phenotypic shifts via the activation of the HIF pathway in microglia are involved in the chronic condition of AD pathology.12) BV-2 microglia have a genetic background of c57BL/6 mice and are widely used in neurodegenerative disease research because of their immune functions similar to primary microglia and in vivo microglia.13) Moreover, in common with primary microglia, BV-2 microglia exhibit phenotypic shifts via the activation of the HIF pathway induced by LPS.14,15) Therefore, in order to investigate the effect of E22P-Aβ42 on the microglia of chronic conditions in NL-P-F mice, we used BV-2 microglia as an alternative cellular model of microglia in vitro. The chronic conditions of microglia can be mimicked with cultured cells exposed to LPS or Aβ for 24 h, which was followed by 3–5 d of culture without additional stimuli.10) Aberrant toll-like receptor activation has been associated with chronic inflammatory conditions of diseases such as asthma, rheumatoid arthritis, and cancer.16) Therefore, simultaneous stimulation of LPS and Aβ was used in inducing the chronic condition of BV-2 microglia. This study investigated the effects of E22P-Aβ42 treatment on the LPS-induced inflammatory response and HIF expression in BV-2 microglia in chronic conditions.
The cell culture protocol was followed in a previous study.17) To induce the chronic condition of BV-2 microglia, we administered the following treatment: BV-2 microglia (1 × 105 cell/well) were cultured in 12-well plates overnight and preincubated for 24 h with LPS from Escherichia coli O111 (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) and E22P-Aβ42. After that, the BV-2 microglia were washed and cultured in the culture medium for three days. Experiments using this cell culture method mimicked the chronic condition.10) Thereafter, BV-2 microglia were incubated with vehicle (VH) or restimulated with LPS and/or E22P-Aβ42 for 24 h, which was defined as chronic treatment (Fig. 1A). After restimulation for 24 h, cells and supernatant were collected for experiments. Drug treatments were conducted as follows. LPS and Aβ were dissolved in 0.1% NH4OH distilled water to a concentration of 1 µg/mL and 200 µM, respectively, and added to the culture medium just before treatment. The final LPS and Aβ peptide concentrations were adjusted to 10 ng/mL and 2 µM respectively followed by previous study.11)
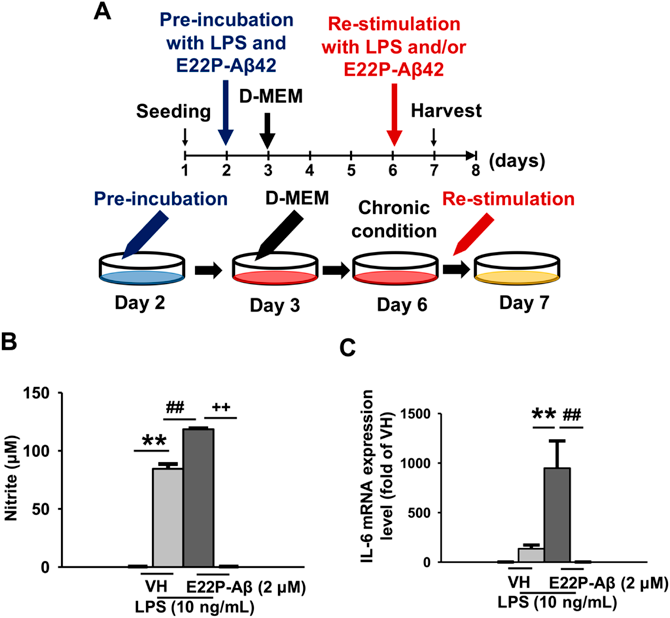
(A) A schematic illustration of chronic treatment in investigating the effects of restimulation with LPS (10 ng/mL) and/or E22P-Aβ42 (2 µM) in the chronic condition of BV-2 microglia. BV-2 microglia in chronic conditions were retreated with vehicle, LPS (10 ng/mL), and/or E22P-Aβ42 (2 µM) for 24 h. (B) Nitrite level in the extracellular medium, n = 6. (C) IL-6 mRNA expression, n = 10. The values indicate the mean ± S.E.M. ** p < 0.01, ## p < 0.01, ++ p < 0.01, two-way ANOVA followed by Tukey’ s test.
The Griess assay was conducted based on a previous study.17) The concentration of nitrite, as an indicator of nitric oxide (NO), in the medium released by BV-2 microglia was determined.
Real-Time RT-PCRPCR was conducted in accordance with a previous study.17) The primer sequences of Interleukin-6 (IL-6), HIF-3α, and Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) used for PCR were based on previous studies.6,17)
Western BlotBV-2 microglia were lysed in radioimmunoprecipitation assay buffer (50 mM Tris–HCl [pH 7.6], 50 mM NaCl, 1% Nonidet P40 Substitute, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate (SDS)) with 1% protease inhibitor cocktail (FUJIFILM Wako Pure Chemical Corporation) and 1 mM phenylmethylsulphonyl fluoride on ice. A Western blot experiment was then conducted as previously described with minor modifications.17) The membranes were blocked for 90 min by 5% skim milk and were then reacted with the primary antibody (anti-HIF-1α (NB100-479, 1 : 1000 dilution; Novus Biologicals, Centennial, CO, U.S.A.) or anti-GAPDH (016-25523 1 : 10000 dilution; FUJIFILM Wako Pure Chemical Corporation).
ImmunocytochemistryImmunocytochemistry was performed based on a previous study with minor modifications.17) Fluorescence images were taken using a confocal laser microscope (Zeiss LSM 900 with an airy scan, Carl Zeiss, Oberkochen, Germany). The ratio of NF-κB nuclear translocation was analyzed using the previous method.17)
Statistical AnalysisThe data were expressed as the mean±standard error of measurement (S.E.M.). The statistical significance of the differences was determined using a two-way ANOVA followed by Tukey’s post hoc test using Prism software version 7 (GraphPad Software, Inc., San Diego, CA, U.S.A.). The criterion for statistical significance was p < 0.05.
We show a schematic illustration of the culture method which was used in this study in examining the effects of chronic treatment (Fig. 1A). LPS activates microglia, which releases NO and produces inflammatory cytokines. Therefore, we measured NO production and IL-6 mRNA expression as an index of microglial activation and inflammatory response. Nitrite level as an indicator of NO production significantly increased by LPS treatment and was further increased by cotreatment of E22P-Aβ42 and LPS (Fig. 1B). Furthermore, the IL-6 mRNA expression level was increased in the simultaneous treatment of E22P-Aβ42 and LPS. However, LPS alone had little effect on IL-6 levels (Fig. 1C), whereas E22P-Aβ42 alone did not affect NO or IL-6 levels (Figs. 1B, C).
Chronic Treatment with E22P-Aβ42 and LPS Decreases HIF-1α Protein and HIF-3α mRNA in BV-2 MicrogliaWe previously reported that mice producing E22P-Aβ42 exhibited the increased expression of HIF-1α and decreased expression of HIF-3α with phenotypic changes which are associated with AD.6) Therefore, we examined the expression of HIF molecules. The simultaneous treatment of E22P-Aβ42 and LPS significantly decreased HIF-1α protein and HIF-3α mRNA expression levels (Figs. 2A, B). The HIF-1α level in the simultaneous treatment of E22P-Aβ42 and LPS significantly exceeded that in LPS alone (Fig. 2A).
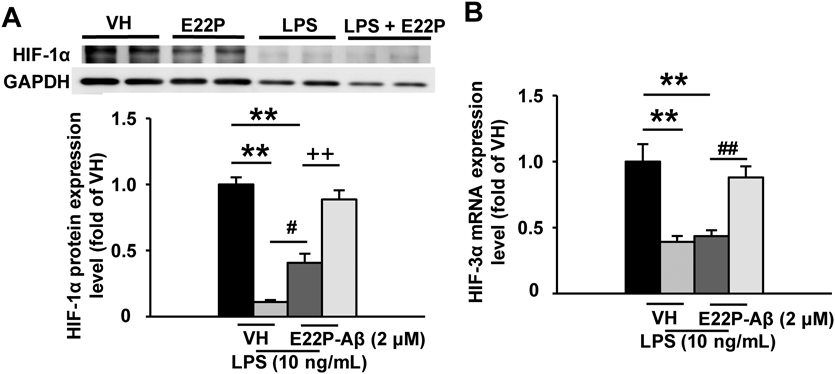
BV-2 microglia in chronic conditions were retreated with vehicle, LPS (10 ng/mL), and/or E22P-Aβ42 (2 µM) for 24 h. (A) HIF-1α protein expression (Western blot), n = 4. (B) HIF-3α mRNA expression (real-time RT-PCR), n = 10. GAPDH was used as a control for housekeeping protein and gene expression. The values indicate the mean ± S.E.M. ** p < 0.01, # p < 0.05, ## p < 0.01, ++ p < 0.01, two-way ANOVA followed by Tukey’s test.
NF-κB signaling is involved in LPS-induced microglial activation and inflammatory response. Therefore, we conducted immunocytochemical staining of NF-κB with 4′,6-diamidino-2-phenylindole (DAPI) and investigated whether the nuclear translocation of NF-κB contributes to the activation of BV-2 microglia by chronic treatment. The simultaneous treatment of E22P-Aβ42 and LPS significantly increased the nuclear translocation of NF-κB (Fig. 3).

BV-2 microglia in chronic conditions were retreated with vehicle, LPS (10 ng/mL), and/or E22P-Aβ42 (2 µM) for 24 h. (A) Immunocytochemical measurement of NF-κB nuclear translocation in BV-2 microglia after chronic treatment. The scale bar indicates 50 µm. (B) Quantitative data of the nuclear translocation of NF-κB. n = 6. The values indicate the mean ± S.E.M. ** p < 0.01, ## p < 0.01, two-way ANOVA followed by Tukey’s test.
LPS treatment with or without E22P-Aβ42 increased NO production (Fig. 1B) in the chronic condition of BV-2, which suggest that the microglia were activated by LPS treatment. However, IL-6 mRNA expression was low in LPS treatment alone but significantly increased in cotreatment with E22P-Aβ42 (Fig. 1C). LPS-induced chronic conditions of microglia were reported to be in an activated state with increased expression of ionized calcium-binding adapter molecule 1 (Iba1) protein but decreased IL-6 level.10,18) As previously reported, BV-2 microglia were probably rendered immunotolerant to LPS, and the inflammatory response to LPS treatment was low in this study. However, chronic treatment with LPS and E22P-Aβ42 may cause aberrant activation in microglia, amplifying inflammatory responses that are suppressed by immune tolerance. NF-κB nuclear translocation was induced by LPS to a lesser extent but drastically increased by cotreatment with E22P-Aβ42 (Fig. 3). LPS increased NO production in BV-2 microglia (Fig. 1B). NO is produced by inducible nitric oxide synthase (iNOS), which is induced by LPS.19) NF-κB activation is suppressed in rats tolerant to LPS, but iNOS mRNA expression is increased.20) Notably, the contribution of NF-κB activation to NO production by LPS treatment is probably minimal in chronic conditions. LPS treatment with E22P-Aβ42 remarkably increased IL-6 mRNA expression (Fig. 1C). IL-6 increases the transcriptional activity of NF-κB.21) Additionally, activation of the NF-κB pathway increases NO production and HIF-1α protein expression.22) In this study, HIF-1α protein and HIF-3α mRNA expression levels were significantly decreased in the chronic treatment of LPS with or without E22P-Aβ42 compared with VH treatment (Figs. 2A, B). Normally, acute treatment of LPS or Aβ in microglia increases HIF-1α protein expression, but restimulation in microglia in a chronic condition does not activate the HIF pathway.10,11) There is likely a dysregulation of HIF molecule expression in response to stimulation in the chronic condition even though the mechanism for the decreased expression of HIF molecules upon restimulation of BV-2 microglia in the chronic condition is unclear. Furthermore, simultaneous treatment with LPS and E22P-Aβ42 in BV-2 microglia of the chronic condition increases HIF-1α protein expression without altering HIF-3α mRNA expression compared to LPS alone (Fig. 2A). Compared to wild-type Aβ42, E22P-Aβ42 has potent neurotoxicity via significantly increased production of intracellular reactive oxygen species (ROS).3) Increased production of ROS in mitochondria stabilizes HIF-1α protein expression via inhibition of HIF-degrading enzyme activity.23) Although the chronic treatment of LPS caused a decrease in HIF expression, it is possible that the increased production of ROS by E22P-Aβ42 contributed to the HIF expression level. In this study, the inflammatory response via IL-6/NF-κB signaling, which was minimal with LPS treatment, was dramatically increased by cotreatment with E22P-Aβ42 (Figs. 1C, 3). Thus, it is possible that E22P-Aβ42 increased the activation of HIF-1α pathway through IL-6/NF-κB inflammatory signaling without altering HIF-3α mRNA in LPS-induced inflammation in BV-2 microglia. However, HIF-3α gene expression is normally increased downstream of HIF-1α activation.24) HIF-3α then negatively regulates HIF-1α transcriptional activity.7,25) This suggests that in chronic conditions, the feedback loop of the HIF pathway is disrupted by increasing HIF-1α protein via IL-6/NF-κB inflammatory signaling without altering HIF-3α mRNA expression in microglia. As previously demonstrated in NL-P-F mice, this study validated the inflammatory responses and dysregulation of HIF molecules in microglial cells.6) In the hippocampus of NL-P-F mice, neuroinflammation in microglia occurred at three months of age. HIF-1α protein expression was increased, and HIF-3α mRNA was decreased at six months of age, which is the period of chronic neuroinflammation and pathogenesis of AD-related pathology in NL-P-F mice although there were no changes at three months of age. Consistent with the results of this study, a decrease in HIF-3α mRNA expression was commonly observed, at least in the chronic phase of inflammation rather than in the early phase. Therefore, decreased HIF-3α mRNA expression in microglia and an increased inflammatory response can be involved in phenotypic changes in the chronic stage of AD pathology which was caused by E22P-Aβ42 with the toxic turn. However, HIF-1α protein expression was increased in the NL-P-F mice.6) In this study, LPS treatment with or without E22P-Aβ42 decreased HIF-1α protein expression. The increase in HIF-1α protein expression in the hippocampus of NL-P-F mice is likely influenced by cells other than microglia in the brain. Further investigation is required in determining the relationship between increased HIF-1α protein expression and neuroinflammation in AD pathology.
E22P-Aβ42 treatment concentration followed previous studies, but E22P-Aβ42 alone did not affect BV-2 microglia.11) Simultaneous treatment with LPS and Aβ in preincubation may have induced a different behavior in BV-2 microglia than previously reported. This study has some limitations. In this study, we preincubated BV-2 microglia with LPS and Aβ. Thus, we could not compare the results with those of preincubation with LPS or Aβ alone, as in previous studies.10,11) More studies are needed in determining the effects of regulating HIF and NF-κB signaling on BV-2 microglia in chronic conditions. E22P-Aβ42 treatment also inhibits cell proliferation in THP-1 macrophage cells at concentrations lower than 2 µM.26) Therefore, it is possible that changes in the number of cells in BV-2 microglia due to each chronic treatment may affect the experiment. It is necessary to assess the cell viability of BV-2 cells in future studies to determine the effect of cell number on the experiment. In summary, treatment with E22P-Aβ42 altered the LPS-induced inflammatory response and HIF-1α expression in BV-2 microglia in the chronic condition. In NL-P-F mice, increased neuroinflammation by glial cells was followed by dysregulation of HIF molecule expression in the hippocampus, which suggests that the dysregulation of HIF expression associated with AD pathology in NL-P-F mice is partially caused by microglia.
This work was supported by JSPS KAKENHI Grant Number JP19K07119 (T.K.), 19K09323 (D.U.), and JST SPRING, Grant Number JPMJSP2145 (T.M.). This study was partially supported by the Hoansha Foundation and the Smoking Research Foundation (T.K.).
Conceptualization, T.K. and K.I.; methodology, T.M. and T.K.; formal analysis, M.S. and T.M.; investigation, T.M.; resources, K.I. and T.K.; data curation, T.M.; writing—original draft preparation, T.M.; writing—review and editing, M.S., T.K., and K.I.; visualization, T.M.; supervision, M.S. and T.K.; project administration, T.M., M.S., D.U. and T.K.; funding acquisition, T.M., D.U. and T.K. All authors have read and agreed to the published version of the manuscript.
The authors declare no conflict of interest.