2023 年 46 巻 5 号 p. 725-729
2023 年 46 巻 5 号 p. 725-729
Epidermal keratinocytes protect themselves by cooperating with neighboring cells against internal and external stresses, which leads not only to the maintenance of cell homeostasis but also to the prevention of skin aging. Although it is known that nuclear factor (NF)-E2-related factor 2 (Nrf2) signaling plays a pivotal role in ameliorating oxidative stress and inflammatory responses under stress situations, it is unclear whether Nrf2 signaling in keratinocytes cooperates with neighboring cells such as dermal fibroblasts. Thus, this study was conducted to examine the influence of dermal fibroblasts on Nrf2 signaling in epidermal keratinocytes using a co-culture system. The results show that expression levels of Nrf2-regulated antioxidant factors, such as glutathione and heme oxygenase-1, in HaCaT keratinocytes (HaCaT KCs) are up-regulated in the presence of normal human dermal fibroblasts (NHDFs). In addition, the secretion of pro-inflammatory molecules, including interleukin-1α (IL-1α) and prostaglandin E2 (PGE2), is suppressed in co-cultures of NHDFs and UVB-irradiated HaCaT KCs. Interestingly, the localization of Nrf2 protein in HaCaT KCs was immediately translocated from the cytoplasm to the nucleus after the co-culture with NHDFs. These results suggest the possibility that Nrf2 signaling in keratinocytes is regulated in cooperation with dermal fibroblasts.
Epidermal keratinocytes are located in the outermost layer of the skin and are continuously affected by external stimuli such as sunlight, air pollution and changes of humidity and temperature due to seasonal variations. Among those, UV radiation causes DNA damage in keratinocytes due to the formation of cyclobutane pyrimidine dimers and (6-4) photoproducts, which increases the frequency of skin disorders including skin cancers.1) Also, reactive oxygen species (ROS) in keratinocytes, which are generated by UV through the influx of calcium, promote skin aging characterized by the formation of hyperpigmented spots, deep wrinkles and sagging via autocrine and paracrine signaling.2–4) Therefore, the protection of keratinocytes against those stresses is quite important not only to maintain cell homeostasis but also to prevent skin aging.
Keratinocytes have mechanisms to protect themselves against external stimuli not only by themselves but also by cooperating with neighboring cells, including epidermal melanocytes and fibroblasts located in the papillary layer of the dermis just below the basement membrane. For instance, keratinocytes protect their nuclear DNA against UV damage by incorporating melanosomes into the nuclei of surrounding cells by activating melanogenesis in melanocytes.5) In addition, it has been reported that the proliferation and migration of keratinocytes is activated by the presence of dermal fibroblasts during the process of wound healing due to the secretion of growth factors from fibroblasts.6) Thus, it is considered that the protective reactions of keratinocytes are complemented by cooperating neighboring cells under stress situations.
It is known that the one of fundamental mechanisms of keratinocytes to protect themselves against stresses is nuclear factor (NF)-E2-related factor 2 (Nrf2)-Kelch-like ECH-associated protein 1 (Keap1) signaling.7) When cells are exposed to oxidative stress, Nrf2 dissociates from Keap1, translocates to the nucleus and up-regulates the expression of antioxidant factors such as glutathione (GSH), heme oxygenase-1 (HO-1) and reduced nicotinamide adenine dinucleotide phosphate (NADPH) quinone oxidoreductase 1 (NQO1), which results in ameliorating oxidative stress.7,8) In addition, Nrf2 improves inflammatory situations by suppressing the production of pro-inflammatory molecules such as interleukin-1α (IL-1α) and prostaglandin E2 (PGE2).9,10) On the other hand, several studies have reported that skin aging symptoms are increased by reductions of Nrf2,11,12) and in fact, the amount of Nrf2 is reduced in elderly skin and in regions at solar lentigos, which are typical characteristics of photoaged skin.13) The sum of these facts indicates that Nrf2 signaling plays a pivotal role in the skin aging process.
Since Nrf2 expression and activity in keratinocytes is easily affected by stresses, it is possible that Nrf2 signaling in keratinocytes is modulated by neighboring cells such as dermal fibroblasts. In this study, we examined whether the presence of fibroblasts affects Nrf2 signaling in keratinocytes.
Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Sigma-Aldrich (MO, U.S.A.). Fetal bovine serum (FBS) was purchased from Hyclone (UT, U.S.A.). The Power SYBR Green Cells-to-CT kit and PCR primers were purchased from Thermo Fisher Scientific (MA, U.S.A.). The anti-Nrf2 polyclonal antibody (ab31163) and immunoglobulin G (IgG) H&L (Alexa Fluor 594, ab150080) were purchased from Abcam (U.K.). Hoechst 33342 was purchased from Dojindo (Japan). Diethyl Maleate (DEM) was purchased from Tokyo Chemical Industry (Japan). The human IL-1 alpha/IL-1F1 Quantikine enzyme-linked immunosorbent assay (ELISA) kit was purchased from R & D Systems (MN, U.S.A.). The prostaglandin E2 ELISA kit was purchased from Cayman Chemicals (MI, U.S.A.).
Cell CultureHaCaT keratinocytes (HaCaT KCs) were purchased from CLS Cell Lines Service GmbH (Eppeiheim, Germany), and normal human dermal fibroblasts (NHDFs) derived from human foreskin tissues were obtained from Kurabo (Japan). All cells were cultured in DMEM containing 10% FBS at 37 °C in a 5% CO2 atmosphere.
Co-culture of HaCaT KCs with NHDFsHaCaT KCs were seeded in transwell plates (6-Well Millicell, Hanging Cell Culture Insert, 1.0 µm PET, Millipore, MA, U.S.A.) in DMEM containing 10% FBS, and NHDFs were seeded in 6-well plates in DMEM containing 10% FBS. The transwells seeded with HaCaT KCs were set on the top of 6-well plates seeded with NHDFs and were used for the following examinations.
UVB IrradiationAfter incubating HaCaT KCs with or without NHDFs using the co-culture system for 24 h, only the transwells with HaCaT KCs were moved to other plates and were exposed to 60 mJ/cm2 UVB. HaCaT KCs pretreated with DEM for 24 h were also exposed to 60 mJ/cm2 UVB. The UVB source used was a FL20SE UVB broadband lamp (Toshiba, Japan) and radiation energies were measured using a DERMARY UV DETECTOR (Terumo Clinical Supply, Japan).
Real-Time PCRTotal RNAs were extracted from HaCaT KCs, and cDNAs were synthesized using a Power SYBR Green Cells-to-CT kit. Real-time PCR was performed with SYBR Green Master Mix using an Eco Real-Time PCR System (Illumina, CA, U.S.A.). The following primer sets for NQO1, HO-1, glutamate-cysteine ligase catalytic subunit (GCLC) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were used as follows: NQO1 forward: 5′-GTGGCAGTGGCTCCATGTACTC-3′, NQO1 reverse: 5′-GAGTGTGCCCAATGCTATATGTCAG-3′, HO-1 forward: 5′-TTGCCAGTGCCACCAAGTTC-3′, HO-1 reverse: 5′-TCAGCAGCTCCTGCAACTCC-3′, GCLC forward: 5′-GCATTATTGACGAACTGGCTACA-3′, GCLC reverse: 5′-CTTAATCAATTTCTGGCTCACTGG-3′, GAPDH forward: 5′-GCACCGTCAAGGCTGAGAAC-3′, GAPDH reverse: 5′-TGGTGAAGACGCCAGTGGA-3′. Data were analyzed according to the ΔΔCt method.
ImmunostainingHaCaT KCs were fixed with 4% formaldehyde for 15 min at room temperature. After blocking non-specific binding with 1% IgG-free bovine serum albumin (BSA), 2 µg/mL anti-Nrf2 antibody were used for immunofluorescent staining. Two micrograms per milliliter IgG H&L (Alexa Fluor 594) were used to label antibodies that had reacted with Nrf2. Cell nuclei were stained with Hoechst 33342, and confocal images were obtained using a FLoid® Cell Imaging Station (Thermo Fisher Scientific, U.S.A.).
Quantification of GSHAfter preparing cell lysates of HaCaT KCs using 0.5% Triton X-100, the amount of total GSH in each lysate was quantified using the GSH recycling assay.14)
Quantification of IL-1α and PGE2 SecretionThe amounts of IL-1α and PGE2 that were secreted into the medium by HaCaT KCs were quantified using ELISA kits according to the instruction manuals.
Statistical AnalysisAll data are expressed as means ± standard deviation (S.D.). Comparisons between two groups were performed by Student’s t-test and Tukey’s test. A p-value <0.05 is considered statistically significant.
In order to investigate whether dermal fibroblasts are involved with Nrf2 signaling in keratinocytes, we examined the intracellular antioxidation ability via Nrf2 of HaCaT KCs co-cultured with NHDFs. The mRNA expression levels of NQO1, HO-1 and GCLC, which are regulated downstream of Nrf2,7,8) were significantly up-regulated by the co-culture of NHDFs with HaCaT KCs (Figs. 1a–c). Intracellular GSH levels in HaCaT KCs also increased significantly in the presence of NHDFs (Fig. 1d). Interestingly, the localization of Nrf2 protein in HaCaT KCs was translocated from the cytoplasm into the nucleus at 2 h after the co-culture with NHDFs (Fig. 1e). These results indicated that the intracellular antioxidant capacity via Nrf2 in HaCaT KCs was enhanced in the presence of NHDFs.

Transwell inserts seeded with HaCaT KCs were placed on top of 6-well plates seeded with NHDFs and were co-cultured. After co-culture for 6 h, the mRNA expression levels of (a) NQO1, (b) HO-1 and (c) GCLC in HaCaT KCs were measured using real-time PCR. (d) After co-culture for 24 h, the amount of total GSH in HaCaT KCs was quantified using the GSH recycling assay. Bars indicate means ± S.D. (n = 3). Significance: * p < 0.05, ** p < 0.01. (e) After co-culture for 2 h, Nrf2 protein in HaCaT KCs was immunostained and fluorescent images were obtained using a Floid® Cell Imaging Station. Red: Nrf2, Blue: Hoechst 33342 (nucleus). Scale bar: 100 µm.
It is known that Nrf2 attenuates inflammatory responses, such as the excess secretion of IL-1α and PGE2, under stress conditions like UV irradiation.9,10,12) Thus, we examined the influence of NHDFs on the expression of inflammatory molecules in UVB-irradiated HaCaT KCs. Although the secretion of IL-1α and PGE2 by HaCaT KCs was increased at 48 h after UVB irradiation, those secretion levels were significantly suppressed by the co-culture with NHDFs (Figs. 2a, b). Pretreatment of HaCaT KCs with DEM, which is an activator of Nrf2 signaling that induces the translocation of Nrf2 into the nucleus,15) also suppressed the secretion of IL-1α and PGE2 induced by UVB (Figs. 2c, d). In addition, the mRNA expression level of cyclooxygenase-2 (COX-2), which is an important enzyme in the synthesis of PGE2,16) was down-regulated in HaCaT KCs by the presence of NHDFs at 24 h after UVB irradiation (Fig. 3a). Further, the up-regulated mRNA levels of IL-1β, IL-6 and tumor necrosis factor-α (TNF-α), which are regulated by Nrf2,17) were induced by UVB and were attenuated by the presence of NHDFs (Figs. 3b–d). These results indicated the possibility that the inflammatory response in UVB-exposed HaCaT KCs is ameliorated by interactions with NHDFs.
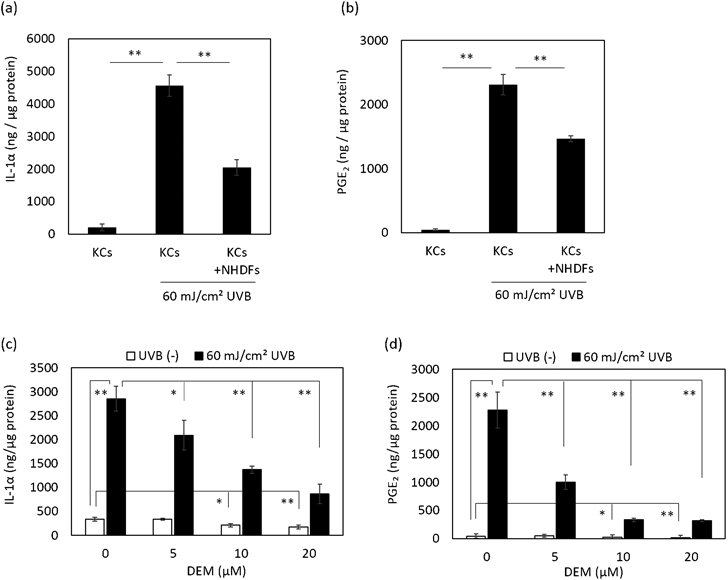
Transwell inserts seeded with HaCaT KCs were placed on top of 6-well plates seeded with NHDFs and were co-cultured for 24 h, after which only the HaCaT KCs were exposed to 60 mJ/cm2 UVB. At 48 h after UVB irradiation, the amounts of (a) IL-1α and (b) PGE2 protein secreted from HaCaT KCs into the medium were quantified by ELISA. Bars indicate means ± S.D. (n = 3). Significance: ** p < 0.01. After treatment of HaCaT KCs with DEM for 24 h, the cells were exposed to 60 mJ/cm2 UVB. At 48 h after the UVB irradiation, the amounts of (c) IL-1α and (d) PGE2 protein secreted from HaCaT KCs into the medium were quantified by ELISA. Bars indicate means ± S.D. (n = 3). Significance: * p < 0.05, ** p < 0.01.
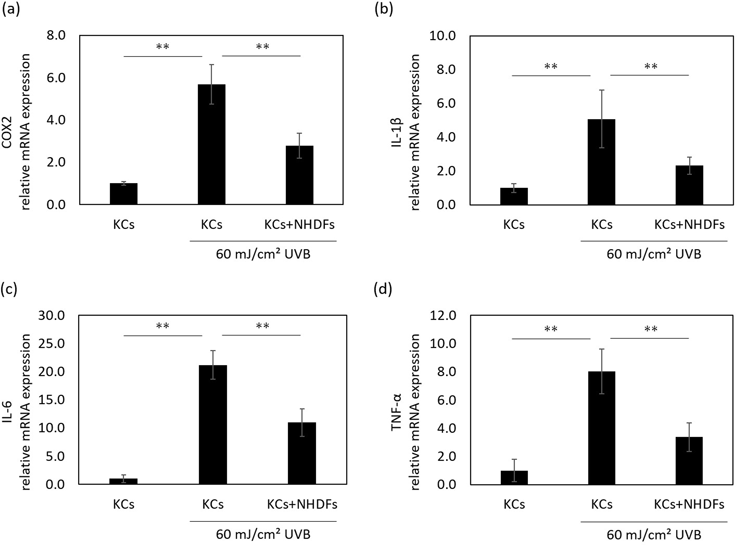
Transwell inserts seeded with HaCaT KCs were placed on top of 6-well plates seeded with NHDFs and were co-cultured for 24 h, after which only the HaCaT KCs were exposed to 60 mJ/cm2 UVB. At 24 h after the UVB irradiation, mRNA expression levels of (a) COX-2, (b) IL-1β, (c) IL-6 and (d) TNF-α in HaCaT KCs were measured using real-time PCR. Bars indicate means ± S.D. (n = 3). Significance: ** p < 0.01.
The results of this study show that inflammatory responses, such as the secretion of IL-1α and PGE2, are suppressed in UVB-irradiated HaCaT KCs by the co-culture with NHDFs (Fig. 2). It has been reported that IL-1α and PGE2, which are secreted by keratinocytes, are involved with the progression of skin aging via paracrine signaling by fibroblasts. IL-1α activates activator protein-1 (AP-1) via extracellular signal-regulated kinase (ERK)/c-Jun N-terminal kinase (JNK) signaling, which results in suppressing the synthesis of type I collagen and increasing the expression of matrix metalloprotease-1 (MMP-1).18–20) PGE2 down-regulates the production of type I collagen through binding to EP receptors in fibroblasts.21) Also, it has been reported that type I collagen and fibrillin-1 fibers formed by fibroblasts were diminished due to the treatment with PGE2.22) These facts suggest that the behavior of IL-1α and PGE2 is directly related to the progression of skin aging. Meanwhile, it is known that ROS is the main factor that stimulates the secretion of IL-1α and PGE2, and in fact, some reports have mentioned that they are regulated by Nrf2.9,10) Furthermore, the expression levels of IL-1α and PGE2 are higher but the expression level of Nrf2 is lower in elderly skin,13,21,23) which indicates that Nrf2 is a key regulator of skin aging.
In this study, we found that Nrf2 signaling in HaCaT KCs is enhanced by the presence of dermal fibroblasts. In particular, Nrf2 in HaCaT KCs was immediately translocated into the nucleus after the co-culture with NHDFs (Fig. 1e). It has been reported that the expression of Nrf2 and substances downstream of Nrf2 signaling in keratinocytes are decreased immediately by UVB,24) and results in the enhancement of excess inflammatory responses. In this study, the UVB-induced secretion of IL-1α and PGE2 was suppressed in HaCaT KCs co-cultured with dermal fibroblasts as well as in HaCaT KCs treated with DEM, which stimulates the translocation of Nrf2 into the nucleus (Fig. 2). Those results indicated that the activation of Nrf2 signaling in HaCaT KCs co-cultured with fibroblasts (Fig. 1) ameliorated the inflammatory responses stimulated by UVB. Thus, it is anticipated that Nrf2 in HaCaT KCs is activated but the effect on its expression is not yet determined. At present, the mechanism by which fibroblasts stimulate Nrf2 signaling in HaCaT KCs is still unknown. In general, Nrf2 activation is caused by the dissociation of Nrf2 from Keap1 due to the oxidation of SH groups in Keap1 by ROS.25,26) Therefore, it is possible that the activation of Nrf2 in keratinocytes may be affected by oxidative stress derived from the presence of fibroblasts. It is also a possibility is that some factor(s) secreted from fibroblasts may activate Nrf2 signaling in HaCaT KCs. It has been reported that transforming growth factor-β (TGF-β) activates Nrf2/HO-1 signaling, and the expression of TGF-β in dermal fibroblasts is reduced in elderly skin.27,28) Those facts suggest that growth factors such as TGF-β secreted from fibroblasts may be involved in the activation of Nrf2 and the impairment of Nrf2 signaling with age in keratinocytes.
Of course, there are several types of cells in the skin, such as melanocytes and Langerhans cells in the epidermis, and mast cells, dendritic cells and macrophages in the dermis. In this study, we investigated the involvement of fibroblasts in the activation of Nrf2 in HaCaT KCs. However, it is currently unclear whether the activation of Nrf2 is a specific effect of fibroblasts on keratinocytes. Therefore, we will continue to study this system in order to demonstrate the mechanism of Nrf2 activation by fibroblasts and its specificity for other types of cells in the skin, and will report those findings in the future.
The authors declare no conflict of interest.