2023 Volume 46 Issue 5 Pages 672-683
2023 Volume 46 Issue 5 Pages 672-683
Evidence suggests that CXC motif chemokines are involved in neuronal injury and inflammatory processes. Bioinformatics analysis by using data from the Gene Expression Omnibus (GEO) database was performed and identified CXC motif chemokine ligands (CXCLs) as associated with diabetic peripheral neuropathy (DPN). The present study focused on CXC motif chemokine ligand 2 (CXCL2), and the role and potential mechanisms of CXCL2 in DPN were investigated. The DPN rat model was generated by streptozotocin (STZ) injection in vivo, and high-glucose (HG)-stimulated Schwann cell RSC96 was considered a cell model of DPN in vitro. Neuropathic symptoms of DPN were explored by neurological tests and histological examinations. DPN rats showed a decreased level of motor nerve conduction velocity (MNCV) along with typical histological changes. CXCL2 expression was significantly increased in STZ-induced DPN rat sciatic nerve and HG-induced RSC96 cells. Functionally, CXCL2 knockdown inhibited cell apoptosis and inflammation activation under diabetic conditions in vitro and in vivo. CXCL2 knockdown increased cell viability in HG-treated RSC96 cells and reduced apoptosis concerning the decreased expression of cleaved Caspase 3/9. In addition, CXCL2 knockdown protected against NOD-like receptor protein 3 (NLRP3) inflammasome activation and reduced levels of pro-inflammatory cytokines, interleukin (IL)-1β and IL-18. The repressive effects of CXCL2 knockdown on inflammasome activation under HG conditions were significantly abolished by treatment of the NLRP3 activator nigericin. In conclusion, these results indicated that CXCL2 knockdown exhibited amelioration of hyperglycemia-induced DPN by inhibiting cell apoptosis and NLRP3 inflammasome activation, suggesting that targeting CXCL2 might be a potential strategy for DPN treatment.
Diabetic peripheral neuropathy (DPN) is one of the serious complications of diabetes with complicated pathogenesis.1) DPN is associated with several clinical symptoms such as varying degrees of nerve damage, paraesthesia, and sensory loss, and affects approximately half of adults with diabetes.2,3) However, effective therapies are lack specificity and not available currently.4) Thus, it is necessary to find efficient approaches against DPN based on the exact mechanism involved in the pathogenesis. Although the precise pathogenesis of DPN is complex, inflammation and Schwann cell dysfunction are thought to be the major pathogenic factors.2,5)
The inflammatory response is one of the essential pathologic features that link to the onsets and progression of DPN.6,7) Inflammasome-driven inflammation contributes to a wide range of inflammatory reactions, and targeting the NOD-like receptor protein 3 (NLRP3) inflammasome pathway is vital for the treatment of inflammation-related diseases, such as pulmonary disease, asthma, coronavirus disease 2019, and DPN.8–10) NLRP3 inflammasome is activated after stimulation, followed by a series of immune responses, such as NLRP3 inflammasome proteins (molecule NLRP3, the adaptor molecule ASC, and Caspase-1) production, Caspase 1-dependent release of the pro-inflammatory cytokines, and pyroptotic cell death.11–13) Additionally, Schwann cell loss or apoptosis commonly occurred in both clinic DPN patients and experimental animals, and inhibition of Schwann cell dysfunction ameliorated DPN progression.14,15) Therefore, the reduction of inflammasome activation and Schwann cell apoptosis might be potential strategies for treating DPN.
CXC motif chemokine ligand 2 (CXCL2) is a neutrophil chemoattractant that belongs to the CXC chemokine family.16) Chemokines are commonly produced at inflammation sites and mediate inflammatory responses by recruiting and promoting neutrophils to injury tissues under pathological stimuli.17) Recent reports have indicated that CXCL2 exerts extensive physiological functions by mediating inflammation.18,19) Blockade of CXC chemokine and deficiency of its receptors provide a level of protection against inflammation by reducing neutrophil recruitment.20–22) The regulatory roles of CXCL2 in tumor progression and osteoblast differentiation have also been described in previous reports.23,24) Excess CXCL2 activation triggered by continuous inflammatory stimuli could lead to severe tissue damage and inflammation-associated diseases.25,26) Previous study reported that the expression of interleukin (IL)-1β-mediated CXCL2 exacerbated islet inflammation by promoting neutrophil migration.27) In addition, CXCL2 is involved in neuroinflammation and is upregulated in injured sciatic nerves.28) However, the potential roles of CXCL2 in the progression of DPN remain unclear. Interestingly, recent research reported that CXCL2 might be a candidate biomarker of DPN and involved in the progression of DPN by performing a microarray assay.29) Given all these data, we speculated that CXCL2 might regulate DPN progression through modulation of Schwann cell dysfunction and inflammation process.
Bioinformatics analysis was performed to identify the potential biomarker of DPN. CXCL2 was identified as the candidate gene, and the effect of CXCL2 on experimental hyperglycemia-induced DPN and the underlying molecular mechanisms were investigated. CXCL2 was found to be highly expressed in the rat sciatic nerve of DPN and high-glucose (HG)-induced Schwann cell RSC96 cells. Moreover, the functional role of CXCL2 gene deficiency in hyperglycemia-induced DPN was investigated both in vitro and in vivo. Mechanistically, CXCL2 knockdown attenuated inflammasome activation by decreased NLRP3 inflammasome expression. Overall, the results of the present study indicated that CXCL2 might be a candidate biomarker for DPN by suppressing cell apoptosis and the action of NLRP3 inflammasome.
Array data of GSE147732 was obtained from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/). The differentially expressed genes were screened by GEO2R (http://www.ncbi.nlm.nih.gov/geo/geo2r/). CXC chemokines expression in DPN tissues was visualized using heatmaps. The p value <0.05 and |log2FC| > 1 were set as significant. Gene Ontology (GO) enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways assays were performed using the DAVID (https://david.ncifcrf.gov/) to annotate the biological roles of CXC chemokines. In addition, a protein–protein interaction (PPI) network was developed to evaluate the correlation among selected proteins.
Animal ModelAll procedures that involved animals were approved by the Use Committee of the First Affiliated Hospital of Zhengzhou University (No. KS-2020-06-07). Male Sprague Dawley rats (Six-week-old) were maintained under a standard condition. The DPN model was established by administering 55 mg/kg streptozotocin (STZ, MP Biomedicals, China), and normal rats received citrate buffer. Rats with blood glucose levels more than 16.7 mmol/L were defined as diabetic. In addition, related neurological changes were detected by measuring motor nerve conduction velocity (MNCV) at 0, 2, 4, and 6 weeks after the diabetes induction. Rats were randomly assigned to four groups and treated as follows: (1) Control, (2) DPN, (3) DPN + negative control short hairpin RNAs (shRNAs) (sh-NC), DPN rats treated with 1 × 108 TU/mL empty lentiviral vectors, and (4) DPN + sh-CXCL2, DPN rats that treated with 1 × 108 TU/mL lentivirus carrying shRNAs specific for CXCL2. After 6 weeks of induction, the sciatic nerves and serum were collected for further experiments. The sciatic nerves were processed and embedded in paraffin for morphological measurement.
Histological ExaminationParaffin-embedded sciatic nerves were processed on slides for morphological study. The sections were successively deparaffinized, dehydrated, and stained with hematoxylin–eosin (H&E) or toluidine blue (TB) for morphological assessment following the method routinely. The histologic sections of the sciatic nerve from the DPN rat were viewed using light microscopy (Olympus, Japan). In addition, the myelin sheath structure in the rat sciatic nerves was detected by transmission electron microscopy (TEM) observation.
Cell Culture and TreatmentSchwann cell RSC96 was obtained from iCell Bioscience (iCell-r030, China) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) medium (G4528, Servicebio, China). RSC96 cells were cultivated in a medium with normal glucose (5.6 mM, D-glucose; NG) or high glucose (25 mM, D-glucose, HG) for 48 h. To elucidate the function of CXCL2, RSC96 cells were transfected with two desired small interfering RNAs (siRNAs) (si-CXCL2-1 and si-CXCL2-2) or a negative control siRNA (si-NC) using Lipofectamine 3000 (Invitrogen, U.S.A.) and siRNA sequences were shown in Supplementary Table 1. After transfection for 24 h, cells were treated with the high glucose, followed by incubation with nigericin (20 µM, Yuanye Bio-Technology, China) or vehicle for 30 min to evaluate the effects of NLRP3 agonists on RSC96 reactions. To investigate the effect of CXCL2 on apoptosis, HG-treated RSC96 cells were co-cultured with recombinant CXCL2 (rCXCL2; 50 ng/mL, 400–11, PeproTech, U.S.A.) for 24 h.
Quantitative Real-Time PCR (qRT-PCR)Total RNA was extracted from sciatic nerves and HG-treated RSC96 cells with the TRIpure reagent (RP1001, BioTeke, China) and reverse-transcription was performed to gather cDNA. The real-time PCR reactions were conducted using SYBR Green PCR system (Exicycler96, BIONEER, Korea). The PCR program consisted as: 94 °C, 5 min; 94 °C for 10 s; 60 °C for 20 s, for 40 cycles. Relative quantities were calculated using the 2−ΔΔCt method with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as inner control. The primers used for PCR were as follows: CXCL2 sense, 5′-ACTGGTCCTGCTCCTCCT-3′, and anti-sense, 5′-TTAGCCTTGCCTTTGTTC-3′; GAPDH sense, 5′-CGGCAAGTTCAACGGCACAG-3′, and anti-sense, 5′-CGCCAGTAGACTCCACGACAT-3′.
Western Blot and Enzyme-Linked Immunosorbent Assay (Elisa) AnalysisProtein samples were extracted using radio immunoprecipitation assay (RIPA) buffer (R0010, Solarbio, China) and quantified by BCA Protein Assay Kits (PC0020, Solarbio). The proteins were electrophoretically separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes. After blocking with 1% bovine serum albumin (BSA), the samples were reacted with the primary antibodies overnight at 4 °C prior to incubation with the corresponding horseradish peroxidase (HRP)-conjugated secondary antibody for 1h. Bands were then visualized using an enhanced chemiluminescence solution (PE0010, Solarbio). Antibodies used in the Western blot were shown in Supplementary Table 2. The concentrations of CXCL2 in rat serum were evaluated using the commercial quantitative Rat CXCL2 Elisa Kit (EK3142, Multisciences biotech, China). The concentration of cytokines in the cell supernatant including IL-1β (EK301B, Multisciences biotech) and IL-18 (ER0036, Wuhan Fine Biotech, China) were analyzed by Elisa Kits, as per the manufacturer’s protocols.
ImmunohistochemistryParaffin embedded-sciatic nerve sections were immune-blocked with 1% BSA and then incubated with CXCL2 antibody (bs-1162R, 1 : 100, Bioss, China) at 4 °C overnight and then reacted with HRP-conjugated secondary antibody (#31460, ThermoFisher, U.S.A.). After staining with the dimethylaminoazobenzene (DAB) solution, the sections were counterstained with hematoxylin and mounted. The pictures were obtained under a light microscope (Olympus, Japan).
Cell Viability AssayRSC96 cells were seeded onto a 96-well plate at a density of 3 × 103 cells per well. The transfected cells were then treated with high glucose. Cell counting kit-8 (CCK-8, C0037, Beyotime, China) reagent was added and cultured for another 2 h. The optical density values were measured using a microplate reader (Biotek, U.S.A.) at 450 nm.
Flow Cytometry DetectionRSC96 cells were seeded in a 6-well plate. Annexin V-propidium iodide (PI) staining was carried out using an Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection Kit (KGA106, KeyGEN, China). RSC96 cells were stained with PI staining solution in the dark, and the stained cells were analyzed by flow cytometry (NovoCyte, U.S.A.).
ImmunofluorescenceFor immunofluorescence analysis, HG-treated RSC96 cells were permeabilized with 1% Triton X-100 (ST795, Beyotime) for 30 min. After blocking with 1% BSA, cells were incubated with antibodies against NLRP3 (bs-6655R, Bioss) and cleaved Caspase-3 (AF7022, Affinity, China) overnight at 4 °C, and nuclei were counterstained with 4′-6-diamidino-2-phenylindole (DAPI). The immunofluorescence images were obtained under a microscope (Olympus, Japan).
FAM-FLICA Caspase-1 AssayFor determination of the presence of active Caspase-1, RSC96 cells were incubated with FAM-FLICA Caspase-1 probe (#9161, ImmunoChemistry Technologies, U.S.A.) as per the manufacturer’s protocols. For nuclear staining, the cell nuclei were stained with DAPI and mounted. The slides were viewed under a fluorescent microscope (Olympus, Japan).
Terminal Deoxynucleotidyl Transferase-Mediated Deoxyuridine Triphosphate Nick-End Labeling (TUNEL) AssayParaffin embedded-sciatic nerve sections were permeabilized with 0.1% Triton X-100 (ST795, Beyotime) for 8 min. Samples were stained with TUNEL staining reagents for 1 h at 37 °C and then counterstained with DAPI. TUNEL-stained cells were visualized with a microscope (Olympus, Japan) and calculated.
Statistics AnalysisResults in this study were presented as mean ± standard deviation (S.D.). Statistical differences between the two groups were analyzed by Student’s t-test, and one-way ANOVA was used, followed by the Tukey’s post hoc test for multiple comparisons in GraphPad Prism software. p < 0.05 was taken as significant.
We first screened DPN-related differential genes in the GSE147732 dataset. GEO is a freely accessible database, from which DPN tissue gene expression profile of the GSE147732 dataset was obtained. Specifically, in the GSE147732 dataset, using p-value <0.05 and |log2FC| > 1 as a cut-off criterion, a total of 12 CXC chemokine ligands were identified from the GSE147732 profile datasets (Fig. 1a), including 4 downregulated CXC chemokine ligands and 8 upregulated CXC chemokine ligands in the DPN nerve tissues. Hierarchical cluster analysis was performed and a heat map was drawn to show the expression of the 12 CXC chemokine ligands from the GSE147732 dataset in the DPN and control group (Fig. 1b). Among these genes, CXCL2 was highly expressed in the DPN group. Enriched GO and KEGG pathway analyses of identified CXC chemokines were performed using the online biological tool DAVID. As indicated in Fig. 1c, the enriched GO terms including biological process and molecular function were primarily enriched in chemokine-mediated signaling pathway and chemokine activity. In addition, in the KEGG pathway analysis, CXC chemokines were mainly enriched in the chemokine signaling pathway and inflammation-related pathway. Furthermore, to further screen hub genes, a PPI network was performed to investigate the interaction and expression among these differentially expressed genes. A significant module from the PPI network complex was selected, which was mainly associated with CXC chemokines (Fig. 1d).

(a) A total of 12 CXC chemokine ligands were identified from the GSE147732 profile datasets. Graph showing gene expression between the control group and DPN group, red bars represent upregulated CXC chemokines and blue bars represent downregulated CXC chemokines. p-Value <0.05 and |log2FC| > 1 were considered significant. (b) Heat maps of CXC chemokine ligands positively and negatively correlated with DPN. (c) GO and KEGG analysis of CXC chemokines in biological process and molecular function using the online biological tool DAVID. (d) PPI network of the significant module for DPN vs. control.
DPN model was established by STZ induction and the morphological changes in the DPN rat sciatic nerves were explored. The representative images of sciatic nerve tissue were shown in Fig. 2a. Macroscopically, as demonstrated by H&E and TB staining of the sciatic nerve, control rats showed normal morphological myelinated nerve fibers. Notably, obvious phenotypical alterations were observed in the sciatic nerve of DPN rats, such as loose and disorganized myelinated fibers, and decreased nerve fiber density. In addition, TEM analysis also confirmed that STZ-induced DPN rats displayed disorganized myelin structure and obvious demyelination along with a thinner myelin sheath (Fig. 2a). Results of the neurological test revealed that STZ treatment caused a downregulation level of MNCV compared to the control group at 6 weeks after DPN induction (Fig. 2b). Furthermore, the expression of CXCL2 in the DPN rat was investigated. The concentration of CXCL2 in serum was higher in the DPN model than that in the normal rat (Fig. 2c). Consistently, CXCL2 protein and mRNA levels were significantly increased in the sciatic nerve tissues of DPN rats (Figs. 2d, e). Immunohistochemistry analysis was performed to explore CXCL2 expression in the sciatic nerve. As indicated in Fig. 2f, CXCL2 was widely expressed in STZ-induced DPN rat sciatic nerves.

(a) Neuromorphology images of H&E staining, TB staining, and TEM of the sciatic nerve sections from control and DPN rats. (b) MNCV in the STZ-induced DPN rats at 0, 2, 4, and 6 weeks. (c) Elisa assay results of the serum CXCL2 concentration in control and DPN rats. (d) The mRNA levels of CXCL2 in sciatic nerves of the STZ-induced DPN model. (e) The protein expression and relative quantitative data of CXCL2 in sciatic nerve tissues of the STZ-induced DPN model. (f) Immunohistochemical staining of CXCL2 in sciatic nerves of the STZ-induced DPN model. Data were expressed as mean ± S.D. p < 0.0001 compared with the Control group (n = 6). Red triangle presents the single data results in the bar graph.
RSC96 cells were treated with HG to generate a cell model of DPN in vitro. CXCL2 and its receptor CXCR2 protein expression was remarkably upregulated after treatment of HG in vitro (Fig. 3a). Elisa and qRT-PCR assays further confirmed the increased expression of CXCL2 in HG-induced RSC96 cells (Figs. 3b, c). To investigate the potential role of CXCL2 deficiency in DPN, CXCL2 knockdown was carried out using siRNA in Schwann cells, and transfected cells were then exposed to HG. The interfered efficiency was verified as shown in Fig. 3d. CCK-8 assay was conducted and HG-treated RSC96 cells showed an obvious decrease in cell viability compared to the NG group, while the decreased cell viability of HG-treated cells was significantly increased following CXCL2 knockdown (Fig. 3e).

RSC96 cells were treated with 5.6 mM (NG group) or 25 mM (HG group) glucose for 48 h. (a) Protein expression and quantitation of CXCL2 and CXCR2 in HG-induced RSC96 cells. (b) The mRNA levels of CXCL2 in HG-induced RSC96 cells. (c) Elisa assay results of the CXCL2 concentration in HG-induced RSC96 cells. (d) Protein expression and quantitation of CXCL2 in the HG-induced RSC96 cells after transfection with CXCL2 siRNA. (e) Cell viability in the HG-induced RSC96 cells after transfection with CXCL2 siRNA. * p < 0.05, ** p < 0.01 versus NG group; # p < 0.05, ##p < 0.01 versus HG + si-NC group (n = 3). Red triangle presents the single data results in the bar graph.
We then investigated the function of CXCL2 in the apoptosis of Schwann cells under HG conditions. HG treatment caused an increase in RSC96 cell apoptosis, and RSC96 cells incubating with recombinant CXCL2 further promoted apoptosis under HG conditions (Supplementary Figure), indicating that secreted CXCL2 induced apoptosis. In addition, HG-induced apoptosis was blocked by CXCL2 downregulation (Figs. 4a, b). These data suggested that CXCL2 was associated with HG-induced apoptosis in RSC96 cells. Apoptosis-related protein expression was further investigated in HG-cultured RSC96 cells. As expected, CXCL2 knockdown significantly downregulated cleaved Caspase 3/9 expression, and statistical analysis indicated that downregulation of CXCL2 decreased the cleaved Caspase-3/Caspase-3 and cleaved Caspase-9/Caspase-3 ratio in HG-treated RSC96 cells (Fig. 4c). Moreover, we further marked cleaved Caspase-3 by performing an immunofluorescence assay to verify the effect of CXCL2 deficiency on Caspase-depended apoptosis. The result implicated that the expression of cleaved Caspase-3 in HG-treated RSC96 cells was significantly higher than that in the NG group, while the knockdown of CXCL2 decreased cleaved Caspase-3 levels (Fig. 4d).

(a) Percentages of apoptotic RSC96 cells analyzed by flow cytometry. (b) Apoptotic rate of HG-induced RSC96 cells after transfection with CXCL2 siRNA. (c) Protein expression apoptosis-related proteins cleaved-Caspase-3, cleaved-Caspase-9, Caspase-3, and Caspase-9 in the HG-induced RSC96 cells after transfection with CXCL2 siRNA. Statistical analysis of the cleaved Caspase-3/Caspase-3 and cleaved Caspase-9/Caspase-3 ratio. (d) Immunofluorescence staining of cleaved-Caspase-3 in the HG-induced RSC96 cells after transfection with CXCL2 siRNA. * p < 0.05, ** p < 0.01 versus NG group; #p < 0.05, ## p < 0.01 versus HG + si-NC group (n = 3). Red triangle presents the single data results in the bar graph.
The effect of CXCL2 deficiency on the inflammatory reaction was further investigated. The expression of NLRP3 inflammasome following CXCL2 knockdown under HG conditions was evaluated. As shown in Fig. 5a, NLRP3, ASC, and Caspase-1 p20 protein expression levels were markedly increased in HG-induced RSC96 cells compared to the NG group. CXCL2 knockdown markedly attenuated NLRP3 inflammasome production under HG conditions. In addition, pro-inflammatory cytokines IL-1β and IL-18 were considered biological markers for Caspase-1-induced inflammation.11) Elisa assay indicated that HG-induced RSC96 cells exhibited an elevated concentration of IL-1β and IL-18 in the cell supernatant, and CXCL2 downregulation reversed the elevated expression of IL-1β and IL-18 (Fig. 5b). A fluorescence-labelled Caspase-1 probe was further used to detect active Caspase-1 expression. As indicated in Fig. 5c, the expression level of Caspase-1 was increased under HG conditions, and CXCL2 knockdown decreased Caspase-1 activation (Fig. 5c). Immunofluorescence staining of NLRP3 was further conducted, and NLRP3 was primarily located in the cytoplasm (Fig. 5d). In addition, CXCL2 knockdown by siRNA decreased NLRP3 expression under HG conditions.
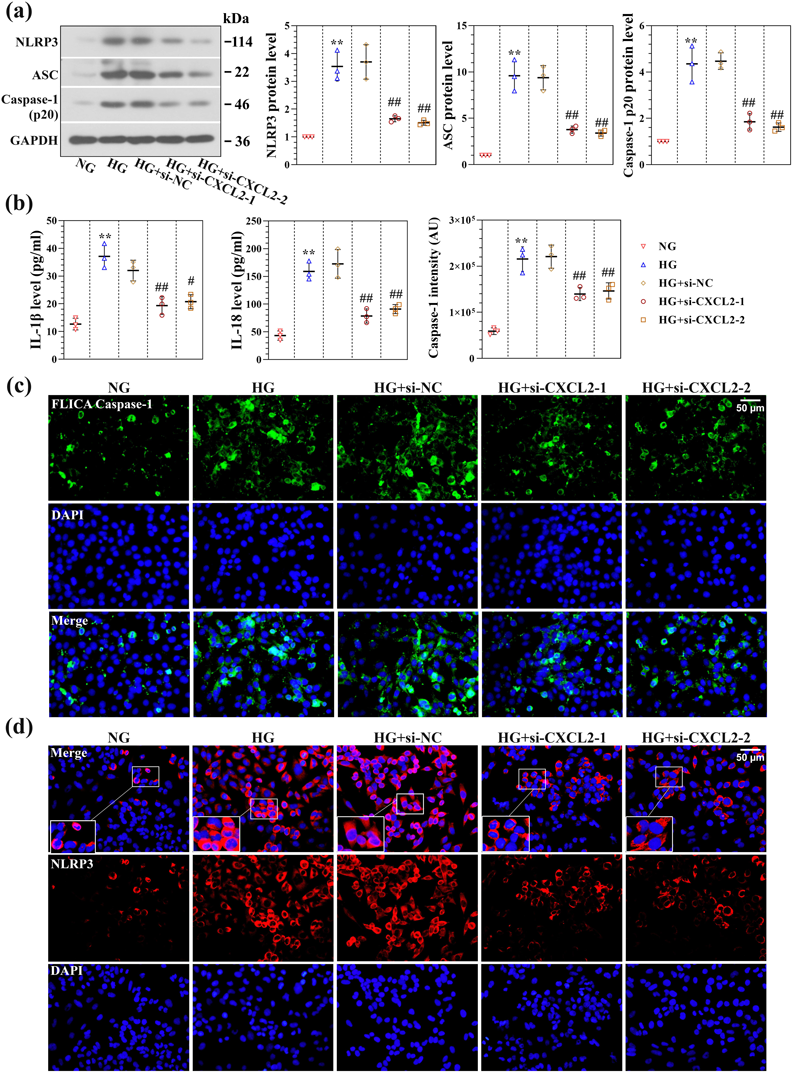
(a) Protein expression and quantitation of NOD-like receptor protein 3 (NLRP3) inflammasomes including NLRP3, ASC, and Caspase-1 p20 in the HG-induced RSC96 cells after transfection with CXCL2 siRNA. (b) Elisa assay results of the IL-1β and IL-18 concentration in the HG-induced RSC96 cells after transfection with CXCL2 siRNA. (c) Caspase-1 activity was detected by FAM-FLIC, and quantification of FAM-FLICA Caspase-1 intensity. (d) Immunofluorescence staining of NLRP3 in the HG-induced RSC96 cells after transfection with CXCL2 siRNA. * p < 0.05, ** p < 0.01 versus NG group; #p < 0.05, ## p < 0.01 versus HG + si-NC group (n = 3). Red triangle presents the single data results in the bar graph.
To further confirm the impact of CXCL2 knockdown on inflammatory reaction, HG-induced RSC96 cells were incubated with nigericin to activate NLRP3 in the absence of CXCL2. As expected, the expression of NLRP3 in CXCL2 silenced RSC96 cells exposed to HG was significantly increased after nigericin treatment (Fig. 6a), suggesting that nigericin reversed the reduction role of CXCL2 deficiency in NLRP3 inflammasome activation. Moreover, IL-1β and IL-18 expression in the cell supernatant, which was reduced by CXCL2 knockdown in HG-induced RSC96 cells, were remarkably elevated upon nigericin treatment (Fig. 6b). NLRP3 inflammasome activation contributes to multiple cell death pathways.30) Therefore, combined staining with Annexin V and PI detected by flow cytometry was performed to investigate the effect of CXCL2 on NLRP3-dependent cell death following NLRP3 inflammasome activation.31–33) As indicated in Fig. 6c, there was a decreased number of Annexin V+/PI+ and Annexin V+/PI− cells in the CXCL2-deficient RSC96 cells, and nigericin stimulation reversed the decreased Annexin V+/PI− and Annexin V+/PI+ cells. The results suggested that CXCL2 knockdown could reduce the mortality of Schwann cells after NLRP3 inflammasome activation.
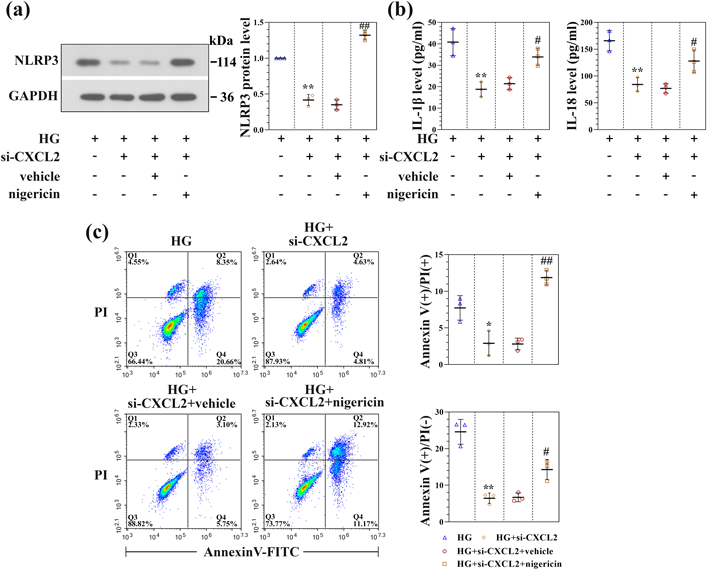
HG-induced RSC96 cells after transfection with CXCL2 siRNA were treated with nigericin or vehicle for 30 min. (a) Protein expression and quantitation of NLRP3 protein in HG-induced RSC96 cells of each group. (b) Elisa assay results of the IL-1β and IL-18 concentration in HG-induced RSC96 cells of each group. (c) Cell death was detected using Annexin V-FITC/PI staining. Statistical analysis of Annexin V+ /PI+ and Annexin V+ /PI − in HG-induced RSC96 cells of each group. * p < 0.05, ** p < 0.01 versus HG group; # p < 0.05, ## p < 0.01 versus HG + si-CXCL2 + vehicle group (n = 3).
Given that CXCL2 was associated with cell apoptosis and NLRP3 inflammasome activation in vitro, we then validated the role of CXCL2 in STZ-induced DPN rats. DPN rats were treated with the lentivirus carrying shRNAs specific for CXCL2, and the expression of CXCL2 in DPN nerve tissues was verified by Western blot assay (Fig. 7a). TUNEL assay was performed to investigate the effect of CXCL2 on cell apoptosis. As indicated in Fig. 7b, CXCL2 knockdown inhibited STZ-induced cell apoptosis in DPN nerve tissues, as indicated by the decreased number of TUNEL-positive cells. Consistently, the increased expression of NLRP3 and ASC in DPN nerve tissues was significantly decreased following CXCL2 knockdown (Fig. 7c).

DPN rats were treated with the lentivirus carrying shRNAs specific for CXCL2. (a) Protein expression and quantitation of CXCL2 protein in STZ-induced DPN rats of each group. (b) Representative images of TUNEL stained sciatic nerve. Statistical analysis of TUNEL-positive cells in STZ-induced DPN rats of each group. (c) Protein expression and quantitation of NLRP3 and ASC protein in STZ-induced DPN rats of each group. ** p < 0.01 versus control group; # p < 0.05, ## p < 0.01 versus DPN + sh-NC (n = 6).
DPN is a common diabetic complication with complicated pathogenesis such as neuroinflammation and Schwann cell dysfunction.34) In the present study, a bioinformatics assay was performed to identify candidate biomarkers in DPN. Interestingly, CXC motif chemokines were screened as associated genes in the STZ-induced DPN model. Among these identified CXC chemokine ligands, CXCL2 was highly up-regulated in DPN tissues, which was selected as a candidate marker in DPN. CXCL2 is also known as macrophage inflammatory protein and exerts its function by binding and activating CXCL2 receptor CXCR2.24) Chemokine CXCL2 regulated a variety of cell functions and mediated the inflammation process.24,35,36) In the present study, a hyperglycemia-induced DPN model was conducted to demonstrate the functional role of CXCL2 in DPN progression. CXCL2 expression was evaluated and we observed an enhanced expression of CXCL2 in the DPN model. Furthermore, Schwann cells were treated with high glucose to mimic DPN in vitro. Functional experiments suggested that conditional knockdown of CXCL2 promoted cell viability, and inhibited cell apoptosis and inflammasome activation under HG conditions. Moreover, the inhibitory effects of CXCL2 knockdown on inflammatory reaction could be eliminated after treatment with nigericin. Accordingly, the protective effect of CXCL2 deficiency against hyperglycemia-induced DPN was confirmed in this study, thus, targeting CXCL2 might be a great choice for the treatment of hyperglycemia-induced DPN.
Previous studies reported that chemokine CXCL2 and chemokine receptors expression were markedly upregulated in diabetes and in inflammatory diseases.37,38) More importantly, CXCL2 was one of the top 10 upregulated genes among the differentially expressed genes in experimental DPN and was considered a candidate biomarker in DPN progression.29) CXCL2 was associated with a peripheral nerve injury, and CXCL2 was increased after induction of trigeminal neuropathic pain and decreased gradually, conversely, anti-CXCL2 alleviated neuropathic pain.39) Dorsal root ganglia neurons lesion is one of the pathological changes of DPN, and it has been indicated that CXCL2 was expressed in rat dorsal root ganglia neurons and inhibited axon growth.40) In accordance with the results of previous research, we now provided the same results that CXCL2 was significantly increased in the sciatic nerve of the DPN model. In addition, HG-induced Schwann cells were created and CXCL2 was also highly expressed in vitro, consistent with earlier studies.41)
DPN is tightly implicated in multiple etiological factors, including neuroinflammation and neuronal apoptosis.42) Numerous studies reported that hyperglycemia could mediate Schwann cell apoptosis during DPN progression.14,43) Importantly, apoptosis of Schwann cell is a vital mechanism or characteristic of the pathogenesis of DPN, and preventing cell apoptosis or damage is an important strategy for DPN treatment.44) For example, a recent study demonstrated that nerve growth factor exerted neuroprotective effects through decreasing apoptosis of Schwann cells in the DPN rat model.45,46) CXCL2 was involved in neuronal apoptosis,47) and inhibition of the CXCL2 receptor attenuated neuronal death.48) Therefore, the effect of CXCL2 on the apoptosis of Schwann cells was clarified in the present study. Schwann cells were incubated with recombinant CXCL2 to mimic the exogenous secretion of CXCL2, and secreted CXCL2 promoted cell apoptosis. CXCL2 deficiency protected against loss of cell proliferation and could inhibit Schwann cell apoptosis. These data indicated the secreted CXCL2 promoted apoptosis in Schwann cells, and the regulation of Schwann cell dysfunction by CXCL2 might be a potential strategy for DPN.
The inflammatory reaction is one of the critical pathogenetic features of DPN.49) Forced production of CXCL2 through macrophages and β-cells promoted pancreatic islets inflammation in diabetes, and blocking of CXCL2 expression by CXCR2 antagonist alleviated inflammatory response and the development of diabetes.50) Another excellent study further indicated that reduced expression of CXCL2 inhibited neutrophil infiltration and suppressed encephalomyelitis progression.51) The formation and activation of NLRP3 inflammasome contribute to a series of inflammatory reactions such as the activation of inflammatory cells, production of pro-inflammatory cytokines, as well as to NLRP3-dependent cell death.9,13,52) It is widely known that activation of NLRP3 and gasdermin D-mediated pyroptosis involve in the development of DPN.53) Moreover, chemokines CXCL2 has been found to regulate NLRP3 inflammasome in activation in macrophages.54) Here, our study demonstrated that CXCL2 might be involved in the development of DPN by regulating the activation of the NLRP3 inflammasome, the expression of pro-inflammatory cytokines, as well as Schwann cell death. Upon NLRP3 inflammasome activation, cell membrane integrity was destroyed and cell death was progressively triggered that releasing pro-inflammatory cytokines to activate inflammatory responses.30) The crosstalk between pyroptosis and apoptosis after NLRP3 inflammasome activation has been described in previous studies.55–57) We found that treatment of stimulator of NLRP3 abrogated the protective effects of CXCL2 deficiency in the activation of NLRP3 and corresponding cell death. There is no evidence that CXCL2 can attenuate DPN progression by directly restraining pyroptosis, the possibility of CXCL2 to affect pyroptosis in the development of DPN is not excluded, and the mechanism underlying NLRP3 inflammasome activation and pyroptosis in DPN require further studies in the future.
An integrated bioinformatics assay is a useful approach for screening potential biomarkers in human diseases. Bioinformatics analysis was performed to explore the meaningful bioinformatics of CXC chemokines in DPN. However, the samples in the gene expression profile were relatively low in the present study. In addition, the cellular component pathways of CXC chemokine in DPN were undefined in the present study, which is to be addressed in the future. The effect of CXCL2 on cell apoptosis was detected by the Annexin V/PI staining method, although fluorescence compensation strategies have been attempted, it is difficult to avoid fluorescence leakage. This is an important limitation of this study.
In summary, our findings implicated that blockage of CXCL2 could ameliorate DPN progression through suppressing cell apoptosis and the activation of NLRP3 inflammasome. Our study indicates that inflammatory chemokine CXCL2 might be associated with the pathogenesis of DPN, and targeting CXCL2 might be a potential strategy for the treatment of DPN.
This work was supported by the Joint Construction Project of Henan Medical Science and Technology Research Plan (Grant No. LHGJ20220973).
The authors declare no conflict of interest.
The datasets are available from the corresponding author on reasonable request.
This article contains supplementary materials.